Environmental
Communication
Biosci. Biotech. Res. Comm. 9(4): 680-688 (2016)
Use of ultraviolet and ultraviolet /peroxide hydrogen
processes for degradation of humic substances from
aqueous solutions
Mohamadreza Massoudinejad
1,2
, Sohrab Golmohammadi
3
and Mansour Ghaderpoori
4
1
Member of Safety Promotion and Injury Prevention Research Center, Shahid Beheshti University of Medical
Sciences, Tehran, Iran
2
Department of Environmental Health Engineering, School of Public Health, Shahid Beheshti University of
Medical Science, Tehran, Iran
3
Environmental engineering (Trends water and wastewater), Kurdistan rural water & wastewater CO
(Kamyaran).
4
Students Research Commitee, Department of Environmental Health, Faculty of Health, Shahid Beheshti
University of Medical Sciences, Tehran, Iran
ABSTRACT
In this study, the degradation of humic acid was studied using advanced photochemical oxidation by exposing humic
acid aqueous solution with low-pressure mercury vapor lamp as a UV light source after the addition of hydrogen
peroxide. The effect of different parameters such as H
2
O
2
dosage, pH, and initial concentration of humic acid on the
removal ef ciency of UV/H
2
O
2
was evaluated and investigated in detail. Increase of initial H
2
O
2
dosage (up to opti-
mum dosage) and also increase of humic acid concentration resulted in the decrease of humic acid degradation. The
residual concentrations of humic acid were measured for assessing the process performance and understanding the
process reaction behavior. The results showed that humic acid was degradable in the presence of hydrogen peroxide
under UV irradiation. In the absence of H
2
O
2
, the degradation ef ciency was very negligible. The results show 91%
humic acid removal in 60 min of reaction time when 30 mmol L
-1
of H
2
O
2
aqueous solution was added to the solution
compared with only 20% of removal in similar conditions and in the absence of H
2
O
2
. Investigation of the kinetics
of the UV/H
2
O
2
process demonstrated that the semi-log plot of the humic acid concentration versus time was linear,
which suggested a rst order reaction.
KEY WORDS: HUMIC ACID, WATER SOLUTIONS, UV, PEROXIDE HYDROGEN
680
ARTICLE INFORMATION:
*Corresponding Author: mghaderpoori@gmail.com
Received 18
th
Sep, 2016
Accepted after revision 5
th
Nov, 2016
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2015: 3.48 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2016. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
Mohamadreza, Sohrab and Mansour
INTRODUCTION
Humic acid (HA) is one of the major components of
humic substances which arise from the microbial degra-
dation of biomolecules. Natural organic matters (NOMs)
such as HA enters water from natural and arti cial
sources. These materials are caused a variety of problems
in treatment operations and distribution systems due
to having unpleasant smell and taste, yellow to brown
color, reaction with chlorine, and formation of disin-
fection by-products (DBPs). NOMs could also stimulate
the growth of the micro-organisms in water distribution
pipelines as well as production of biologically unstable
water and other unwanted water quality issues such as
metal complexes (Vilhunen et al. 2010; Alkan et al. 2007
Wang et al. 2014).
THMs exhibit mutagenic properties during the chlo-
rination step water treatment. The guideline for THMs in
drinking water announced by World Health Organiza-
tion (WHO) states that DBPs should not exceed 100 gL
-1
(Zhang and Minear, 2006). Consequently, the removal
of HA from surface waters or wastewaters is important
because of health and environmental concerns (Jacan-
gelo et al. 1995).
HA may account for up to 90% of NOM. Actually, it is
not possible to completely remove NOM by conventional
methods in water treatment plants. Different treatment
technologies have been used in practice to improve NOM
removal such as ion exchange, sorption, and membrane
processes. But, many of these are not acceptable in terms
of economy and ef ciency. Advanced oxidation pro-
cesses (AOPs) are frequently applied for the oxidation of
organic and toxic materials. These processes are based
on the production of hydroxyl radicals which demon-
strate great ef cacy in breaking down organic materi-
als (Katsumata et al. 1987; Magdaleno & Coichev 2005;
Panyapinyopol et al. 2007; Golmohammadi et al. 2016).
AOPs are de ned as the processes that involve highly
reactive species, speci cally hydroxyl radicals (oxida-
tion potential = 2.8 V) in suf cient quantities to oxidize
the majority of complex organic chemicals in water and
wastewater. Hydroxyl radicals have a signi cant role in
the treatment of organic materials due to their high reac-
tivity and lack of selectivity toward organic compounds.
UV photolysis and UV/H2O2 have been successfully
used in removing NOMs from water solutions. However,
if the UV absorbance of water is considerably high, the
ef cient treatment might require great reaction time
of UV. In such cases, removing the majority of organic
materials by other methods prior to oxidation treatment
leads to decreased UV absorbance and improved NOM
mineralization (Zepp et al. 2007; Vilhunen et al. 2010;
Hui et al, 2011; Bazri et al. 2012; Hiroshi et al. 2013 and
Yazdanbakhsh et al. 2014).
The advantage of UV/H
2
O
2
including high reactivity,
low sludge production, and capability to achieve com-
plete destruction (mineralization) of pollutants to less
harmful by-products (Hui et al, 2011; Bazri et al. 2012;
Hiroshi et al. 2013). In UV/ H
2
O
2
process, optimum H
2
O
2
dosage has been reported for each target compound,
because the characteristics and concentration of the
organic compounds can directly in uence H
2
O
2
utiliza-
tion for degrading organic matters (Kruithof et al.2007;
Pereira et al. 2007; Kim et al. 2009).
Upon the UV irradiation, H
2
O
2
absorbs UV energy and
results in the production of HO•. The excess H
2
O
2
can
react with HO• radicals (Sheikhmohammadi et al. 2013)
and serve as an HO• inhibiting agent in the UV/H
2
O
2
process (Vilhunen et al. 2010; Lamsal et al. 2011; Jung
et al. 2012; Chandran et al. 2014). Many studies have
illustrated the effectiveness of the UV/H
2
O
2
process in
the oxidation and mineralization of various organic pol-
lutants and this process has been widely studied for the
remediation of both ground and drinking waters (Lamsal
et al. 2011; Jung et al. 2012). Therefore, the aim of this
study was investigate the suitability and ef ciency of
the UV and UV/H
2
O
2
processes for the removal of HA
as an important problem in treatment operations and
distribution systems
MATERIAL AND METHODS
The chemical materials were of reagent grade. Commer-
cially, available HA was extracted from peat coal. HA
was contained 56.2% of C, 3.91% of H, 36.1% of O, and
1.07% of N. It was dried for 1 h at 110˚C. A stock solu-
tion was prepared by dissolving 1.0 g of HA in 1000
ml deionized water. Then, it was ltered through a 0.45
µm glass- ber-membrane lter and stored in 4˚C condi-
tions. The as-synthetic sample was prepared by adding
the measured amount of HA stock solution to the deion-
ized water. 30% hydrogen peroxide (H
2
O
2
, DUKSAN) was
also employed. HA and H
2
O
2
solutions were accurately
prepared daily to the required diluted concentrations.
Ultra-pure water was employed for all the dilutions.
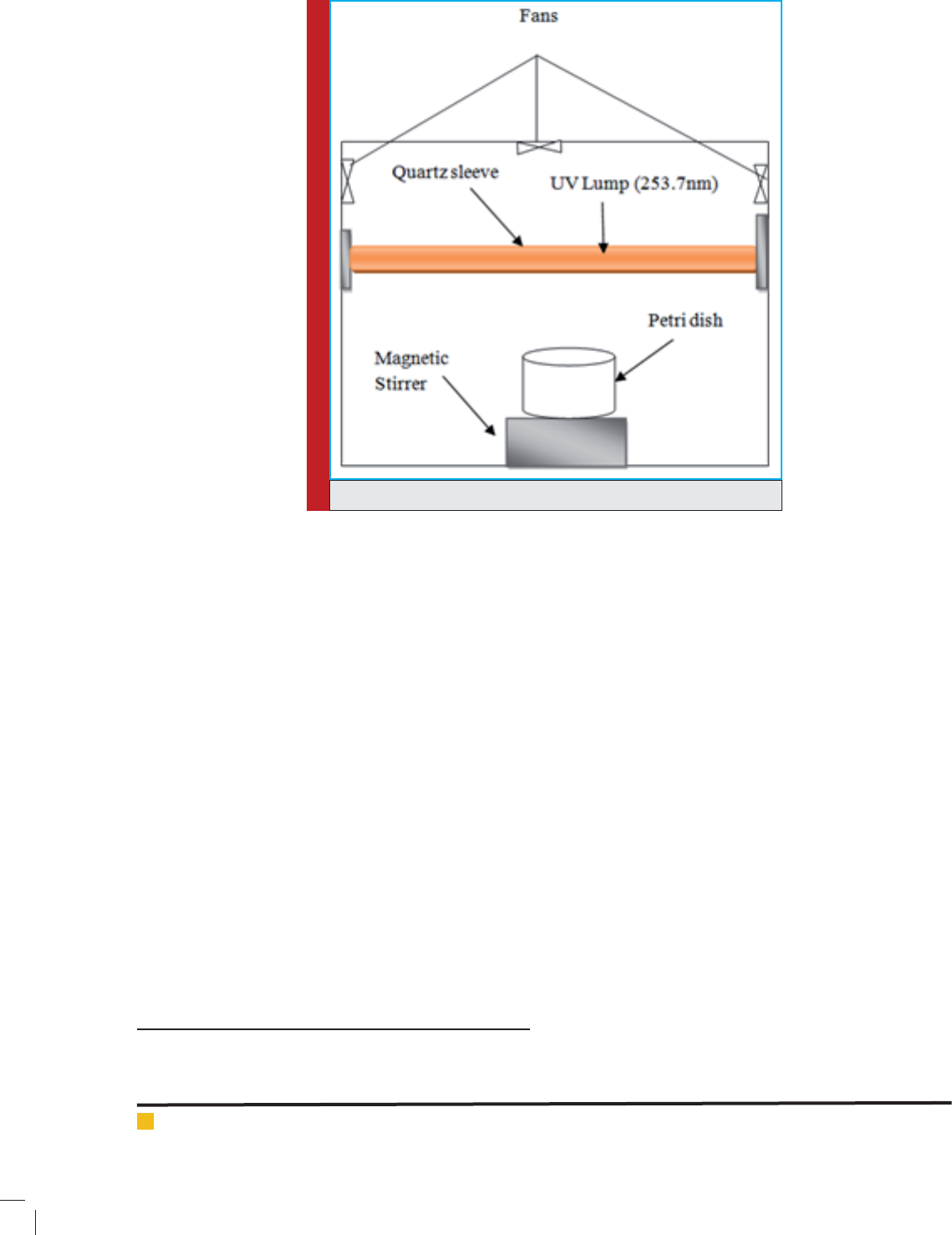
All the photochemical experiments were carried out
in a batch photo-reactor, which is schematically shown
in Fig. (1) R-52 Mineralight
®
Lamp which was used a grid
design and produces a highly uniform 254 nm UV light
with high intensity was used as a light source. A J-225
Black Ray intensity meter was employed to measure the
UV irradiation intensity (I). Interior surface of the photo-
reactor was made of stainless steel (20 cm diameter and
30 cm depth) for the photo-oxidation of HA in aqueous
solutions. UV lamps were xed parallel at the center of
the reactor and covered with a quartz sleeve. The UV
lamps were turned on 10 min before performing every
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS USE OF ULTRAVIOLET AND ULTRAVIOLET /PEROXIDE HYDROGEN PROCESSES FOR DEGRADATION 681
Mohamadreza, Sohrab and Mansour
experiment. The total UV intensity was controlled by
turning on different numbers of UV lamps. Air cool-
ing system, with electrical fans, was used to prevent the
lamp from overheating and to maintain the temperature
at 25
o
C.
The UV/H
2
O
2
experiments were carried out in a photo-
reactor batch type made of a Pyrex-based material with
2 L volume capacity and a UV-C lamp from LIGHTTECH
(
max
= 254 nm with the light intensity of approximately
0.05 W cm
-2
) attached to the center of the reactor. An
aqueous HA solution was prepared and subjected to the
UV/H
2
O
2
process. The selected levels to run the experi-
ments were: H
2
O
2
(10-100) Mmol L
-1
, pH (3-11), reaction
time (5-60 min), and initial HA concentrations (2.5-8 mg
L
-1
), set at the beginning of the reactions. The removal
ef ciency of HA by the UV/H
2
O
2
process was determined
at the optimum values of pH, H
2
O
2
dosage, and reaction
time. Reactions were quenched by removing the excess
of H
2
O
2
by catalase or NaHSO
3
according to the analysis.
A Perkin-Elmer (Lambda-II) double beam spectrophotom-
eter was used for the absorption measurements at 254 nm
using the 10 mm quartz cell (Rosenfeldt et al. 2006).
RESULTS AND DISCUSSION
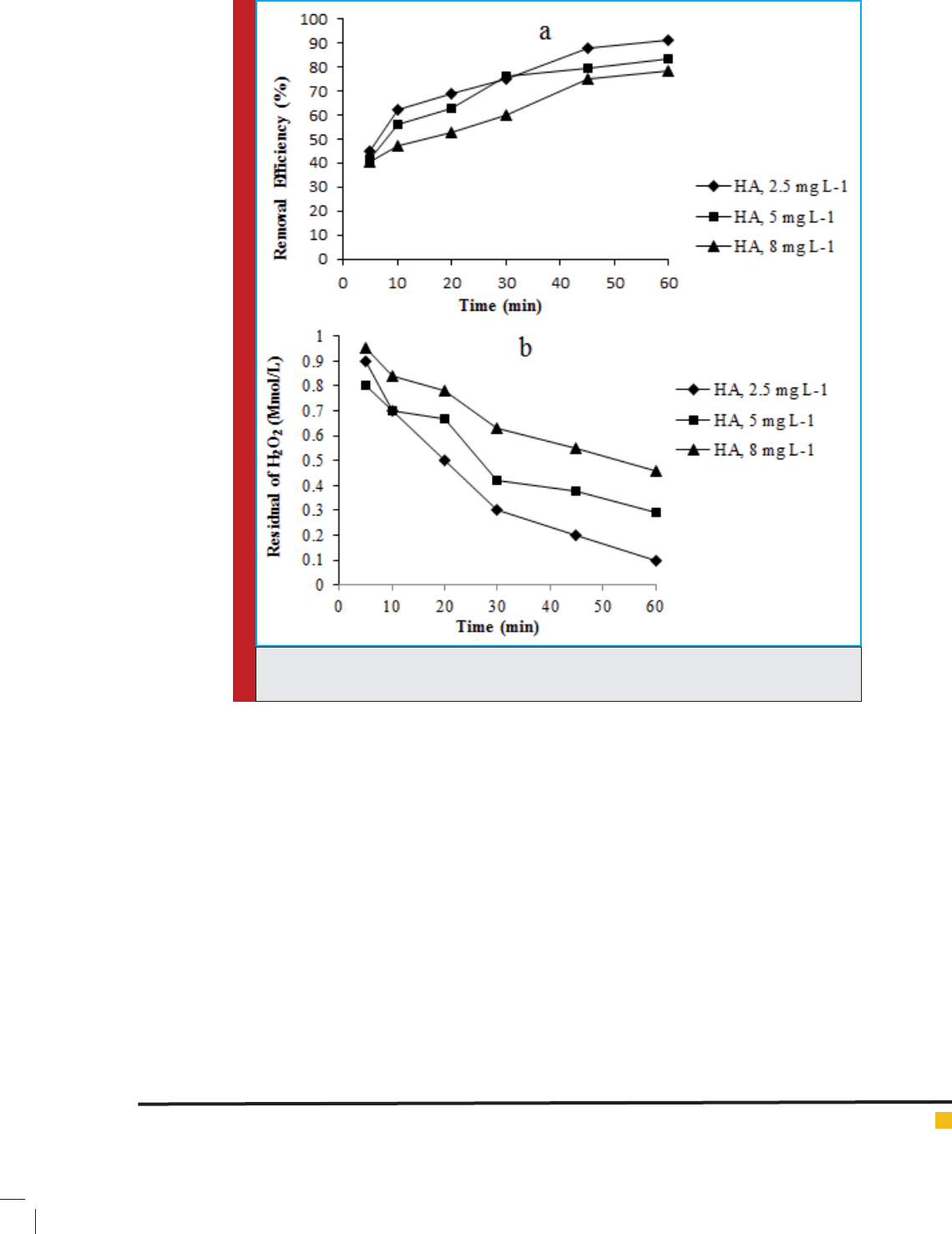
Fig. (2) presents the HA residual fraction at three differ-
ent concentrations with initial HA of 2.5, 5, and 8 mg
L
-1
. At rst, H
2
O
2
concentration was set xed in the reac-
tion mixture as 30 Mmol L
-1
. pH was adjusted at 7.0. As
shown in Fig. (2a), it seems that the removal ef ciency
was less for all the concentrations within the initial
reaction for the 5 min period. With increasing reaction
time, it was observed that the low concentrations of HA
had higher removal ef ciency than high concentrations.
Removal ef ciency for the concentrations of 2.5 and 8
mg L
-1
was 78 and 91% after 60 min, respectively. As
in Fig. (2b), the residual fraction of H
2
O
2
was higher for
HA 8 mg L
-1
than HA 2.5 mg L
-1
; therefore, the solution
with higher HA concentration was content of higher the
residual H
2
O
2
. Reduction of HA was related to the exist-
ence of HO• radicals in the solution; therefore, consider-
ing the dual roles of humic substances at UV light pho-
ton absorption and HO• scavenging, higher removal of
HA at lower concentrations was expected. Also, at high
humic concentration, HA competed with H
2
O
2
for UV
absorption and resulted in the reduced light absorption
by H
2
O
2
to a greater degree. However, the high genera-
tion of H
2
O
2
at high concentration of HA might be due
to the formation of H
2
O
2
as the by-product of sunlight-
induced reactions in natural waters, which would more
appear when HA with certain concentration was irradi-
ated by UV light (Matilainen & Sillanpa 2010; Chang
et al. 2010; Haji et al. 2011; Jung et al. 2012; Gonzalez
et al. 2013).
FIGURE 1. A scheme of batch photo-reactor
682 USE OF ULTRAVIOLET AND ULTRAVIOLET /PEROXIDE HYDROGEN PROCESSES FOR DEGRADATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Mohamadreza, Sohrab and Mansour
FIGURE 2. Effect of initial HA concentration on removal ef ciency by UV/H
2
O
2
(a); Residual
H
2
O
2
to different concentrations at reaction times 5-60 min (b).
The HA solution with the concentration of 2.5 mg
L
−1
was exposed to UV radiation in the absence of H
2
O
2
.
Fig. (3) shows relative residual concentrations for HA
versus time. The samples of the treated solution with-
drawn at different times and the residual concentrations
of HA were measured. As shown in Fig. (3a), only 20%
HA removal was observed after 60 min of the solution
exposure to UV radiation. However, the result of this
research was different from that of some studies by other
researchers, which can be related to the dissimilarity
in the experimental conditions, mainly the UV radia-
tion intensity (Vilhunen et al. 2010; Lamsal et al. 2011).
Because it is deduced that the residual concentration of
HA in the presence of UV radiation linearly decreased
versus time, the reaction of HA with UV radiation was
of rst order (Lamsal et al. 2011), indicating that the
removal of HA was affected by UV may be because
of UV energy absorbed by HA. Another reason for the
improvement of HA removal could be explained by •OH
radicals generated by UV photolysis of water (water
could be dissociated into a hydrogen atom and hydroxyl
radical when exposed to UV irradiation with the wave-
length of less than 200 nm (Shemer&Linden 2007; Alkan
et al. 2007; Comninellis et al. 2008; Hu et al. 2008).
UV (occurring at 185 nm) is more effective than UV
(occurring at 254 nm) in the photolysis of water, because
more energy is transferred to the aqueous solution via
UV (6.72 eV) vs UV (4.89 eV). Since this research was
focused on the degradation of HA by UV, as shown in
Fig. (3a), HA degradation by UV was very slow. Accord-
ingly, the presence of a UV sensitive agent (an oxidiz-
ing reagent, for example H
2
O
2
) was necessary to enhance
the HA degradation rate. Therefore, during the UV
treatment, the effect of H
2
O
2
addition on HA degrada-
tion was investigated. The presence of H
2
O
2
is neces-
sary to accelerate the HA decomposition process by UV.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS USE OF ULTRAVIOLET AND ULTRAVIOLET /PEROXIDE HYDROGEN PROCESSES FOR DEGRADATION 683
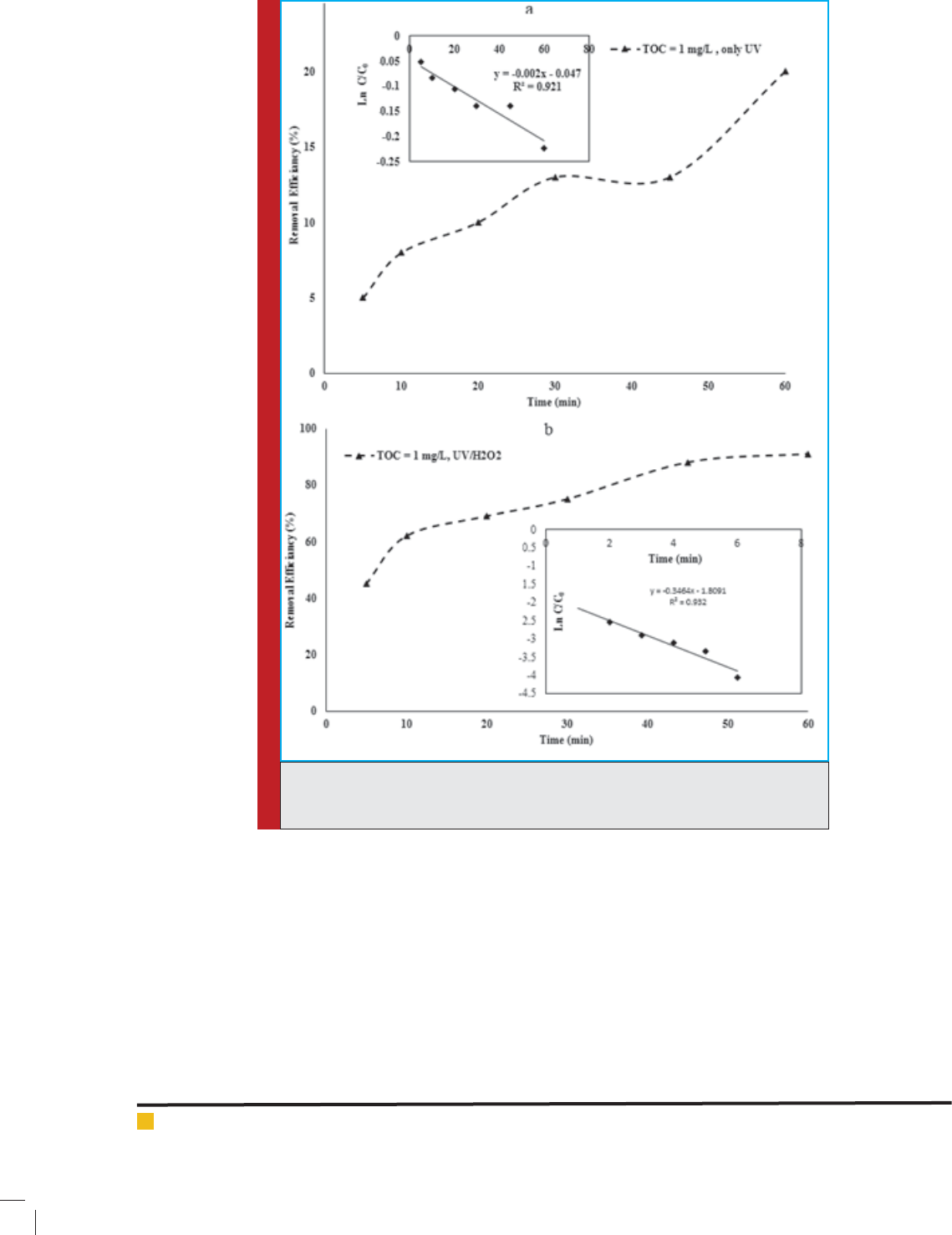
Mohamadreza, Sohrab and Mansour
FIGURE 3. Decomposition of HA by UV radiation (a) and UV/H
2
O
2
(b) (experimental
conditions: Initial H
2
O
2
concentration: 30 mmol L
-1
; pH: 7.0; HA concentration: 2.5
mg L
-1
).
The solution temperature was maintained at 25˚C and
pH of the solution was adjusted to 7.0 (Kruithof et al.
2007; Li et al. 2008). In accordance with Fig. (3b), the
highest removal ef ciency of HA was 91% in the pres-
ence of H
2
O
2
and 60 min of exposure to UV radiation
which could be compared with the percentage of HA
removal by UV radiation in the absence of H
2
O
2
due to
provoking the generation of •OH by UV with
max
=254
nm in the presence of H
2
O
2
. Fig. (3b) shows that relative
residual concentrations of HA decreased linearly over
time. Therefore, it can be concluded that adding H
2
O
2
to the solution subjected to UV radiation could result
in the generation of •OH radicals by the photolysis of
H
2
O
2
molecules (Sheikhmohammadi et al. 2013; Yazdan-
bakhsh et al. 2014); consequently, these radicals oxi-
dized HA molecules.
The cost of H
2
O
2
accounts for most of the cost of AOP
processes, so the determination of optimum H
2
O
2
amount
is quite important in AOP processes. The effect of ini-
tial H
2
O
2
concentration on the residual fractions of HA
and H
2
O
2
is presented in Fig. (4). To determine optimum
H
2
O
2
concentration, the experiments were conducted by
684 USE OF ULTRAVIOLET AND ULTRAVIOLET /PEROXIDE HYDROGEN PROCESSES FOR DEGRADATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
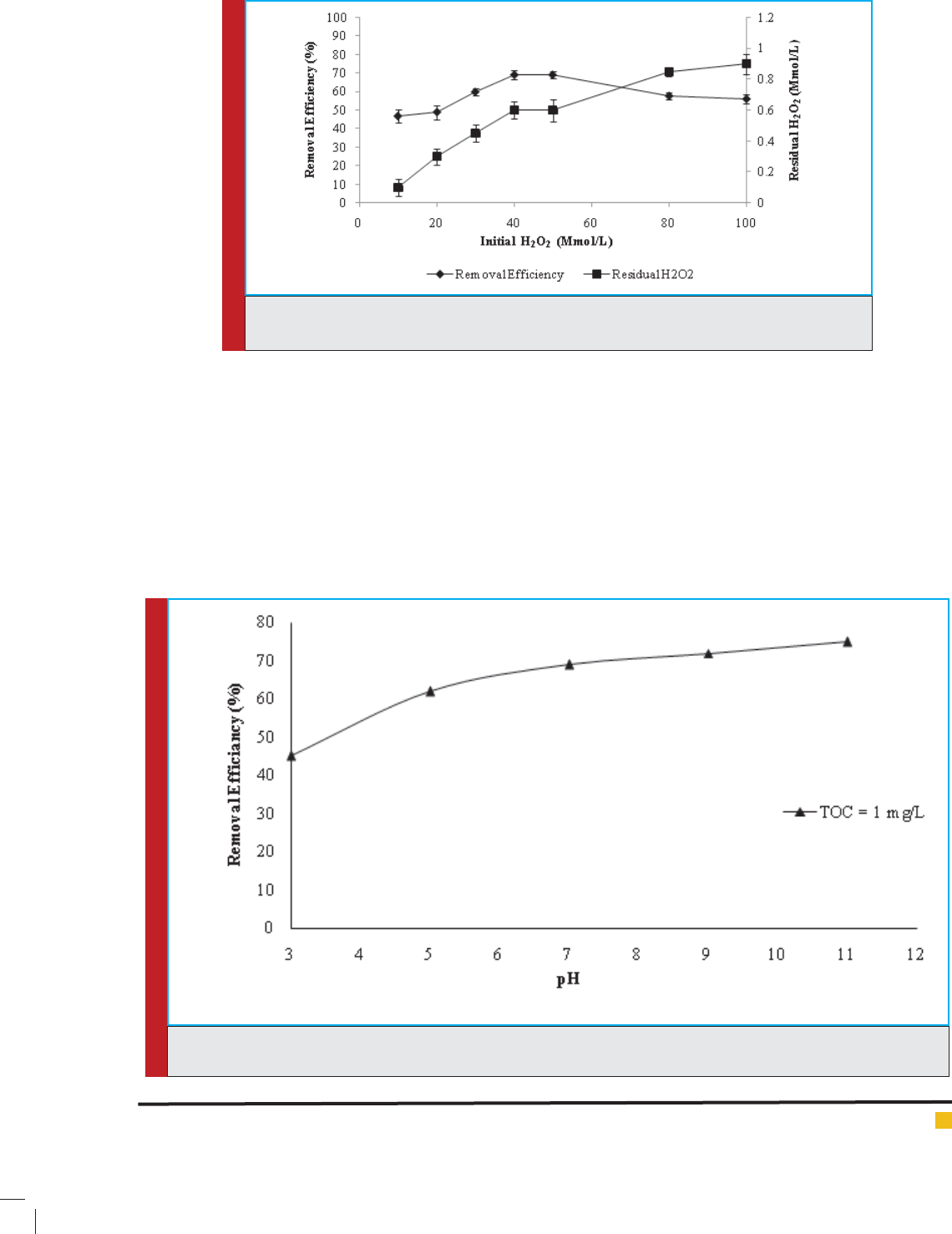
Mohamadreza, Sohrab and Mansour
FIGURE 4. Effect of initial H
2
O
2
on removal ef ciency of HA by UV/H
2
O
2
(experimental condi-
tions: pH: 7.0 and HA concentration: 2.5 mg L
-1
).
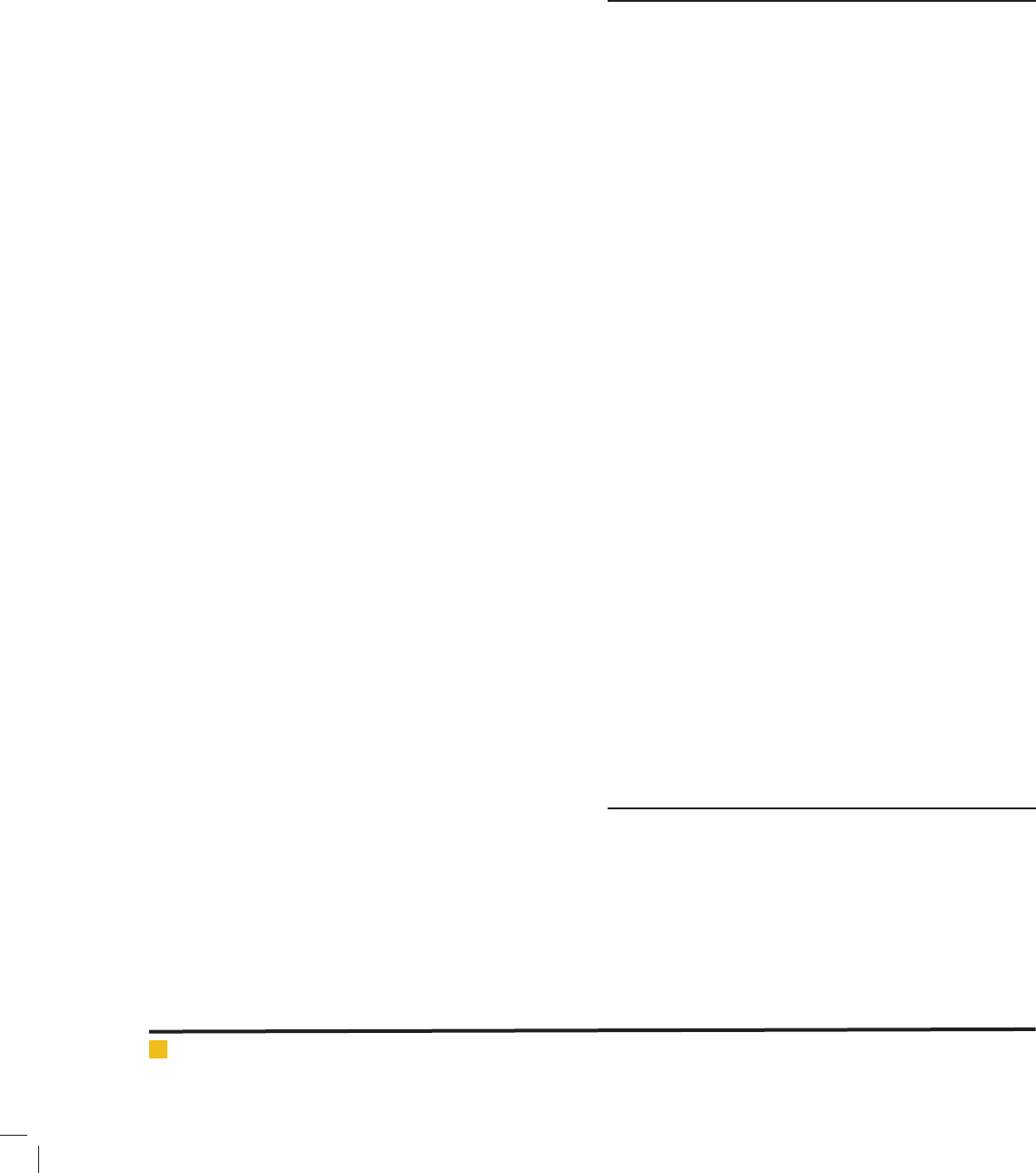
FIGURE 5. Effect of pH on removal ef ciency of HA by UV/H
2
O
2
(experimental conditions: Initial H
2
O
2
concentration: 30
mmol L
-1
; Reaction time: 60 min; HA concentration: 2.5 mg L
-1
).
varying the amount of H
2
O
2
from 10 to 100 Mmol L
-1
(pH was xed at 7 and HA concentration was 2.5 mg
L
-1
). Fig. (4) shows that the ef ciency of HA removal
was maximum at 40 Mmol L
-1
of H
2
O
2
. It was observed
that HA removal ef ciency increased from 47% to 70%
as H
2
O
2
concentration went up from 10 to 40 Mmol L
-1
.
An increase in the concentration of H
2
O
2
up to 40 Mmol
L
-1
did not signi cantly affect the removal of HA and
there was no signi cant difference between the dosages
of 40 and 50 Mmol L
-1
(Chan et al. 2010). It is interesting
to nd that, with increasing H
2
O
2
concentration up to
50 Mmol L
-1
, the degradation rate of HA was decreased,
since with increasing H
2
O
2
from 50 to 100 Mmol L
-1
,
removal ef ciency decreased from 70% to 56% at 30
min, which might be due to three reasons: 1- At exces-
sive amounts of H
2
O
2
, it
acts as a scavenger of
•
OH to
produce perhydroxyl radical (HO
2
•
) (Yazdanbakhsh et al.
2014), which might be due to three reasons: 1- At exces-
sive amounts of H
2
O
2
, it
acts as a scavenger of
•
OH to
produce perhydroxyl radical (HO
2
•
) (Yazdanbakhsh et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS USE OF ULTRAVIOLET AND ULTRAVIOLET /PEROXIDE HYDROGEN PROCESSES FOR DEGRADATION 685
Mohamadreza, Sohrab and Mansour
2014). According to reaction 4 (HO
2
•
has much lower
oxidization capacities than
•
OH).
2- The second reason might be due to the reaction
pathways of HA degradation, mineralizing HA through
UV photolysis alone, and
•
OH radical oxidation as fol-
lows:
3- As shown in Fig. (3), the residual fraction of H
2
O
2
increases with the increasing initial concentration of
H
2
O
2
that is due to decrease of decomposition H
2
O
2
.
Reduction of H
2
O
2
concentration from 40 to 10 Mmol
L
-1
resulted in a decrease in HA removal ef ciency from
70% to 47%, which might be because of partial oxida-
tion at H
2
O
2
concentrations of lower than optimum val-
ues. At the optimum concentration of H
2
O
2
, the genera-
tion of hydroxyl radicals increased (Sheikhmohammadi
et al. 2013; Yazdanbakhsh et al. 2014).
Therefore, an H
2
O
2
dosage of 40 Mm L
-1
with the
HA removal ef ciency of 70% was chosen as the opti-
mum dosage. Reactions were quenched by removing
the excess of H
2
O
2
by catalase or NaHSO
3
. Massche-
lein study group used a diluted enzyme catalyst solu-
tion made from Micrococcus lysodeikticus was used to
destroy unreacted H
2
O
2
and the amount of residual H
2
O
2
was determined via the molybdate-catalyzed iodometric
spectrophotometry method (Rosenfeldt & Linden 2007;
Elmolla & Chaudhuri. 2010; Nie et al. 2010; Ghaderpoori
& Dehghani 2015).
To determine the optimum pH for the UV/H
2
O
2
pro-
cess, pH was varied from 3 to 11. Fig. (5) shows that
HA removal by the UV/H
2
O
2
process was affected by
pH. As presented in Fig. (5), maximum HA removal was
achieved at pH 7-11, 30 Mmol L
-1
H
2
O
2
, and 60 min reac-
tion time. Higher HA removal at pH 7-11 can be com-
pared with pH <9. After the 30 min reaction, 70% of HA
was degraded at the pH of 7.0 as compared with less
than 45% at the pH of 3.0. At pH < 7.0, a substantial
decrease in the ef ciency of HA removal was observed
(Yuan et al. 2009; Nie et al. 2010).
These phenomena could be explained by: 1- UV
reacts more slowly with H
2
O
2
; therefore, the degrada-
tion of H
2
O
2
is slow 2- Scavenging effect of
•
OH by H
+
becomes signi cant in very low pH ranges (the ions of
H
+
may have inhibited the generation of hydroxyl radi-
cals necessary to achieve the UV/ H
2
O
2
oxidation (Li et
al. 2008; Yuan et al. 2009; Nie et al. 2010).
At higher pH values (alkaline), a rapid increase in HA
removal was observed, because in this pH At higher pH
values (alkaline), a rapid increase in HA removal was
observed, because in this pH range (alkaline solutions),
the UV/H
2
O
2
process was most effective and HA removal
increased signi cantly. Increased removal ef ciency at
optimum pH might be because of two possible reasons:
H
2
O
2
stability is less disturbed, which provides further
support to earlier work that HA can be totally removed
in the pH range of 7–11 in 30 min by UV/H
2
O
2
system,
resulting in an increase in the oxidation potential of
•
OH. Also, it might produce intermediate products that
cause an increase in OH radicals (Li et al. 2008; Yuan
et al. 2009; Nie et al. 2010).
CONCLUSION
In this study, the UV and UV/H
2
O
2
oxidation processes
were employed for the degradation of HA in water solu-
tions. The residual concentration of humic acid was
measured for assessing the process performance and
understanding the process reaction behavior. The results
showed that HA was degradable in the presence of
hydrogen peroxide under UV irradiation. In the absence
of H
2
O
2
, the degradation ef ciency was very negligible.
It was achieved 91% degradation of HA in 60 min when
40 Mmol L
-1
of H
2
O
2
was added to the solution compared
with only 20% degradation achieved in 60 min in the
absence of H
2
O
2
. The effects of H
2
O
2
, pH, and initial con-
centration of HA were investigated in this research. It
was concluded that HA degradation was decreased with
excess H
2
O
2
amounts and higher HA concentrations. On
the other hand, not necessarily guarantee the improve-
ment of the HA degradation in excess H
2
O
2
values
because of scavenging effect of the
•
OH to produce the
per-hydroxyl radical and mineralizing of HA through UV
photolysis alone (the UV oxidation of humic acids might
dominate the removal of HA in the UV/H
2
O
2
process)
and
•
OH radical oxidation. Hence, the degradation of HA
was retarded when the H
2
O
2
concentration increased and
when the humic degradation was dominated by UV oxi-
dation. Also, the solution with higher humic concentra-
tion is content of the high residual H
2
O
2
. Reduced HA at
high concentration was due to the presence of
•
OH in the
solution and UV light photon absorption; therefore, con-
sidering the dual roles of humic substances at UV light
photon absorption and
•
OH scavenging, higher removal
of HA was expected at lower concentrations. At higher
humic concentrations, HA competed with H
2
O
2
for UV
absorption and resulted in reduced light absorption by
H
2
O
2
to a greater degree.
ACKNOWLEDGEMENT
This research was supported by Students Research com-
mittee of Shahid Beheshti University of Medical Science
of Iran (Grant No. 7874).
REFERENCES
Alk an U, Teksoy A, Ateply A, Bapkaya HS (2007). Ef ciency
of the UV/H
2
O
2
process for the disinfection of humic surface
waters, J. Environ. Sci. Health, 16: 21-28.
686 USE OF ULTRAVIOLET AND ULTRAVIOLET /PEROXIDE HYDROGEN PROCESSES FOR DEGRADATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Mohamadreza, Sohrab and Mansour
Ars lan-Alaton I, Olmez-Hanci T, Kartal Z (2010). H
2
O
2
/UV-C
Treatment of the Economically Important Naphthalene Sul-
fonate J-Acid:Process Optimization, Kinetic Evaluation and
Activated Sludge Inhibition, Journal of Advanced Oxidation
Technologies,13: 27-35.
Bazri MM, Barbeau B, Mohseni M (2012). Impact of UV/H
2
O
2
advanced oxidation treatment on molecular weight distribution
of NOM and biostability of water, Water research, 46:5297-
04
Chandran P, Netha N, Sudheer Khan S (2014). Effect of humic
acid on photocatalytic activity of ZnO nanoparticles, Journal
of Photochemistry and Photobiology B: Biology, 138: 155–159
Cha ng MW, Chung CC, Chern JM & Chen TS, Tia Shang C.
(2010). Dye decomposition kinetics by UV/H
2
O
2
: initial rate
analysis by effective kinetic modelling methodology, Chem.
Eng. Sci, 65: 135-40.
Com ninellis C, Kapalka A, Malato S, Parsons SA, Poulios I,
Mantzavinos D (2008). Advanced oxidation processes for water
treatment: advances and trends for R & D, J. Chem. Technol.
Biotechnol, 83:769-776.
Elm olla ES, Chaudhuri M (2010). Photocatalytic degradation
of amoxicillin, ampicillin and cloxacillin antibiotics in aque-
ous solution using UV/TiO
2
and UV/H
2
O
2
/TiO
2
photocatalysis,
Desalination, 252: 46-52.
Mag daleno GB, Coichev N (2005). Chemiluminescent determi-
nation of humic substances based on the oxidation by peroxy-
monosulfate, Analytica Chimica Acta, 552: 141-6.
Gha derpoori M, Dehghani MH (2016). Investigating the
removal of linear alkyl benzene sulfonate from aqueous solu-
tion by ultraviolet irradiation and hydrogen peroxide process,
Desalination and Water Treatment, 57: 15208-15212.
Golmohammadi S, Ahmadpour M, Mohammadi A, Alinejad A,
Mirzaei N, Ghaderpoori M, Ghaderpoori A (2016). Removal of
blue cat 41 dye from aqueous solutions with ZnO nanoparticles
in combination with US and US-H
2
O
2
advanced oxidation pro-
cesses. Environmental Health Engineering and Management
Journal, 3: 107-113
Gon zalez O, Justo A, Bacardit J, Ferrero E, Malfeito JJ, Sans
C (2013). Characterization and fate of ef uent organic mat-
ter treated with UV/H
2
O
2
and ozonation, Chemical Engineering
Journal, 226: 402-8.
Haj i S, Benstaali B, Al-Bastaki N (2011). Degradation of methyl
orange by UV/H
2
O
2
advanced oxidation process, Chemical
Engineering Journal, 168: 134-9.
Hu Q, Zhang C, Wang Z, Chen Y, Mao K, Zhang X, Xiong
Y, Zhu M (2008). Photodegradation of methyl tert-butyl ether
(MTBE) by UV/H
2
O
2
and UV/TiO
2
, I. Hazard. Mater, 154: 795-
803
Kim I, Tanaka HT (2009). Photodegradationcharacteristics of
PPCPs in water with UV treatment, Environment international,
35: 793-802.
Kru ithof J, Kamp PC, Martijn BJ (2007). UV/H
2
O
2
treatment:
a practical solution for organic contaminant control and pri-
mary disinfection, Ozone Sci. Eng, 29: 273-80.
Jun g YJ, Kim WG, Yoon Y, Kang J.W, Hong YM, Kim HW
(2012). Removal of amoxicillin by UV and UV/H
2
O
2
processes,
Science of the Total Environment, 420: 160-7.
Kan g SF, Yen HY, Liao CH, Yao YC (2010). Decolorization and
Mineralization of Textile Ef uent by H
2
O
2
/Ultraviolet Pro-
cesses, Environmental Engineering Science, 27: 357-63.
Lamsal R, Walsh ME, Gagnon GA (2011). Comparison of
advanced oxidation processes for the removal of natural
organic matter, Water Research, 45: 3263-69.
Li K, Hokanson D, Crittenden J, Trussell R, Minakata D (2008).
Evaluating UV/H
2
O
2
processes for methyl tert-butyl ether and
tertiary butyl alcohol removal: effect of pretreatment options
and light sources, Water Res, 42: 5045-53.
Lin HC, Wang GS (2011). Effects of UV/H
2
O
2
on NOM frac-
tionation and corresponding DBPs formation, Desalination,
270: 221 –226.
Mat ilainen A, Sillanpa M (2010). Removal of natural organic
matter from drinking water byadvanced oxidation processes,
Chemosphere, 80: 351-65.
Nie Y, Hu C, Zhou L, Qu J, Wei Q, Wang D (2010). Degradation
characteristics of humic acid over iron oxides/Fe
0
core–shell
nanoparticles with UVA/H
2
O
2
, J. Hazard. Mate, 173: 474-9.
Per eira VJ, Weinberg HS, Linden KG, Singer PC (2007). UV
degradation kinetics and modeling of pharmaceutical com-
pounds in laboratory grade and surface water via direct and
indirect photolysis at 254 nm, Environmental science & tech-
nology, 41,1682-8.
Ros enfeldt EJ, Linden KG, Canonica S, Gunten U (2006). Com-
parison of the ef ciency of *OH radical formation during ozo-
nation andthe advanced oxidation processes O
3
/H
2
O
2
and UV/
H
2
O
2
, Water research, 40: 3695-704.
Ros enfeldt E, Linden K (2007). The ROH, UV concept to charac-
terize and the model UV/H
2
O
2
process in natural waters, Envi-
ron. Sci. Technol, 41: 2548- 53.
Sakai H, Autin O, Parsons S (2013). Change in haloacetic acid
formation potential during UV and UV/H
2
O
2
treatment of
model organic compounds, Chemosphere, 92: 647–651.
Sheikh Mohammadi A, Sardar M, Almasian M (2013). Fenton’s
oxidation of para-chlorophenol with zero-valent iron, Desali-
nation and Water Treatment, 12: 1-7
She mer HS, Linden KG (2007). Aqueousphotodegradation and
toxicity of the polycyclic aromatic hydrocarbons uorene,
dibenzofuran and dibenzothiophene, Water research, 41: 853-
61
Wan g W, Wang W, Fan Q, Wang Y, Qiao Z, Wang XC (2014).
Effects of UV radiation on humic acid coagulation characteris-
tics in drinking water treatment processes, Chemical Engineer-
ing Journal, 256: 137-43.
Vilhunen Sari, Vilve Miia, Vepsäläinen Mikko, Sillanpää Mika
(2010). Removal of organic matter from a variety of water
matrices by UV photolysis and UV/H
2
O
2
method, Journal of
Hazardous Materials, 179: 776–782.
Yazdanbakhsh AR, Amir SM, Mahdieh S, Godini H, Almasian
M (2014). COD removal from synthetic wastewater containing
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS USE OF ULTRAVIOLET AND ULTRAVIOLET /PEROXIDE HYDROGEN PROCESSES FOR DEGRADATION 687
Mohamadreza, Sohrab and Mansour
azithromycin using combined coagulation and a fenton-like
process, 13: 2929-2936
Yeon J, Gi KW, Yeojoon Y, Kang JW, Min HY, Wook KH (2012).
Removal of amoxicillin by UV and UV/H
2
O
2
processes, Science
of the Total Environment, 420: 160–167.
Yua n F, Hu C, Hu X, Qu J & Yang, M. (2009). Degradation of
selected pharmaceuticals in aqueous solution with UV and UV/
H
2
O
2
, Water Res, 43: 1766-74.
Zhang X, Minear RA (2006). Formation, adsorption and sepa-
ration of high molecular weight disinfection byproducts result-
ing from chlorination of aquatic humic substances, Water Res,
40: 221– 230.
Zep p RG, Braun AM, Hoigne J, Leenheer JA (1987). Photo-
production of hydrated electrons from natural organic sol-
utes in aquatic environments, Environ. Sci. Technol, 21:
485-90.
688 USE OF ULTRAVIOLET AND ULTRAVIOLET /PEROXIDE HYDROGEN PROCESSES FOR DEGRADATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS