Green Catalysis and Process Technology Research laboratory, Department of Chemical Engineering, Maulana Azad National Institute of Technology, Bhopal-462 051
Article Publishing History
Received: 17/11/2016
Accepted After Revision: 27/12/2016
Plants extract have a vital role for the synthesis of different nanocatalysts like TiO2, ZnO, Fe2O3 etc. due to its non-toxicity, low cost. Many of these nanocatalysts have been used for the degradation of water pollutants. Some of the plants extract are used as reducing and stabilizing agent in the synthesis of nanoparticles. No work has been found on Butea monosperma (Palash) flower extract with ZnO nanoparticles. Phenol is found in many industrial effluents and excessive exposure of its may cause coma, cyanosis and even death. This research work focuses on the synthesis of ZnO/SiO2 with palash flower extract nanocatalyst by using single pot process for degradation of phenol from aqueous solution. The synthesized nanocatalyst of ZnO/SiO2 palash flower extract was characterized by using BET surface area, FEG-SEM, EDAX, XRD, and FTIR. BET surface area of nanocatalyst is 157.23 m2/g and FEG-SEM micrograph shows that spot of nanorange particles. Optimum percent removal of acenaphthylene was found to be 81% at 0.5g/500ml catalyst loading, 30oC temperature and 4h reaction time. Synthesis of ZnO/SiO2 nanocatalyst with palash flower extract by single-pot process is eco-friendly and cost effective method than other chemical methods.
Synthesis, Zno/Sio2, Nanocatalyst, Palash, Flower, Extract, Acenaphthylene, Degradation
Bharati R, Suresh S. Synthesis of Green Zno/Sio2 Nanocatalyst and its Application to Reduce Acenaphthylene from Refinery Waste Water. Biosc.Biotech.Res.Comm. 2016;9(4).
Bharati R, Suresh S. Synthesis of Green Zno/Sio2 Nanocatalyst and its Application to Reduce Acenaphthylene from Refinery Waste Water. Biosc.Biotech.Res.Comm. 2016;9(4). Available from: https://bit.ly/2sxJejE
INTRODUCTION
Palash tree is also known as Butea monosperma, is a very use full tree which is easily available in India (Hegde et al., 2014). From literature it has been found that palash flower has many biomolecules some are triterpene, flavonoids, glycosides carbohydrate and protein (Jamkhade et al., 2013). It has been found that solubility of palash flower is more in water than other solvents for extractive value (Hegde et al., 2014). It has been found that using plants extract as reducing and stabilizing agents for nanocatalysts synthesis is eco-friendly and cheap method than other methods which use chemicals (Dizaji et al., 2015).
Single pot method also known as one step method is the bioreduction processes in which plant extracts are used as reducing and stabilizing agent to reduce metal ions in nano range (Gdikandula et al., 2016). Palash flower extract contains a broad variability of biomolecules so this method is faster than other methods (Kumar and Yadav, 2012).
No research article has been found in the literature for the synthesis of ZnO/SiO2 based nanocatalyst by using an aqueous palash flower extract as reducing and stabilizing agent. This work is different from other works because palash flower extract contains many metals naturally which increases catalysts photocatalytic activity. SiO2 has been found naturally in palash flower extract. Incorporation of ZnO with SiO2 increases its photocatalytic activity for visible light. So ZnO/SiO2 nanocatalyst synthesized by this method will be more effective under sunlight to remove pollutant than ZnO nanocatalyst synthesized by other methods. We can use this nanocatalyst to treat effluents from industries under sunlight also. ZnO/SiO2 with palash flower extract nanocatalyst was used for acenaphthylene removal from refinery wastewater in an annular reactor. We have found Hexagonal crystal structure of ZnO nanocatalyst which is the most stable in the environment. Synthesized nanocatalysts were characterized by Brunauer-Emett-Teller (BET), energy dispersive atomic X-ray (EDAX), Field Emission Gun-Scanning electron microscope (FEG-SEM), and X-ray diffraction (XRD) and Fourier transform infrared spectroscopy ( FTIR) to determine their Surface area, size, shape, particle distribution and functional group.
MATERIALS AND METHODS
ZnO bulk material (Molychem from Mumbai, 99.5% purity), acenaphthylene (Ranbaxy fine chemicals from New Delhi, 99% purity),Sodium hydroxide (A. B. Enterprises, Mumba,, 98% purity),HCL (Central drug house Ltd. From Delhi, 35% purity), Palash flowers, (Butea monosperma) were collected from MANIT Bhopal campus, Double distilled water. ZnO bulk material was used as a precursor to make 1mM ZnO aqueous solution; HCL acid was used to making 1 M solution to dissolve ZnO in water. NaOH to make the 1N solution to maintained pH. Phenol was used to make aqueous solution of Phenol to remove by catalyst and find Percentage removal of Phenol by HPLC.
All chemicals used for the experimental work were analytical grade (AR). Palash flowers were collected from the Campus of MANIT Bhopal, Madhya Pradesh, India. The collected flowers were cleaned by washing many times with double distilled water and then dried in sunlight. Dried flower were powdered in a mixer and stored in a container to prevent from moisture and this powder was preserved for experimental work. 250 mL Erlenmeyer flask has been taken for boiling 10 g of palash flower powder and 200 mL of double distilled water for 1 h (Yadav and Khurana, 2015). Whatman filter paper (pore size >0.5µm) has been used for filtration of extract and it has been stored at 4°C in a deep freezer which was used later for experimental work. We have chosen water for extracting agent due to an extractive value of palash flower in water is 17.5 ± 0.5% which is comparatively maximum than other solvents.
ZnO has been bought from Molychem, Mumbai, and the aqueous palash flower extract has been used for the bioreduction process. To make nanoparticles from palash flower 35 mL of the aqueous flower extract was carefully added to 90 mL of 1 mM aqueous ZnO solution in 250 mL Erlenmeyer flasks. Colour of the solutions has changed from brownish yellow to dark brownish, confirming the green synthesis of ZnO. We have performed this experiment at pH 9.30; at this pH we got more precipitate of ZnO nanocatalyst. After the reaction, nanoparticles in the form of the precipitate had been settled at the bottom of the conical flasks. We separated ZnO nanoparticles in the form of precipitate by centrifuging it at 10000 rpm for 15 min (Awwad et al., 2013). Then we have used these nanoparticles for characterization and experimental work.
Characterization of ZnO nanocatalyst with palash flower extract was done by standard methods (Sarma and Sarma, 2014). Brunauer–Emmett–Teller (BET) surface area of the nanoparticle was found by nitrogen adsorption at 77.15K using an automatic pulse chemisorptions system (Micromeritics Chemisorb 2720) using the software available with the instrument. To determine the surface morphology and chemical composition, SEM and EDAX tests were done in the SAIF, IIT Mumbai. The phase identification and crystal structure have been analysed by X-ray powder diffraction (XRD) test. Functional groups present in synthesized nanocatalyst were found by FTIR test. The XRD and FTIR tests have been done in central laboratory of North Maharashtra University, Jalgaon, India.
Removal of acenaphthylene by synthesized nanocatalyst
Concentric Annulus photocatalytic reactor has been used for acenaphthylene removal from refinery wastewater by synthesized nanocatalyst. In this batch reactor, which we have used for experiment; two coaxial cylinders are there. The inner with reaction zone and lamp is placed on the symmetry axis. The total volume of the reactor is 1 litre and working volume is 0.7 litres. Practically, all the photons emitted by UV lamp reach the reaction medium if the outer walls of the reactor are reflective. This geometry has been selected for the photoreactor used in this study and offers a number of advantages viz. efficient photon captures, practical to fluidize the photocatalyst in a cylindrical geometry and an outer jacket can be used for meeting the cooling requirements of UV lamp. At the bottom of the reactor, the magnetic stirrer is fitted for uniform mixing. The temperature was maintained around 28±2 oC.
Acenaphthylene is one of the polycyclic aromatic hydrocarbons that is found as pollutant in refinery wastewater (Uddeen et al., 2011). In this experimental work for treatment of refinery waste water, 500 ml refinery wastewater has been treated in concentric annular reactor with 1000 ml capacity. For this 0.5/500 ml catalyst loading was used for photocatalytic reaction. The solution of 500 ml was used to treat in a 1000ml of the total volume of the reactor. The mixture solution was placed into an annular reactor and started the UV light (6W) to study the degradation or removal for duration of 6 h. The temperature was maintained at 30 oC. The samples were withdrawn from annular reactor periodically in an interval of 0.5, 1.5, 1, 2, 3, 4, 5, 6 hrs. After appropriate degradation, the samples were filtered and then obtained residual concentrations of acenaphthylene were analysed with the help of high performance liquid chromatography (HPLC) (Waters Pvt. Ltd., India) C-18 column.The removal of acenaphthylene (%) was determined by following relationships (Hassan et al.,2015).
Acenaphthylene degradation (%) = 100 (Ci(0)-Cf(t))/Ci(0)
Where, Ci(o) is the initial acenaphthylene concentration (mg/l), Cf(t) is the final acenaphthylene concentration (mg/l) at times t.
RESULTS AND DISCUSSION
The mechanism of ZnO nanocatalyst synthesis with palash flower extract is that we have taken 1mM ZnO solution for synthesis ZnO particles in nano range. Initially, ZnO (Bulk powder) was insoluble in water but when we added few drops of one molar HCL acid, it was completely soluble in water. The ZnO was degraded by acid and formed ZnCl2. ZnCl2 salt worked here as precursor of Zn+2 ions.Zn+2 ions were reduced in nano range by biomolecules present in palash flower extract. Than functional groups present in palash flower extract naturally worked as the capping agent for stabilization of synthesized nanoparticles (Akhtar et al., 2013). The mechanism and role of palash flower extract to synthesize ZnO/SiO2 nanocatalyst in nanorange is shown in Fig. 1. And SiO2 was obtained from palash flower extract which is present in palash flower naturally.
BET surface area of nanocatalyst has been found 157.23 m2/g. Reduction of Zn+2 ions into nanorange by palash flower extract can be seen by colour change. When we added palash flower extract and mixed it with the aqueous solution of the ZnO, the change in colour of solution from brownish yellow to dark brownish was observed and it has been confirmed that formation of ZnO nanocatalyst has been occurred (Shukla and Vankar, 2012). Figs.1-2 shows the EDAX spectra and FEG-SEM images of synthesised ZnO/SiO2 with palash flower extract. The Hexagonal Crystal structure of ZnO nanoparticles has been found by XRD pattern. The FEG-SEM image shown in Fig.2 Shows spherical shape of nanoparticles has been formed and Fig2-3 shows that nanocatalysts have been found in the range of 3 nm to 45 nm with 23 nm mean particle diameter (Khalil et al., 2014). At some places aggregation of nanoparticles have been shown in FEG-SEM image Fig.2.
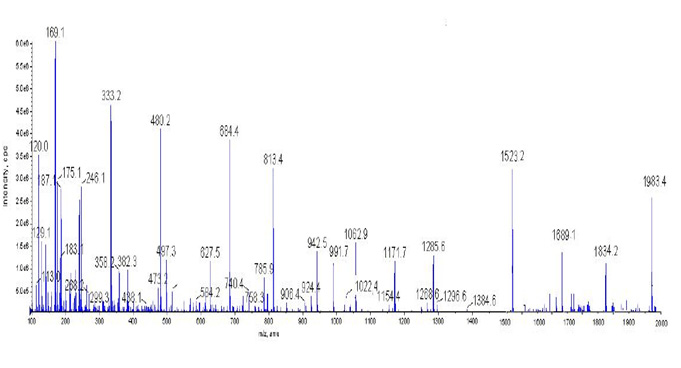 |
Figure 1: EDAX spectra of ZnO/SiO2 palash flower extract nanoparticles |
 |
Figure 1: FEG-SEM image of ZnO/SiO2 palash flower extract nanocatalyst. No. of particles Diameter |
 |
Figure 3: XRD pattern of ZnO/ palash flower extract nanocatalyst |
EDAX test has been done to find chemical composition of ZnO/palash flower extract nancatalyst .EDAX spectra found for a sample of ZnO/palash flower extract nanoparticles and the weight percentage of Zn and O are found to be 15.79 % and 38.05% respectively (Khalil et al., 2014). From EDAX it can be seen that Si is also present palash flower extract. It confirms that we have got ZnO/SiO2 with Palash flower extract nanocatalyst. It has been shown in EDAX spectra that we have got strong signal of energy peaks for Zn and O atoms in the range 0.5–1.5 keV and weaker signals for Zn atoms in the range 8-10 keV (Khalil et al., 2014; Sarma et al., 2014). It has been also found that we have got weaker signals for N, Mg, Si, P, Cl, K, and Ca, which were present in palash flower extract and due to presence of many metals naturally in palash flower extract it increases photocatalytic activity of ZnO/SiO2 with palash flower extract nanocatalyst.
XRD peaks shown in Fig.3 confirmed the presence of ZnO corresponding to PDF No. 80-0075. The lattice parameters a and c were calculated to be about 3.25 A0 and 5.209 A0 respectively. The minor amount of Si (SiO2) was also found in the XRD pattern. The crystal structure of the SiO2 was found to be matching with low quartz. The peaks of Si, as well as ZnO, were found to be broadened indicating nanocrystalline or amorphous nature of the crystal. In our experiment, the X-ray pattern of synthesized ZnO nanoparticles matches the hexagonal structure of the ZnO nanoparticles with the broad peaks at 2è = 36.243, 56.568. These are corresponding to (101), (110) and (103), respectively. Similar peaks were found in the ZnO based nanoparticles (Rekha et al., 2010, Khalil et al., 2014 and Sarma and Sarma, 2014).
Fig. 4 Shows FTIR spectra of ZnO/palash flower extract nanocatalyst , which has been done to find various functional groups present in nanocatalyst .IR frequency from 2697.54 to 3068.85 is due to O-H stretching , 1061.85 and 1248.95 for Si-O-Si starching ,1538.28 to 1664.62 for C=O stretching ,1016.52 for ZnO stretching , this stretching is slightly shifted from 1000 which is for metal oxide due to the presence of other functional groups(Uddeen et al.,2011).
 |
Figure 4: FTIR spectra of ZnO/palash flower extract nanocatalyst |
Hence XRD pattern confirms that ZnO nanoparticles have been formed by this bioreduction process. Phases of the sample were matched with standard XRD pattern of ZnO nanoparticles. From EDAX, XRD and FTIR test it has been found that SiO2 has been also found in the sample which increases photocatalytic activity of this catalyst.
Percentage removal of pollutant
ZnO/SiO2 nanocatalyst made by biosynthesis or green single pot method are very good nanocatalyst for degradation of acenaphthylene from refinery wastewater. Fig. 5 shows the degradation of phenol from aqueous solution by using ZnO/SiO2palash flower extract. Acenaphthylene degradation from refinery wastewater has been done in an annular photocatalytic reactor and it has been found that optimum percent removal of acenaphthylene was found to be 81% at 0.5g/500ml, 30oC and 4h of catalyst loading, temperature and reaction time respectively. Fig. 6 shows optimum percent removal of acenaphthylene with pH at 9.5 pH.
 |
Figure 5: Degradation of acenaphthylene from refinery wastewater with time by ZnO/SiO2 with palash flower extract nanocatalyst |
 |
Figure 6: Degradation of acenaphthylene from refinery wastewater at different pHby ZnO/SiO2 with palash flower extract nanocatalyst |
CONCLUSION
In present research work the characterization of nanocatalyst shows that we have synthesized ZnO/SiO2 nanocatalyst with palash flower extract in nanorange. We have used palash flower extract as reducing and stabilizing agent in place of chemical to synthesize ZnO nanocatalyst so it is eco-friendly method. Palash flower contains many biomolecules which are responsible for reduction and stabilization of Zn+2 ions in nanorange so it is more fast method than other methods. It contains SiO2 naturally catalyst synthesized by palash flower extract is more efficient to remove pollutant than other methods. So Palash flower is easily available in India at low cost and we need very simple techniques to synthesize nanocatalyst by this method so it is cheap process than other processes. We have found from EDAX and XRD test that many metals present naturally in palash flower extract so incorporation of these metals with ZnO nanocatalyst increases its photocatalytic activity .Results shows that ZnO/palash flower extract nanocatalyst is very good photocatalyst to remove phenol from an aqueous solution of phenol. The percent removal of phenol can be optimized by controlling affecting parameters such as reaction time, catalyst size, catalyst loading, pH and temperature. The optimum percent removal of phenol has been found 81% at 0.5/500ml catalyst loading at 30oC temperature and 4h reaction time.
ACKNOWLEDGMENT
The authors wish to thank Ministry of Human Resource Development, Government of India (MHRD) and Maulana Azad National Institute of Technology (MANIT) Bhopal, India to provide fund and facilities to carry out experimental work.
REFERENCES
Ahmed, S.; Ahmad, M.; Swami, B. L.; Ikram, S. (2016): A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications. A green expertise, Journal of Advanced Research, 7(1), 17–28.
Akhtar, M. S.; Panwar, J.; Yun, Y.S. (2013): Biogenic Synthesis of Metallic Nanoparticles by Plant Extracts. American Chemical Society Sustainable Chemistry & Engineering, 1, 591−602.
Awwad, A. M.; Salem, N. M. and Abdeen, A. O. (2013): Green synthesis of silver nanoparticles using carbon leaf extract and its antibacterial activity. International Journal of Industrial Chemistry, 4(1):29, 1-6.
Balachandran, Y. L.; Peranantham, P.; Selvakumar, R.; Arno, C.; Girija, G. S. (2012): Size-Controlled Green Synthesis of Silver Nanoparticles using Dual Functional Plant Leaf Extract at Room Temperature. International Journal of Green Nanotechnology, 4(1), 310–325.
Dizaji, A. N.; Yilmaz, M.; Piskin, E. (2015): Silver or gold deposition onto magnetite nanoparticles by using plant extracts as reducing and stabilizing agents. Artificial Cells, Nanomedicine, and Biotechnology, Early Online, 1–7.
Gudikandula, K. and S. C., Maringanti (2016): Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. Journal of experimental nanoscience, doi: 10.1080/17458080.2016.1139196.
Hassan, S. E. D.; Desouky, S. E.; Fouda, A.; Mamdouh, S. E. and Ahmed A. (2015): Biodegradation of Phenanthrene by Klebsiella sp Isolated from Organic Contaminated Sediment. Journal of Advances in Biology & Biotechnology, 4(4), 1-12.
Hegde, S. V.; Hegde, G. R.; Mannur, S.; Poti, S. S. (2014): Pharmacognostical studies on Butea monosperma (Lam.) Taub (Faboideae) flower. International Journal of Pharmaceutical and Phytopharmacological Research, 4(1), 2250-1029.
Jamkhande, P. G.; Patil, P.H.; Tidke, P. S. (2013): In Vitro Antioxidant activity of Butea monosperma Flowers Fractions. International Journal Drug, Delivery& Research, 5 (3), 245-255.
Khalil, M. I.; M. Qunaibit, M. A.; Zahem, A. M. A.; Labis, J. P. (2014): Synthesis and characterization of ZnO nanoparticles by thermal decomposition of a curcumin zinc complex. Arabian Journal of Chemistry, 7(1), 1178–1184.
Kumar, V. and S. K., Yadav (2012): Characterization of gold nanoparticles synthesized by leaf and seed extract of Syzygium cumini L. Journal of Experimental Nanoscience , 7(4), 440–451.
Rekha, K.; Nirmala, M.; Nair, M. G.; Anukaliani, A. (2010): Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles, Physica B, 405 (1), 3180–3185.
Sarma, H.; Chakrabortty, D.; Sarma, K.C. (2014): Structural and Optical Properties of Zno Nano Particles. IOSR Journal of Applied Physics, 6 (4), 08-12.
Sarma, H.; Sarma, K.C. (2014): X-ray Peak Broadening Analysis of ZnO Nanoparticles Derived by Precipitation method. International Journal of Scientific and Research Publications, 4(3), 2250-3153.
Shukla, D. and P. S., Vankar (2012): Synthesis of Plant Parts Mediated Gold Nanoparticles. International Journal of Green Nanotechnology, 4(1), 277–288.
Uddeen, B.H.D.; Daud, W.M.A.W.; Aziz, A.R.A.(2011): Treatment technologies for petroleum refinery effluents. A review, Process safety and environmental protection, 89(1), 95-105.
Wang, P.; Menzies, N. W.; Lombi, E.; Sekine, R.; Blamey, F. P. C.; Soriano, M. C. H.; Cheng, M.; Kappen, P.; Peijnenburg, W. J. G. M.; Tang, C.; Kopittke P. M. (2015): Silver sulfide nanoparticles (Ag2S-NPs) are taken up by plants and are Phytotoxic. Nanotoxicology, Early Online: 1–9.
Yadav S. and Khurana, J. M. (2015) Cinnamomum tamala leaf extract-mediated green synthesis of Ag nanoparticles and their use in pyranopyrazles synthesis. Chinese Journal of Catalysis, 36 (1), 1042-1046.


