1Department of Botany, Christian College, Kattakada, Thiruvananthapuram, Kerala
2Department of Botany, Bishop Moore College, Mavelikkara, Kerala – 690110
3Plant Biochemistry and Molecular Biology Lab, Department of Botany, University College, Trivandrum
695 034, Kerala
Article Publishing History
Received: 05/11/2016
Accepted After Revision: 20/12/2016
Desiccation-tolerance (DT), the ability to lose virtually all free intracellular water and then recover normal function upon rehydration, is one of the most remarkable features of ferns. The phenomenon of desiccation tolerance in pteridophytes is well known and many species can with stand drying with water content to 10–20% of their dry weight and return to normal metabolism and growth following rehydration. The time required to recover from desiccation increases with duration of desiccation. Hence the present investigation was carried to reveal the biochemical adaptations against desiccation and also subsequent rehydration in Adiantum latifolium Lam.a fern. Experimental design for desiccation periods was1 D (24 h), 2 D (48 h) and 3 D (72 h) in a desiccator contain PEG. Desiccation caused an enhanced production of reactive oxygen species (ROS) and increased lipid peroxidation. The analytical data were substantiated by histochemical localization. Total phenolic content increased with desiccation. Fractionation of phenols revealed a pool of phenolic acids. Further, high induced level of polyphenol oxidase and phenylalanine ammonia lyase enzyme activates provide tolerance to desiccation. Further studies are planned to analyze the molecular mechanism involved in providing desiccation tolerance in the fern.
Desiccation-Tolerance; Rehydration; Reactive Oxygen Species; Lipid Peroxidation
Lubaina A. S, Brijithlal N. D, Murugan K. Unravelling Desiccation and Rehydration Tolerance Mechanism in the Fern, Adiantum Latifolium. Biosc.Biotech.Res.Comm. 2016;9(4).
Lubaina A. S, Brijithlal N. D, Murugan K. Unravelling Desiccation and Rehydration Tolerance Mechanism in the Fern, Adiantum Latifolium. Biosc.Biotech.Res.Comm. 2016;9(4). Available from: https://bit.ly/2qsYvBC
INTRODUCTION
Pteridophytes, the seedless vascular plants characterized with independent heteromorphic alternation of generation and primitive vasculature. These form the conspicuous flora of tropical plant world. The pteridophytes consist of ferns and fern allies; of these, the ferns are predominantly distributed in a wide range of habitats. Out of 1250 species of pteridophytes occurring in India, 173 species have been found to be used as food,
flavor, dye, medicine, bio-fertilizers, oil, fiber and bio-gas production (Manickam and Irudayaraj, 1992). Besides sugar, starch, proteins and amino acids, ferns contain alkaloids, glycosides, flavonoids, terpenoids, sterols, phenols, sesquiterpenes as potential raw materials used in various industries.In addition to medicinal properties they play an important role in the ecological niches of forest ecosystem i.e., integral part of biogeochemical cycling of minerals; also they help as ecological indicators of pollution, (Srivastava and Paul, 2016; Bergenon and Pellerin, 2014).
Among the vascular land plants there are some 60 to70 species of ferns and fern allies exhibit some degree of vegetative desiccation tolerance. Oxidative stress is a major hazard of desiccation which inturn leads to the formation of ROS (Reactive Oxygen Species). Most of the studies in ferns indicate that enzyme conservation may be important in the physiological reactivation process; de novo synthesis of some enzymes during rehydration also contributes to full physiological recovery in these plants. Through the years of evolution and climatic changes, this plant group showed a resistance and persistence in maintaining its diversity in the earth. The stress tolerance capacity of these plants is the reason for its established biodiversity in the earth (Pereira et al., 2014; Scoffoni et al., 2014; Maia et al., 2014)
The primary response of an organism to an oxidative stress condition includes the activation of antioxidant enzymes, and the use of water-soluble antioxidant compounds and lipid-soluble antioxidant molecules. Antioxidant enzymes include catalase (CAT) and ascorbate peroxidase (AP), which are able to reduce H2O2 to water, and peroxiredoxins (PRXs), expressed mainly in the organelles and involved in detoxification of H2O2 and alkyl hydroperoxides. Antioxidant mechanisms are present in all living organisms, and have been studied with particular emphasis in host–pathogen interactions and to clarify how organisms cope with extreme environmental conditions. Rabara et al., (2015) reported that when water uptake and loss cannot keep balance by primary adaptive responses, different drought mechanisms through abscisic acid and other signaling pathways, may be exploited to avoid and/or tolerate dehydration plants. Similarly, various genes that function as stress sensors in signaling transduction pathways, which comprise a network of protein-protein reactions, transcription factors (TFs) and promoters, are activated in Arabidopsis and other plants (Liu et al., 2016).
In this scenario, the present study was undertaken to evaluate the stress tolerance mechanism in terms of desiccation tolerant capacity of a fern Adiantum latifolium Lam. Commonly known as the maidenhair fern.
MATERIAL AND METHODS
Plant Material
The plant material Adiantumlatifolium collected from the KattakadaTaluk of Trivandrum district, Kerala, was used for the present study. It was identified using the standard flora of Manickam and Irudayaraj (1992). The identification of the fern was further confirmed by herbaria of University of Calicut and a voucher specimen was kept intheDepartment herbarium (CCB 047).
Desiccation Treatment
The fresh and mature sporophyte of Adiantaum latifolium cleaned carefully and the samples were desiccated in a desiccators over polyethylene glycol (PEG) in a controlled environment chamber (Fig.2 a & b) . The selected plants were subjected to three different desiccation regimes like 1D (24 h), 2D (48h) and 3D (72h) (Fig.3 a, b & c). After desiccation a set of desiccated samples were subjected to rehydration for 30 min. Thus the samples were divided into two groups namely desiccated and desiccated subsequently rehydrated. Control plants were maintained in an optimal water conditions in each case during the whole experimental period.
Estimation Of Relative Water Content (RWC)
The water status at each desiccated stage was calculated as relative water content (RWC).
![]()
(FW-Fresh Weight, DW – Dry Weight)
Quantification Of Phenol
Total phenol content was estimated by the method of Mayr et al. (1995).
Reverse Phase High Performance Liquid Chromatography (Rp-Hplc) Of Phenolic Acids
Quantitative fractionation of various phenolic acids in the samples was studied by RP-HPLC analysis. Phenolic acids extracted from fresh and desiccated tissues in aqueous methanol were used for the study.
Preparation Of The Sample
1g leaf tissue was refluxed in boiling 80% methanol for 10min. The tissue was homogenized, filtered through cheesecloth and centrifuged at 15000 rpm for 10 min. The resultant supernatant was made up to 5 ml with 80% methanol and used for RP-HPLC analysis.
Procedure Of Rp-Hplc
A modified method of Beta et al. (1999) was followed for HPLC analysis. An HPLC system (Waters Associates) equipped with a 7725 Rheodyne injector and Waters 510 HPLC pump, 486 tunable absorbance detector and Millennium 2010 software data module were used for the study. An HPLC column of 4.6×250 mm id reverse phase (RP) C8 was used for the fractionation of phenolic acids. Potassium hydrogen phosphate and acetonitrile in a ratio of 75:25 was used as the mobile phase for the isocratic elution. An elution period of 20 min with a flow rate of 0.8 ml min-1 was given.10µl of the sample was injected and the absorbance at 254 nm was recorded. Standard phenolic acids such as gallic, vanillic, p-hydroxybenzoic, ferulic, chlorogenic, sinapic, paracoumarate and cinnamic acids were injected in to the column separately. Comparing with the retention time of the standard identified phenolic acids in the sample. Height of the peaks was taken for quantification. Concentration of the standard and height of the standard peak were taken as the standard parameters.
Quantification Of Hydrogen
Peroxide (H2O2)
H2O2 concentration of the experimental tissues was estimated as per the procedure of Bellincampi et al.
(2000).
Localization Of Hydrogen
Peroxide (H2O2)
Histochemical localization of H2O2 was done by staining the tissues with Tetramethylbenzidine (TMB) reagent, as per the method of RosBarcelo (1998).
Quantification Of Lipid Peroxidation (LPX)
The level of lipid peroxidation in the cells was measured in terms of malondialdehyde (MDA) content determined by the thiobarbituric acid (TBA) reaction as described by Zhang and Kirkham (1996).
Isolation And Assay Of Phenylalanine Ammonia Lyase (Pal) And Polyphenol Oxidase (PPO)
Isolation of PAL was made by the method of Morrison
et al. (1994) and the activity of PAL was estimated by the method of Whetten and Sederoff (1992).Isolation and the assayof PPO determined as per the method of Mayr
et al.(1965).
Statistical Analyses
The values were mean of six independent analysis ± SD and significance of the differences in all parameters tested was determined by two-way analysis of variance (ANOVA).
RESULTS AND DISCUSSION
Pteridophytes gain water in their cells both through external (ectohydric) capillary movement and internal (endohydric) transport. When fully hydrated, their water content is very high, attained several times more than their dry mass. The relative water content of control and 76 h desiccated fern were 80% and 31% respectively (Table 1). Unlike other plants, water content of ferns is highly related to environmental conditions and weakly regulated by their internal and morphological structures. Alpert and Oechel (1987) found that those species that occurred in microsites with lower water availability were able to attain maximum net photosynthetic gain at lower water content and to recover better from prolonged desiccation than those taxa in less xeric microsites.
The total phenolic content in the tissues varied among the experimental ferns. After desiccation (72h), the total phenols increased by 5 fold; following rehydration it was decreased substantially than the value found in desiccated phase (Table 2). The level of phenols may be supported by the activity profile of phenylalanine ammonia lyase (PAL) during desiccation. The waxing and waning pattern of phenols was further investigated by fractionating the phenols by reverse phase high performance liquid chromatography (RP-HPLC). The figures 1a and b represent the HPLC chromatogram of phenolic extracts from leaf tissues of A. latifolium with control and desiccated conditions.
Phenolic acids such as cinnamic acid, caffeic acid, ferulic acid, sinapic acid, coumaric acid, hydroxy benzoic acid, chlorogenic acid, gallic acid and vanillic acid were used as standards for detecting the compounds. It is evident from the figures 1a and b that phenolic extracts of the samples, contain the peaks of most of the standards, indicating the functional compartmentation of phenolic acids during desiccation when compared with the control. The major phenolic acid noticed in the plant under control and desiccated condition was represented in the table 3. Interestingly, the desiccated fern shows significant profile of phenolic acids such as sinapicacid(4105.56μg/g), ferullate (5136.73μg/g), pholorogucinol (3006.56μg/g),cinnamate (617.413 μg/g), gallate(529.21 μg/g), vanillate (617.413 μg/g),catechol (705.615μg/g) and hydroxyl benzoic acid(3301.59μg/g). The antioxidant activity of phenolic acids is related to the acid moiety and the number and relative positions of hydroxyl groups on the aromatic ring structure. Hydroxycinnamic acids are more effective antioxidants than hydroxybenzoic acids due to increased possibilities for delocalization of the phenoxyl radical. Hydroxylation in the 2- and 4-positions or in the 3-, 4- and 5-positions confers the greatest antioxidant activity. Adjacent hydroxyl groups, as found in protocatechuic acid, are less favourable for antioxidant activity than those meta-orientated with respect to each other.
 |
Figure 1 |
Higher plants respond to various stresses by activating secondary metabolic pathways such as phenyl propanoid pathway (Zadworna and Zenkteler, 2006) which plays an important role in plant desiccation. Browning of plant tissue probably connected with phenolic accumulation, is a morphological symptom of drought stress ( Hossain et al., 2013a and Moharramnejad et al., 2015 ).
The total phenolic content in the desiccated fronds showed significant increase. It is possible to suggest that negative impact of desiccation stress was reduced by total phenols. Increasing evidences indicate that phenols enhanced water stress tolerance via the induction of antioxidant defence systems in the plants (Jiang and Zhang, 2004). Moreover, Oliver and Bewley (1997) stated that vascular plants, even belonging to resurrection, could survive desiccation, only when they are dehydrated slowly. Longer rapid desiccation could cause even irreparable damages as suggested by Proctor et al., (2007a) would allow insufficient time for cytological osmotic adjustment, which is critical for plant survival during water stress. In all the fronds, after rehydration following desiccation, phenolic levels decreased, but not below control values. It seems that rehydration was too quick to put in motion the cascade of protection mechanisms in them (Nadernejad et al., 2013; Solti et al., 2014)
H2O2, the reactive oxygen species (ROS) is derived from the O2•−by dismutation reaction. In the present study H2O2 was quantified in the fronds of control plants and experimentals. Table 2 demonstrates the H2O2 content accumulated in the desiccated and rehydrated tissues of A. latifolium. From the data it is clear that, the desiccated tissues showed higher deposition of H2O2 than the control. The assay data of H2O2 in the desiccated tissues corroborates with the deep blue colour deposits in the histochemical analysis (Fig.2 a, b, c and d). Apart from the synthesis of H2O2 in cell system as a normal metabolic process, the over production of H2O2 in the tissues of desiccated plants is possibly a manifestation of oxidative stress. It can be postulated that H2O2 play a central role in plant as a signaling cascade or tolerance to stress (Bhattacharjee 2013; Sathiyaraj et al.,
2014).
 |
Figure 2 |
Irrespective of the comparison regarding the deposition of ROS-H2O2 between desiccated and control the higher accumulation of ROS remains as a physiological impact of the pteridophytes against desiccation stress. The level of H2O2 formation in the pteridophytes can be considered as an indicator of magnitude of abiotic stress in the species. Thus the results clearly indicates physiological impairedness of plants, due to this oxidative burst caused by the higher level of H2O2 production and it also highlights the reaction response of the pteridophytes against abiotic stress. The role of abiotic stress response in the formation of H2O2 in plant tissue has been conclusively proved by several studies (Pukacka and Ratajczak, 2006).
The present data conclusively proved the excess generation of H2O2 in the species as a desiccation effect. As a constitutive metabolite of cell wall for lignin formation, the presence of H2O2 on cell system of plants is a common physiological feature. No lignin content and the tendency of plant cell to accumulate H2O2 in the excessive level, requires further analysis at biochemical level, for detecting the role of the marker enzyme involved in the process (Wang et al., 2014a).
 |
Figure 3 |
 |
Figure 4 |
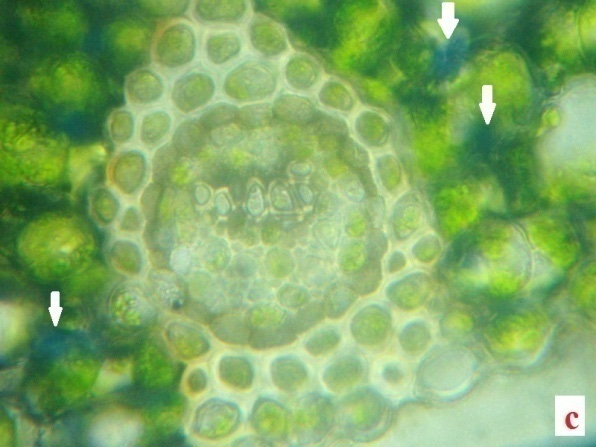 |
Figure 5 |
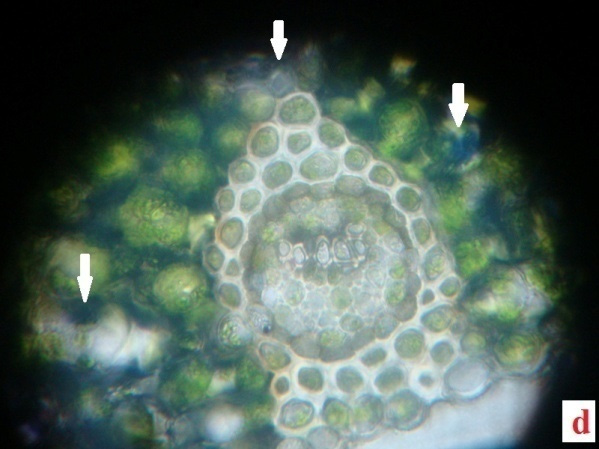 |
Figure 6 |
Accumulation of H2O2 concomitant with lipid peroxidation resulted in significant decrease in cell membrane stability. It has been suggested that decrease in cell membrane stability reflects the extent of lipid peroxidation caused by ROS. Dat et al., (1998) reported that by heat acclimation pre-treatment, accumulation of H2O2 in plants can be reduced, compared with those without heat acclimation pre-treatment, which may be indicative of an enhanced antioxidant potential in leaf tissues and would contribute to enhance thermo tolerance. Desiccation stress injury may involve different mechanisms, depending on environmental conditions, before or during the dehydration stress. Genetic differences may also be important for elevated levels of H2O2 contributing to stress injury in plants.
| Table 1: Influence of desiccation (24, 48 and 72h) stress on the levels of relative water content (RWC) % in Adiantum latifolium. Values are mean ±SD of six independent replications | |
| Stages of desiccation | RWC (%) |
| Control | 80±0.23 |
| 24hour desiccated | 62±0.81 |
| 48 hour desiccated | 48±0.19 |
| 72hour desiccated | 31±0.34 |
Effect of desiccation stress on plant tissues were determined in terms of membrane lipid peroxidation, i.e., estimated as the content of thiobarbituric acid-reactive substances (TBARS). Increase in membrane lipid peroxidation was seen in all the desiccated fronds but at a slow pace right from 24 hour onwards, when compared with control plants (Table 2). Within the experimental period of 72 hour, it increased to a maximum of 12.91 µmolg-1 DW. Thus, desiccation appeared to cause some damage in membrane configuration, but this was reversed and or repaired on rehydration to control values suggesting the active phase of antioxidant machinery in scavenging the ROS in the system.
| Table 2: Hydrogen peroxide (H2O2), lipid peroxidation (LPX) and total phenolic content in Adiantum latifolium subjected to desiccation (24, 48 and 72hours) and rehydration. Values are mean ±SD of three independent replications | |||||||
| Name of the assay | Control | 24D | 24R | 48D | 48R | 72D | 72R |
| H2O2 (µmolg-1DW) | 3.9 ±0.53 | 5.64±0.89 | 4.85±0.24 | 11.03±0.71 | 7.54±0.59 | 16.04±0.53 |
9.35±0.82 |
| LPX (µmolg-1DW) | 3.41±0.23 | 6.87±0.15 | 7.65±0.54 | 8.82±0.48 | 6.73±0.35 | 12.91±0.49 |
7.19±0.98 |
| Total Phenol
(mg g-1 tissue) |
3.2 ±0.21 | 5.4 ±0.35 | 4.1±0.42 | 11.6 ±0.98 | 6.2±0.87 | 15.9 ±0.76 |
10.2±0.57 |
| D-Desiccated R-Rehydrated | |||||||
Sairam et al., (2002) reported that, osmotic stress causes severe damage to the membrane integrity of various tissue types in different plant species. Moreover, membrane integrity is frequently correlated with plant tolerance to stress such as heat, salt, drought and other osmotic stress. Lipid peroxidation is an effective indicator of cellular oxidative damage, and was estimated by the levels of TBARS. The observed increase in TBARS concentration in stressed plants, might indicate lipid peroxidation of cell membrane components, caused by ROS generated by the oxidative stress (Sairam et al., 2002), i.e., LPX data strongly corroborates with the level of H2O2 content in the species (Zhou et al., 2014)
The key enzyme of phenyl propanoid metabolism known as phenylalanine ammonia lyase (PAL) was isolated from the control, desiccated and rehydrated fronds of A. latifolium and assayed spectrophotometrically in order to ascertain their role in secondary metabolite initiation. Being the initial enzyme of PPM, the presence of PAL, in plant tissue becomes an indispensable part for the synthesis of phytoalexins, lignin and phenolics thus involved in the defence response of plant cells (Morrison et al., 1994). Thus, PAL has been generally recognized as a marker of environmental stress in different plant species (MacDonald and D’ Cunha, 2007).
| Table 3: Phenolic acid profile of the control and desiccated A. latifolium. | ||||
| Control | Desiccated | |||
| Retention time(minutes) | Amount
(μg/g tissue)
|
Retention time(minutes) | Amount
(μg/g tissue) |
|
| Catechol | 3.66 | 640.03 | 3.656 | 705.615 |
| Cinnamic Acid | 3.663 | 560.03 | 3.656 | 617.413 |
| Gallate | 3.663 | 480.02 | 3.656 | 529.29 |
| Vanillate | 3.663 | 560.027 | 3.656 | 617.413 |
| Sinapic Acid | 4.284 | 4043.30 | 4.296 | 4105.56 |
| Ferullate | 4.310 | 4877.08 | 4.602 | 5136.73 |
| Hydroxybenzoic Acid | 5.284 | 2339.99 | 5.350 | 3301.589 |
| Pholorogucinol | 4.284 | 2557.85 | 4.296 | 3006.56 |
Effects of desiccation on PAL activity in A.latifolium was shown in Table 4.A significant increase in PAL activity was observed in the fern at all tested desiccation periods, and the highest activity of 96μmg-1 proteins observed at the 72 h desiccation period compared to the control. In few studies, PAL activity was induced by various biotic and abiotic stresses, such as wounding, chilling, heavy metal and infection by viruses, bacteria or fungi (MacDonald and D’Cunha, 2007). The responses of PAL activity subjected to desiccation could be a protective response to the cellular damage provoked by desiccation stress (John et al., 1971). The results suggest that changes in activity of enzyme strongly depend on desiccation periods (Huang et al., 2015)
| Table 4: Phenylalanine ammonia lyase (PAL) and poly phenol oxidase (PPO) content in Adiantum latifolium subjected to desiccation (24, 48 and 72h) and rehydration. Values are mean ±SD of three independent replications | |||||||
| Name of the assay | Control | 24D | 24R | 48D | 48R | 72D | 72R |
| PAL (Umg-1 protein) | 37±0.62 | 59±1.23 | 48±1.01 | 78±1.48 | 54±0.98 | 96±0.89 | 63±1.16 |
| PPO (Umg-1 protein) | 3.25±0.14 | 10.79±0.98 | 6.89±0.36 | 6.81±0.28 | 3.77±0.13 | 2.48±0.11 | 1.23±0.05 |
D-Desiccated R-Rehydrated
PPOs are copper containing metalloenzymes that catalyze the oxidation of hydroxy phenols to their quinone derivatives. Polyphenol oxidase (PPO) showed varied expression in the desiccated fern compared to the control. PPO activity decreased from the 1stto 3rd day after desiccation i.e., 10.79±0.98 and 2.48±0.11respectively (Table 4). Initially the activity of endogenous polyphenol oxidase was higher in desiccated and rehydrated samples followed by a decrease in subsequent days of desiccation and rehydration (Table 4). Sofo et al., (2005) reported that a decrease in PPO activity following abiotic stress was associated with improved antioxidant capacity. The suppression of PPO increased the drought tolerance in tomato plants (Thipyapong et al., 2004a).
CONCLUSION
The results obtained in this study demonstrate that desiccation treatment from 24 to 72 h duration, in Adiantum latifolium, generated an oxidative stress condition, and morphological and biochemical alterations. The activation of different biochemical mechanisms helps to explain the high tolerance to desiccation of this species. In this context, in vitro rehydration experiments demonstrated the rapid capacity of the species to recover from oxidative stress, and provide the functional basis that helps to explain, at least in part, the position of this species at the highest tropical zones.
REFERENCES
Alpert P., and Oechel W. C. 1(987). Comparative patterns of net photosynthesis in an assemblage of mosses with contrasting micro distributions. American Journal of Botany 74 : 1787-1796.
Bellincampi D., Dipierro N., Salvi G., Cervone F., and De Lorenzo G. (2000).Extracellular H2O2 induced by oligogalactouronides is not involved in the inhibition of the auxin regulated ro 1B gene expression in tobacco leaf plants. Plant Physiology 122 : 1379-1385.
Bergenon A., and Pellerin S. (2014). Pteridophytes as indicators of urban forest integrity. Ecological Indicators 38 : 40-49.
Beta T., Rooney L. W., Marovatsanga L. T., and Taylor J. R. N. (1999). Phenolic compounds and kernel characteristics of Zimbabwean sorghums. Journal of Science Food and Agriculture 79: 1003-1010.
Bhattacharjee S. (2013). Heat and chilling induced disruption of redox homeostasis and its regulation by hydrogen peroxide in rice (Oryza sativa L., Cultivar Ratna). Physiology and Molecular Biology of Plants 19: 199-207.
Dat J.F., Foyer C.H., and Scott I. M. (1998).Changes in salicylic acid and antioxidants during induction of thermo tolerance in mustard seedlings. Plant Physiology 118: 1455-1461.
Hossain M. A., Mostofa M. G., Fujita M. (2013a). Cross protection by cold-shock to salinity and drought stress-induced oxidative stress in mustard (Brassica campestris L.) seedlings. Molecular Plant Breeding 4: 50-70.
Huang Y. W., Zhou Z. Q., Yang H. X., Wei C. X., Wan Y. Y., and Wang X. J. (2015). Glucose application protects chloroplast ultrastructure in heat-stressed cucumber leaves through modifying antioxidant enzyme activity. Biologia Plantarum 59: 131-138.
Jiang M. Y., and Zhang J. H. (2004). Abscissic acid and antioxidant defense in plant cells. Acta Botanica Sinica 46: 1-9.
John M., Bardzik H. V., Marsh J. R., and Havis J. R. (1971). Effects of water stress on the activities of three enzymes in Maize seedlings. Plant Physiology 47 : 828-831.
Liu C, Zhang X, Zhang K, An H, Hu K, Wen J, Shen J, Ma C, Yi B , Tu J and Fu T (2015) Comparative Analysis of the Brassica napus root and leaf transcript profiling in response to drought stress. Int. J. Mol. Sci. 2015, 16: 18752-18777.
Mac Donald M. J., and D’Cunha G. B. (2007).A modern view of phenylalanine ammonia-lyase. Biochemistry and Cell Biology 85: 273-282.
Maia J., Dekkers B.J.W., Dolle M., Ligterink W., and Hilhorst H.W.M. (2014). Abscissic acid (ABA) sensitivity regulates desiccation tolerance in germinated Arabidopsis seeds. New Phytologist 203: 81-93.
ManickamV.S., and Irudayaraj V. (1992). Pteridophytic flora of theWestern Ghats-South India. B.I.
Mayr V., Treutter D., Santos-Buelga C., Bauer H., and Feucht W. (1995). Developmental changes in the phenol concentration of golden delicious apple fruits and leaves. Phytochemistry 38: 1151-1155.
Moharramnjad S., Sofalian O., Valizadeh M., and Shiri A .M. (2015). Proline, glycine betaine, total phenolics and pigment contents in response to osmotic stress in maize seedlings. Journal of Bio Science and Biotechnology 4 : 313-319.
Morrison T. A., Kessler J. R., Hatfield R. D., and Buxton D. R. (1994). Activity of two lignin biosynthesis enzymes during development of a maize internode. Journal of the Science of Food and Agriculture 65: 133-139.
Nadernejad N., Ahmadimoghadam A., Hossyinifard J., and Poorseyedi S. (2013). Evaluation of PAL activity, phenolic and flavonoid contents in three pistachio (Pistacia vera L.) cultivars grafted on to three different root stocks. Journal of Stress Physiology and Biochemistry 9 : 84-97
Oliver M. J., and Bewley J. D. (1997). Desiccation tolerance of plant tissues: a mechanistic overview. Horticultural Reviews 18: 171-214.
Pereira W.V.S., Faria J.M.R., Tonetti O.A.O., and Silva E.A.A. (2014). Loss of desiccation tolerance in Copaiferalangs dorffii Desf. seeds during germination. Brazilian Journal of Biology 74: 501-508.
Proctor M. C. F., Ligrone R., and Duckett J. G. (2007a). Desiccation tolerance in the moss Polytrichumformosum: physiological and fine-structural changes during desiccation and recovery. Annals of Botany 99: 75-93.
Rabara R.C, Tripathi P, Reese R.N, Rushton D.L, Alexander D, Timko M. P, Shen Q.J and Rushton P.J (2016). Tobacco drought stress responses reveal new targets for Solanaceae crop improvement BMC Genomics 2015, 16:484, 1-22.
Ros Barcelo A. (1998). Hydrogen peroxide production is a general property of the lignifying xylem from vascular plants. Annals of Botany 82: 97- 103.
Sairam R. K., Rao K. V., and Srivastava G. C. (2002).Differential response of wheat genotypes to long-term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Science 163: 1037-1046.
Sathiyaraj G., Srinivasan S., Kim Y. J., Lee O. R., Balusamy S. D. R., and Khorolaragchaa A. (2014). Acclimation of hydrogen peroxide enhances salt tolerance by activating defense-related proteins in Panax ginseng CA. Meyer. Molecular Biology Reports 41: 3761-3771.
Scoffoni C., Vuong C., Diep S., Cochard H., and Sack L. (2014). Leaf shrinkage with dehydration: coordination with hydraulic vulnerability and drought tolerance. Plant Physiology 164: 1772–1788.
Sofo A., Dichio B., Xiloyannis C., and Masia A. (2005). Antioxidant defenses in olive tree during drought stress: changes in activity of some antioxidant enzymes. Functional Plant Biology 32: 45-53.
Solti A., Mihailova G., Sarvari E., and Georgieva K. (2014). Antioxidative defence mechanisms contributes to desiccation tolerance in Haberlea rhodopensis population naturally exposed to high irradiation. Acta Biologica Szegediensis 58 :11-14.
Srivastava S., and Paul A. K. (2016). Associated microflora of medicinal ferns: biotechnological potentials and possible applications. International Journal of Bioassays 5: 4927-4943.
Thipyapong P., Hunt M.D., and Steffens J.C. (2004a) Antisense downregulation of polyphenol oxidase results in enhanced disease susceptibility. Planta 220:105–117.
Wang Y., Zhang J., Li J. L., and Ma X. R. (2014a). Exogenous hydrogen peroxide enhanced the thermo tolerance of Festuca arundinacea and Lolium perenne by increasing the antioxidative capacity. Acta Physiologiae Plantarum 36: 2915-2924.
Whetten R. W., and Sederoff R. R. (1992). Phenylalanine ammonia lyase from loblolly pine. Purification of the enzyme and isolation of complementary DNA clones. Plant Physiology 98: 380-386.
Zadworna B A., and Zenkteler E. (2006). Ultrastructure of endodermis and stele cells of dehydrated Polypodium vulgare L. rhizomes. Acta Biologica Cracoviensia Series Botanica 49(2): 73-81.
Zhang J. X., and Kirkham M. B. (1996). Enzymatic responses of the ascorbate-glutathione cycle to drought in sorghum and sunflower plants. Plant Science 113: 139-147.
Zhou J., Xia X. J., Zhou Y. H., Shi K., Chen Z., and Yu J. Q. (2014). RBOH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato. Journal of Experimental Botany 65: 595–607.


