1Department of Chemistry, Azadshahr Branch, Islamic Azad University, Azadshahr, Golestan, Iran
2Department of Physics, Behbahan Branch, Islamic Azad University, Behbahan, Iran
Article Publishing History
Received: 22/07/2016
Accepted After Revision: 19/09/2016
The absorption feasibility of toluene molecule in the C24, Si-doped C24, and C20 fullerenes has been studied based on calculated electronic properties of those fullerenes using Density functional Theory (DFT). It is found that energy of toluene adsorption upon the pure and Si-doped C24 fullerenes were in range of -1.80 and -15.72 kJ/mol with slight changes in their electronic structure. The results showed that the C24 and Si-doped C24 fullerenes cannot be used as a chemical adsorbent or sensor for toluene molecule in nature. Also, silicon doping cannot significantly modify both the adsorption energy and electronic properties of C24 fullerene to toluene. On the other hand, toluene molecule exhibits a high sensitivity upon C20 fullerene, so that the energy gap of the fullerene is changed about 91.21% after the adsorption process. We concluded that the C20 fullerene can be served as a reliable material for toluene detection.
C24 And C20 Fullerene, Toluene, Sensor, Dft Study
Baei M. T, Shojaei A. Toluene Adsorption on C24, Si-doped C24, and C20 Fullerenes. Biosc.Biotech.Res.Comm. 2016;9(4).
Baei M. T, Shojaei A. Toluene Adsorption on C24, Si-doped C24, and C20 Fullerenes. Biosc.Biotech.Res.Comm. 2016;9(4). Available from: https://bit.ly/34auG7N
INTRODUCTION
Toluene, a kind of important volatile organic compound (VOC), is used in many kinds of industries, such as painting, printing, coating, automotive, and petrochemical industries. Emission of the toluene from these industries causes air pollution and the environment, odor problem, flammability problem and affects human health. Due to these toxicological effects, air contaminated with toluene needs to be treated before it can be released to atmosphere. Therefore, adsorption and detection of toluene molecule has high importance in environmental systems, (Chang et al 2000, Vandenbroucke et al 2011).
In recent years, a wide variety of investigations have been done upon the adsorption of toluene. For example, the adsorption of toluene on ZSM5 and mordenite zeolites modified with Cs was investigated both theoretically and experimentally. Toluene removal by oxidation reaction in spray wet scrubber has been also studied. It has been shown that the highest toluene removal efficiency was of 91.7%. Their results showed that the sequence to prepare the catalyst affected the adsorption and plasma catalytic of adsorbed toluene, (Chungsiriporn et al 2006, Serra et al 2012 and Qin et al 2016).
After the synthesis of fullerene C60 by Kroto et al (1985) fullerenes have attracted great interest because of their physical and chemical properties and applications in nanomaterials and biomedical science, (Akasaka and Nagase 2002 and Muthukumar and Larsson 2008). Also, they play a fundamental role in medical sciences, chemistry, biology, materials, electronics, and related fields (Senapati et al 2004, Yoon et al 2009, Chamberlain et al 2011).
Among the smaller fullerenes, C20 and C24 fullerenes are a favorable candidate for examining in molecule electronic devices, nanotechnology, and biomedical engineering. Liang Xu et al (2012) have studied the interaction between empty C24 fullerene and the smallest amino acid (glycine). Their results showed that the glycine molecule is energetically favorable to interact on the C24 fullerene through the amino nitrogen active site. Also, orientation effects on the electronic transport properties of C24 fullerene were studied by Wen-Kai Zhao et al (2013) between the electrodes (Au–C24–Au). Their findings showed the application of the C24 fullerene in the field of single molecular devices or nanometer electronics. Prinzbach et al (2000) have synthesized C20 fullerene by using C20H20. They replaced the hydrogen atoms with bromine atoms, and then debrominated to produce C20 fullerene in gas phase.
An et al (2011) have tried to stabilize the highly perfect Ih symmetry C20 fullerene cage by placing interstitial atoms at the center of the fullerene using first-principles density functional theory (DFT). They was also investigated the transport properties of C20 fullerene and the endohedral Li@C20 metallofullerene coupled to three-dimensional electrode system using DFT methods . Using different DFT methods, the transformation processes from the physisorption state to the chemisorption state of a H2 molecule in C20 fullerene and B-doped fullerene C19B system was investigated.
In previous study, we have studied the chemical functionalization of C20 fullerene with NO2 molecule. In summary, there are few studies on the C20 and C24 fullerenes and further study on the structures is of important duties. On the other hand, the doped C20 and C24 fullerenes show dramatic changes in electronic properties with respect to their pristine. Therefore; the aim of this study was to investigate the ability of toluene adsorption onto C24, Si-doped C24, and C20 fullerenes, to determine whether the fullerenes are applicable for filtering or sensing toluene molecule (An et al., 2010, Tian et al 2011 and Baei 2013).
Computational Methods
In this study, the adsorption of toluene on the C24, Si-doped C24, and C20 fullerenes are considered. C24 fullerene with a D6d symmetric form consisting of two 6-membered rings joined by twelve 5-membered rings and C20 fullerene with an Ih symmetric form consisting of 12 pentagons and 30 bonds are selected for this purpose. DFT calculations at the level of B3LYP (Becke 1993), with the standard 6-31G* basis set were carried out on the C24, Si-doped C24 fullerenes and the PBE (Perdew and Ernzerhof,1996) level in GAMESS package, (Schmidt et al 1996) with the standard 6-31G* basis set were performed on the C20 fullerene. For the C20 fullerene, the PBE functional show better results with respect to B3LYP method of (Perdew and Ernzerhof, 1996). These methods were used to calculate the adsorption energy (Ead) of toluene on the surface of the fullerenes using the following equations:
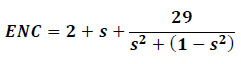
Where Etoluene/C24 and Etoluene/C20 are the total energy of complex of adsorbed toluene on the C24 and C20 fullerenes. EC24, EC20, and Etoluene are the total energy of the pure C24 and C20 fullerenes and toluene molecule. Etoluene/Si-doped C24 is the total energy of complex of adsorbed toluene on the Si-doped C24 fullerene. E Si-doped C24 is the total energy of Si-doped C24 fullerene, and äBSSE is the BSSE correction. The negative values of Ead reveal that the adsorption is exothermic. For the structures, the geometry optimization, natural bond orbital (NBO) density of states (DOS), energies, and frontier molecular orbital (FMO) were calculated according to Glendening et al (1998). The energy gap (Eg) of the optimized structures was obtained by the energy difference between the highest occupied molecular orbital (HOMO) and the lowest un-occupied molecular orbital (LUMO). Also, Fermi level energy (EFL) and work function (Ö) of the considered structures are calculated.
RESULTS AND DISCUSSION
The optimized structure of C24, Si-doped C24, and C20 fullerenes are shown in Fig. 1. The C-C bond lengths of pure C24 and C20 fullerenes are in the range of 1.36-1.53 and 1.44-1.51 Å. The electronic property analysis based on DOS shows a HOMO-LUMO gap (Eg) of 1.82, 1.63, and 0.74 eV for the C24, Si-doped C24, and C20 fullerenes, respectively. Eg of the C24 and C20 fullerenes are very close to the results of Liang Xu et al (2012) and Kumar et al (2011) respectively. Change of Eg of the Si-doped C24 fullerene is about 10.44% with respect to the pristine model, suggesting that the electronic properties of C24 fullerene is not very sensitive on the Si adsorption.
Toluene Adsorption On The C24, Si-Doped C24, And C20 Fullerenes
First, we computed the optimized structures of the individual toluene molecule and C24, Si-doped C24, and C20 fullerenes. Then, for investigation of toluene adsorption on the out surface of the fullerenes, several possible initial adsorption geometries including the parallel and perpendicular orientations of toluene molecule close to the surface of the fullerenes are considered. After full structural optimization without any constraints, the three most stable structures are obtained upon the relaxation processes for toluene adsorption on the C24 fullerene which are shown in Figs. 2 and their electronic properties are shown in Table 1.
| Table 1: Adsorption energy (Ead) of toluene on C24, Si-doped C24, and C20 fullerenes, HOMO energies (EHOMO), LUMO energies (ELUMO), HOMO–LUMO energy gap (Eg), Fermi level energy (EFL), and work function (Ö) for the studied systems. | |||||||||
| Structure | Ead(kJ/mol) | EHOMO(eV) | ELUMO(eV) | Eg(eV) | aΔEg(%) | bQT|e| | EFL(eV) | Ö(eV) | cΔÖ% |
| Toluene | – | -6.40 | 0.14 | 6.54 | – | – | -3.13 | 3.27 | – |
| C24 | – | -5.64 | -3.82 | 1.82 | – | – | -4.73 | 0.91 | – |
| Fig. 2A | -1.80 | -5.68 | -3.86 | 1.82 | 0.00 | -0.01 | -4.77 | 0.91 | 0.00 |
| Fig. 2B | -3.22 | -5.68 | -3.85 | 1.83 | 0.55 | -0.01 | -4.76 | 0.92 | 1.10 |
| Fig. 2C | -3.18 | -5.57 | -3.76 | 1.81 | 0.55 | 0.00 | -4.66 | 0.90 | 1.10 |
| Si-doped C24 | – | -5.62 | -3.99 | 1.63 | – | – | -4.80 | 0.82 | – |
| Fig. 3 | -15.72 | -4.38 | -3.02 | 1.36 | -16.53 | -0.01 | -3.70 | 0.68 | 17.04 |
| C20 | – | -4.45 | -3.71 | 0.74 | – | – | -4.08 | 0.37 | – |
| Fig. 4A | -6.26 | -4.49 | -3.76 | 0.73 | 0.72 | 0.00 | -4.12 | 0.37 | 0.00 |
| Fig. 4B | -60.16 | -4.60 | -3.18 | 1.41 | 91.21 | 0.17 | -3.89 | 0.70 | 91.21 |
| aThe change of Eg of C24, Si-doped C24, and C20 fullerenes after toluene adsorption
bQT is defined as the total natural bond orbital charges on the toluene molecule (positive values show charge transfer from toluene molecule to the fullerenes) cThe change of work function of C24, Si-doped C24, and C20 fullerenes upon toluene adsorption |
|||||||||
In Configurations A and B in Fig. 2, the hydrogen atoms of toluene is interacted perpendicular to 5 and 6-membered rings of the C atoms of the fullerene with the minimum distance of about 3.45 to 3.22 Å, respectively. Calculated Ead values of the configurations are about -1.80 and -3.22 kJ/mol, respectively and a maximum NBO charge of 0.01|e| is transferred from the fullerene to the toluene. The structural parameters of the configurations upon the adsorption process remain unchanged. The results show that the Configurations have a weak interaction between toluene molecule and the C24 fullerene. Also, in the configurations, the influence of toluene adsorption on the electronic properties of the fullerene was investigated and almost remains unchanged. HOMO and LUMO energies, energy gap (Eg), Fermi level energy (EFL), and work function (Ö) of the configurations are shown in Table 1.
The EFL in a molecule is approximately middle of Eg and Ö for a semiconductor is defined as the energy difference between the EFL and the LUMO [24] which is important in field emission applications. The important sensing mechanisms in nanostructure devices is change of Eg the nanostructure and subsequently change of its conductivity upon the adsorption process as per Zhou et al (2010). Therefore, it is very important to calculate the DOS of the C24 fullerene in the configurations before and after toluene adsorption. DOS for the configurations is shown in Fig. 2. In comparison with the pure model, their Eg values remain almost unchanged (changed by about 0.55 %). Also, for the configurations, Fermi level energy (EFL), and work function (Ö) values remain almost unchanged (changed by about 1.10 %). The results indicate that the toluene adsorption via these configurations has no sensible effects on the electronic properties of the fullerene.
 |
Figure 1: Optimized Structures of C24, Si-doped C24, and C20 fullerenes and their density of states (DOS). |
In Configurations C in Fig. 2, the hydrogen atoms of toluene is interacted parallel to 5 and 6-membered rings of the C atoms of C24 fullerene with the minimum distances of about 3.94 Å. The Ead value of the configuration is about -3.22 kJ/mol. In comparison with the pure C24 fullerene, its Eg, EFL, and Ö values remain almost unchanged (see Table 1).
 |
Figure 2: Different models of toluene adsorption on the C24 fullerene and their density of states (DOS). Distances are in Å. |
The above results show that C24 fullerene cannot be a potential efficient adsorbent or sensor for adsorption or determine of toluene from environments systems. Therefore, to solve this problem, introduced various functional groups and or the doping methods, which enables chemical covalent bonding between the fullerene and foreign atoms or molecules. Pure silicon can be doped with other elements to adjust its electrical response by controlling the number and charge of current carriers. Such control is necessary for transistors (Cui and Lieber (2001), solar cells, semiconductor detectors, and other semiconductor devices which are used in electronics and other high-tech applications.
Therefore, doping of C24 fullerene by Si atoms may be able to yield changes in the interactions between the fullerene and foreign atoms or molecules. Fig. 3 shows adsorption configuration of toluene molecule and its density of states (DOS) on the C24 fullerene doped with Si atom. The Ead value of the configuration is about -15.72 kJ/mol, which is stronger than that in the pure C24 states. Nevertheless, the adsorption configuration is in the range of physisorption and cannot be used as potential efficient adsorbent for adsorption of toluene. For further study, the changes of Eg, EFL, and Ö of the configuration is shown in Table 1. The values do not show notable changes. Therefore, the C24 fullerene doped with Si atom cannot be a potential efficient sensor for determine of toluene molecule. Also, Silicon doping cannot significantly improve both the adsorption energy and electronic properties of C24 fullerene to toluene.
 |
Figure 3 |
In the next step, the influence of the toluene adsorption on the electronic properties of the C20 fullerene was studied. After full structural optimization, the two most stable structures are obtained upon the relaxation processes which are shown in Fig. 4. In Configurations A and B in Fig. 4, the toluene is interacted perpendicular and parallel to surface of the fullerene. The Ead value of toluene in Fig. 4A is -6.26 kJ/mol (a weak interaction) and in comparison with the pure C20 fullerene, its Eg, EFL, and Ö values remain almost unchanged (see Table 1). The results showed that in this configuration, C20 fullerene cannot be a potential efficient adsorbent or sensor for adsorption or determine of toluene molecule. However, the Ead value of toluene in Fig. 4B is -60.16 kJ/mol and also, the results show that the toluene adsorption through this configuration has sensible effects on the electronic properties of the fullerene. In this state, the Eg and Ö values are changed about 91.21% after the toluene adsorption. The important sensing mechanisms in nanostructure devices is change of Eg the nanostructure and subsequently change of its conductivity upon the adsorption process, (Zhou et al 2010). Therefore, the presence of the toluene molecule can be detected by computing the conductivity change of the C20 fullerene before and after the toluene adsorption. This behavior can be explained according to the following equation, (Li 2006).
![]()
Where ó is the electric conductivity of the structure and k is the Boltzmann’s constant. According to equation (4), smaller Eg at a special temperature leads to the larger electric conductivity. Therefore, the considerable change in Eg of the C20 fullerene shows the high sensitivity of electronic properties of C20fullerene towards the toluene molecule. Also, it is well known that one of the most important factors in sensor devices is their recovery time (ô) that can be described as:
 |
Figure 4 |
Where kB is the Boltzmann’s constant, T is the temperature, and í0 is the attempt frequency. According to equation (5), more negative Ead will prevent the recovery of the device. In other words, very strong interactions are not favorable in sensor devices due to long recovery times (ô). However, the Ead value of the configuration is -60.16 kJ/mol (Table 1) that is not too large to hinder the recovery of the fullerene. The results show the high sensitivity of the fullerene towards toluene molecule and can be used as toluene sensor.
CONCLUSION
We have investigated the toluene adsorption on C24, Si-doped C24, and C20 fullerenes using DFT calculations. The results show that toluene molecule presents a weak physical adsorption with the pure and Si-doped C24fullerene and the fullerenes are not a suitable adsorbent for toluene molecule. In addition, the results suggest that the pure and Si-doped C24 fullerene has low sensitivity to the presence of toluene and the influence of toluene adsorption on the electronic properties of the fullerenes remain almost unchanged. On the other hand, toluene molecule exhibits a high sensitivity upon C20 fullerene, so that the energy gap of the fullerene is changed about 91.21% after the adsorption process. The results show the high sensitivity of C20 fullerene towards toluene molecule and therefore can potentially be used for toluene sensors.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the financial support received from Islamic Azad University, Azadshahr Branch.
REFERENCES
AkasakaT.S. Nagase A. (2002) New, Family of Carbon Clusters, Kluwer Academic Publisher, Dordrecht, The Netherlands
An YP., C. L. Yang, M. S. Wang, X. G. Ma, D. H. Wang (2010) First-principles study of transport properties of endohedral Li@C20 metallofullerene, Current Applied Physics, 2010, 10, 260–265
An YP., C. L. Yang, M. S. Wang, X. G. Ma, D. H. Wang (2011) Geometrical and Electronic Properties of the Clusters of C20 Cage Doped with Alkali Metal Atoms, J. Clust. Sci.22, 31–39
Baei MT (2013) First-Principles Study of NO2 Adsorption on C20 Fullerene, Heteroatom Chemistry 24 (6), 516–522
Becke AD (1993) The role of exact exchange J. Chem. Phys., 1993, 98, 5648
Chang C, C. Lee, Y. Wu, and F. Jeng (2000) Assessment of the strategies for reducing volatile organic compound emissions in the automotive industry in Taiwan, Resour, Conserv. and Recycl, 34 117-128
Chungsiriporn J., C. Bunyakan, R. Nikom (2006) Toluene removal by oxidation reaction in spray wet scrubber: experimental, modeling and optimization, J. Sci. Technol., 28(6) 1265-1274
Cui Y., C. M. Lieber (2001) Highly polarized assembled using silicon nanowire building blocks Science, 291, 851
Glendening ED., A.E. Reed, J.E. Carpenter, F. Weinhold, NBO(1998) Version 3.1 TCI, University of Wisconsin, Madison
Kim C., B. Kim, S.M. Lee, C. Jo, Y.H. Lee (2002) Electronic structures of capped carbon nanotubes under electric fields, Phys. Rev. B. 65, 165418.
Kroto, HW., J.R. Heath, S.C. O’Brien, R.F. Curl, R.E. Smalleym (1985) C60: Buckmin- sterfullerene, Nature (London), 318, 162
Kumar R., A. Rani, Structure and electronic properties of Hn@C20 molecule (2011) Physica B 406, 1173–1177
Li S. (2006) Semiconductor Physical Electronics, 2nd ed., Springer, USA,
Liang Xu, Li Chao, Li Feng, Li Xiaojun, Tao Shuqing (2012) Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 98, 183–189
Muthukumar K.. J. A.Larsson (2008) Explanation of the different preferential binding sites forCeandLainM2@C80(M¼Ce, La)–a density functional theory prediction, J. Mater. Chem. 18, 3347
Perdew JP., K. Burke, M. Ernzerhof (1996) Generalized gradient approximation made simple, Physical Review Letters 77, 3865–3868
Prinzbach H., A. Weiler, P. Landenberger, F. Wahl, J. Worth, L.T. Scott, M. Gelmont, D. Olevano, B. Issendorff (2000) Gas-phase production and photoelectron spectroscopy of the smallest fullerene, C20, Nature, 407, 60-63
Qin C., C., X. Huang, X. Dang, J. Huang, J. Teng, Z. Kang (2016) Toluene removal by sequential adsorption-plasma catalytic process: Effects of Ag and Mn impregnation sequence on Ag-Mn/ã-Al2O3
Schmidt M., K. Baldridge, J. Boatz, S. Elbert, M. Gordon, J. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. Su, T.L. Windus, M. Dupuis, J.A. Montgomery Jr (1993) J. Comput. Chem., 1993 14, 1347
Senapati L., J. Schrier, K. B. Whaley(2004) Electronic Transport, Structure, and Energetics of Endohedral Gd@C82Metallofullerenes Nano Lett. 4, 2073–2078
Serra RM E. E. Miró, P. Bolcatto, A. V. Boix (2012) Experimental and theoretical studies about the adsorption of toluene on ZSM5 and mordenite zeolites modified with Cs, Microporous and Mesoporous Materials, 147 17–29
Tian C., K Fen Zhao, M. Shan Wang K (2011)Transformation mechanism of a H2 molecule from physisorption to chemisorptions in pristine and B-doped C20 fullerenes, Chemical Physics Letters 511, 393–398
Vandenbroucke, AM R. Morent, N. De Geyter (2011) Non-thermal plasmas for non-catalytic and catalytic VOC abatement. J. Hazard. Matter. 195 30-54.
Yoon M., S. Yang, Z. Zhang (2009) J. Chem. Phys., 2009, 131(6), 64707. Chamberlain TW, N. R. Champness, M. Schrder, A. N. Khlobystov (2011) Chem: Eur. J., 2011, 17, 668–674
Zhao WK., Chuan-Lu Yang, Jing-Fen Zhao, Mei-Shan Wang, Xiao-Guang Ma (2012) Physica B, 2012, 407, 2247–2253
Zhou X., W.Q. Tian, X.-L. Wang (2010) Adsorption sensitivity of Pd-doped SWCNTs to small gas molecules, Sensors and Actuators B: Chemical 151, 56–64.


