1Department of Biochemistry, School of Medicine, Kashan Uiversity of Medical Sciences, Kashan, Iran
2Department of Radiopharmacy, School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
3Herbal Medicine Research Center & Department of Pharmaceutics, School of Pharmacy, Branch of Pharmaceutical Sciences. Islamic Azad Universty, Tehran, Iran
Corresponding author Email: mostafa.saffary@gmail.com
Article Publishing History
Received: 19/01/2017
Accepted After Revision: 22/03/2017
Nowadays there is a substantial interest on the biological activities of essential oils. Zataria multiflora essential oil has a wide spectrum of pharmacological activities. However, essential oils are unstable and susceptible to degradation by oxygen, light and temperature and also low penetration in transdermal administration. Therefore, nanoliposomal systems can be used as promising strategies to overcome these limitations due to their unique set of properties: Nanoliposomes were prepared via two different methods based on thin film hydration method, including extrusion and sonication methods. The physical properties of nanoliposomes such as particle size, polydispersity index, zeta potential, encapsulation efficiency and stability were also determined. In comparison of two methods, sonicated nanoliposomes had smaller mean particle size and better dispersity while mean encapsulation efficiency in extruded ones were higher. It can be deducted by consideration of significance level; physicochemical properties of the vesicles were strongly influenced by essential oil and cholesterol concentration besides the preparation method.
Nanoliposome; Zatariamultiflora; Essential Oil; Thin Film Hydration; Sonication
Arabi M. H, Mirzapour A, Chabok H, Ardestani M. S, Saffari M. Preparation Methods of Nanoliposomes Containing Zataria Multiflora Essential Oil: a Comparative Study. Biosc.Biotech.Res.Comm. 2017;10(1).
Arabi M. H, Mirzapour A, Chabok H, Ardestani M. S, Saffari M. Preparation Methods of Nanoliposomes Containing Zataria Multiflora Essential Oil: a Comparative Study. Biosc.Biotech.Res.Comm. 2017;10(1). Available from: https://bit.ly/2YJrI6E
Introduction
Zataria multiflora is one of the lamiacea family herbs with remarkable pharmacological properties. It natively grows in Iran, Pakistan, and Afghanistan (Saedi Dezaki et al., 2016). It has various therapeutic effects such as antiseptic, antispasmodic, carminative, expectorant, anti-inflammatory, antiparasitic, spasmolytic, antiviral, antibacterial, antifungal and antioxidant properties (Sunar et al., 2009). Most of these properties are related to the main constituents of its essential oils, which are thymol and carvacrol and significant quantities of phenolic monoterpenes (Misaghi & Basti., 2007; Ali et al., 2000). Essential oils are confirmed to possess wide spectrum of pharmacological properties, besides their classical roles as natural food additives, such as the antioxidant, antibacterial, antifungal and anti-inflammatory activities (Shahsavari et al., 2008; Edris, 2007). Unfortunately, essential oils are biologically unstable, poorly soluble in water and are very sensitive to environment (Martin et al., 2007). All these obstacles restrict the application of essential oils as candidates for pharmatherapeutic treatments. Currently, nanoencapsulation of these oils in drug delivery systems, among which is the use of liposomal encapsulation, have been proposed to improve solubility, stability and efficacy of essential oil-based formulations (Belay et al., 2011; Saffaria et al., 2016).
Liposomes represent versatile and progressive nano delivery system for extensive range of biologically active compounds (Hofheinz et al., 2005). Liposome is a lipoidal vesicle composed of a bilayer membrane that have been developed to deliver drug to specific site in the body for more than four decades (Chetanachan et al., 2008); El-Samaligy et al., 2006). Because of biodegradation, nontoxicity, biocompatibility, non-immunogenicity, as well as superior efficacy, they are excellent carrier systems for a variety of applications, and in particular for essential oils (Sinico et al., 2005; Liolios et al., 2009; Saffarib et al., 2016). Encapsulation in liposome protect the essential oil from light, air and humidity, increases the solubility of the oil , enhancing the bioavailability of this drug, drug-targeting and makes the oil easier to handle (Wen et al., 2010; Valenti et al., 2001). The aim of this study was to prepare phosphatidyl choline based nanoliposomes via optimization the concentrations of essential oil, cholesterol and lipid composition through different methods for encapsulating zataria multiflora essential oil. Furthermore, to compare these methods via physicochemical properties of nanoliposomes including size, PDI index, zeta potential, encapsulation efficiency and drug release profile.
Materials and Methods
Lecithin, Egg PC, DOTAP were purchased from LIPOID (Germany). Cholesterol was from Sigma-Aldrich Co. The solvents (chloroform, methanol and ethanol) were purchased from commercial source and were in analytical grade. The essential oils were obtained from Barij essence Co. in Kashan, Iran.
Preparation Of Liposome
Liposomes can be formulated and processed to vary in size, composition and charge (Akbarzadeh et al., 2013). For production of different types of liposomes, many methods are available (Meure et al., 2008). All Preparation methods can be simplified as to involve three basic steps: 1) dissolve the lipids in aqueous media in order to forming liposomes. 2) Purification of prepared liposomes. 3) Analysis of final product. Liposomes in our study prepared with three different methods as describe here:
Thin film hydration
The most conventional and classical method, known by hydration of dried phospholipid films (Bangham et al., 1965). Multilamellar vesicles (MLVs) were prepared based on the thin film hydration method. Briefly the precise amount of lecithin, cholesterol and essential oil, according to several ratios of essential oil to total lipids, molar ratio of cholesterol to lecithin and lipid composition (DOTAP), were dissolved in chloform-methanol (2:1, v/v) in a 500 ml round-bottomed flask. The organic solvent was eliminated by rotary evaporator (heidolph Hei-VAP Germany) under reduced pressure and high vacuum for 2 hour until a thin film was formed on the walls (above the lipid phase transition temperature, Tc). Then the obtained lipid film was dispersed in Phosphate Buffer Saline solution (PBS, pH=7.4). This suspension was allowed to hydrate for 1.5 hour. Finally milky white suspension is formed. During the process, the conditions such as speed (120 rpm) and temperature (above the Tc of lecithin) for conventional liposomes were maintained constant.
Concentration of essential oil
The total lipid concentration to prepare liposomes was 50 mM. Different ratios of essential oil to total lipid (1/2, 1/3, 1/4) with a constant molar ratio of cholesterol to phosphatidyl choline (1: 1) were prepared.
Concentration of cholesterol
After finding optimum concentration of essential oil to total lipid, cholesterol concentration was optimized by various concentrations as 7.1, 6.4, 4.83 mg/ml on a weight basis and 1:1, 1:2, 1:3 (molar ratio of Cholesterol: PC) in the overall formulation.
Charge-inducing lipids
EO-loaded cationic liposomes were formulated with pc, 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), and cholesterol at a 1:1:1 molar ratio of E80: DOTAP: CH.
Sonication method
Sonication is a simple method for reducing the size of liposomes to the nanoscale and manufacture of nanoliposomes. Liposomal suspension of MLVs transferred to a tube and placed in ice bath. Then the suspension was sonicated using prob sonicator (Hielscher UP400S, Germany).Vertically, the probe of a sonicator was fully immersed in the middle of the volume. The liposomal suspension was exposed to ultrasonic irradiation with an output of 70 Watt amplitude and duration of continuous 20 minute (6 times for 5 min). The energy input into liposomal dispersion is very high in this method.
Extrusion method
To obtain large unilamellar vesicles, the liposomal dispersion up to 3 mL was extruded three times through a polycarbonate membrane (100, 200 nm pore size) at above the lipid transition temperature by using an extruder to give a translucent solution.
Different formulation are listed in table 1 based on their method of preparation and their components and composition.
| Table 1: formulation based on method of preparation and composition | |||
| Method | Composition | EO /Total lipid | CH:PC |
| Thin film hydration | PC+CH+EO
PC+CH+EO |
0.5, 0.33,0.25
0.33
0.5, 0.33, 0.25
0.33
0.5, 0.33, 0.25
0.33 |
1:1
1:2,1:3
1:1
1:2,1:3
1:1
1:2,1:3 |
| Sonication | PC+CH+EO
PC+CHO+EO |
||
| Extrusion | PC+CH+EO
PC+CH+EO |
||
| PC: phosphatidyl choline; CH: cholesterol; EO: essential oil | |||
Purification of Liposomes
Entrapped vesicles were separated from non-entrapped vesicles using centrifugation method (Shashi et al., 2012). Vesicular dispersions were centrifuged at 10000 rpm for 10 minutes (laboratory centrifuge Hettich Universal 320 R). The supernatant was removed and the liposomes were reconstituted with PBS buffer. Concentration of essential oil in both fractions was determined.
Physicochemical Characterization Of Liposomes
Particle Size distribution
Mean vesicle size and polydispersity index (PDI) was determined by using Zetasizer (nanoZs Malvern ZEN 3600), which the principle is based on the Brownian motion of particles in medium. Dynamic light scattering is a simple and rapid method to determine the particle size and size distribution of liposomes.
Zeta potential (æ) determination
Zeta potential is another important factor that is responsible for a description of the liposome surface charge and predicts the stability. Charge on loaded vesicles surface and average zeta potential was determined using Zetasizer ZEN 360 Malvern Instruments (Bhatia et al., 2004).
Imaging of liposomes by Scanning Electron Microscopy (SEM)
The shape and morphology of drug-loaded liposomal formulation were observed using a KYKY-EM3200 scanning electron microscopy (SEM, KYKY Instruments, China). Sample was dispersed on glass slide and gold paste used as filament and then viewed using an accelerating voltage of 20 kilovolt at different magnifications. Images of liposomes exhibited the diameter and size of vesicles and this is in agreement with the results of Zetasizer (Figure 4).
Evaluation of the Encapsulation efficiency
In case of purified liposomal suspension, after the centrifugation, by the sediment, the quantity of essential oil was measured using a UV Visible Spectrophotometer at ë = 270 nm. The encapsulation efficiency (EE %) of essential oil was calculated using the following formula:
S3/ L3
Encapsulation Efficiency = ———- × 100
S2/ L2
Which in this equation; S3= EO amount in sediment & L3= phospholipid amount in sediment,
S2= EO amount before centrifuged & L2= phospholipid amount before centrifuged
In vitro essential oil release studies
Release studies were performed using dialysis membrane method. In vitro release was done for selected formulation. In brief, 1000 μL of the 7.1 mg/mL essential oil encapsulated liposome suspension was added in a dialysis bag (MWCO 12kDa, Thermo Fisher Scientific). The dialysis system was suspended in a release volume of 100 mL PBS at 25°C and rotated at 100 rpm (1:100 dilution relation between donor and acceptor compartments). At scheduled intervals, 1 ml of the release medium was collected for the UV- spectrophotometric assay. The same volume of fresh PBS buffer at the same temperature was added immediately to maintain constant release volume. The length of the dialysis tubing was kept consistent for all methods to ensure that the surface area available for dialysis remained constant. To ensure that dilution between the donor and acceptor compartments provided sink conditions, a 1:100 dilution study was conducted and release volume was set at 100 mL PBS pH 6.5.
Statistical Analysis
Result has been reported as mean ± standard deviation of three times repetition assays for each method. The mean values compared by T-Test and one-way analysis of variance (ANOVA), and statistical significance declared at P<0.05. Results and Discussion
Mean Particle Size
The results showed that the mean size of the vesicles was strongly affected by EO and CH concentration, besides of preparation method. Since the mean size of particles prepared by thin film method was around a few microns, 1426.3± 50 nm, particle size reduction in nano scale by two methods was quite impressive. According to the table 2, the mean size sonicated oil loaded vesicles ranged from about 97.8 to about 210 nm, while extruded liposomes were larger (256–489 nm). It’s clearly seen that as the amount of the essential oil increases, size of vesicles increases. Also with decrease of cholesterol content, size of liposomes reduced (Figure 1).
| Table 2: Effect of preparation parameters on Zataria multiflora essential oil Loaded nanoliposomes in two different preparation methods | ||||
| Essential oil concentration mg/ml | Size (nm) | PDI | EE% | |
| Extrusion method | 14.2 | 489.1±21 | 0.38±0.15 | 53.66±0.55 |
| 9.46 | 405.66±67.5 | 0.31±0.03 | 56.26±1.99 | |
| 7.1 | 296.5±127.49 | 0.27±0.09 | 83.03±7.42 | |
| Sonication method | 14.2 | 208.333±7.63 | 0.27±0.006 | 11.91±0.17 |
| 9.46 | 167.333±2.08 | 0.18±0.007 | 14.13±0.36 | |
| 7.1 | 97.866±0.80 | 0.21±0.01 | 16.67±0.30 | |
| All data are mean value ± standard deviation | ||||
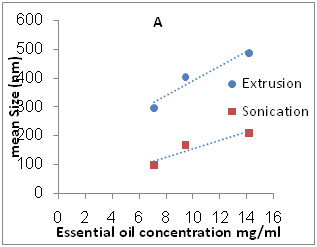 |
Figure 1a: A is effect of essential oil concentration on Size of nanoliposomes and B is effect of cholesterol content on Size of nanoliposomes |
 |
Figure 1b |
Polydispersity index
All MLV liposomes prepared by thin film method had high PDI index (>0.4), which reflects the heterogeneity and dissimilarity of their colloidal system. While the size distribution of sonicated and extruded nanoliposomes was relatively narrow. Nanoliposomes prepared by the sonication method were most homogenous in size distribution than liposomes prepared by two other methods (Table 2).
As seen in figure 2 (A) by increasing the concentration of essential oil in two methods PDI was increased. In the case of cholesterol, figure 2 (B), in extruded method PDI decreases with increased cholesterol levels, this trend is more imperceptibly in sonication method.
 |
Figure 2a: A is effect of essential oil concentration on PDI index and B is effect of cholesterol content on PDI index of nanoliposomes |
 |
Figure 2b |
Zeta potential
The zeta potential measurement of liposomal sample containing DOTAP was +14.7 mV. Other liposomes showed neutral charge that was owing to lack of charge ingredients.
Encapsulation Efficiency
The encapsulation efficiency greatly depends on liposomal content, lipid concentration, method of preparation and the drug which is used. The results show that both two methods have acceptable incorporation efficiency. However sonication method gave a lower content in incorporated essential oil than extrude method (11-18%). Extruded vesicles showed high encapsulation efficiency (53-83%) of the essential oil. The highest mean entrapment efficiency was found at 0.25 of EO/ total lipid (Table 2). Moreover, in the case of cholesterol effect, it was also observed that liposomal formulation PC/Chol (3:1) load the highest amount of the active ingredient in each method (70.56±2.58 % for extruded vesicles and 18.96±0.05 % for sonication method) (Table 3). From figure 3(A), we observed a decrease of incorporated oil with increase of essential oil concentration in each method. Also, it can be appreciated that decrease content of cholesterol lead to increase loading of the essential oil (figure 3 B). Results were summarized in Table 2 and 3. On the whole, ANOVA test showed that oil concentration influence on physicochemical properties of each method significantly (p <0.05).
| Table 3: Effect of cholesterol concentrations on properties of Zataria multiflora essential oil loaded nanoliposomes in two different preparation methods | ||||
| Cholesterol: lecithin | Size(nm) | PDI | EE% | |
| Extrusion method
Sonication method |
1:1 (7.1mg/ml)
1:2 (6.4mg/ml)
1:3 (4.83mg/ml) |
405.66±67.50
302.66±62.54
285.033±15.59 |
0.313±0.03
0.412±0.03
0.424±0.12 |
56.26±1.99
62.39±4.50
70.56±2.58 |
| 1:1
1:2
1:3 |
167.33±2.08
133.66±0.57
138±1 |
0.183±0.01
0.172±0.001
0.174±0.0005 |
14.16±0.31
18.59±0.01
18.96±0.05 |
|
 |
Figure 3a: A is effect of essential oil concentration on encapsulation efficiency and B is effect of cholesterol content on encapsulation efficiency of nanoliposomes |
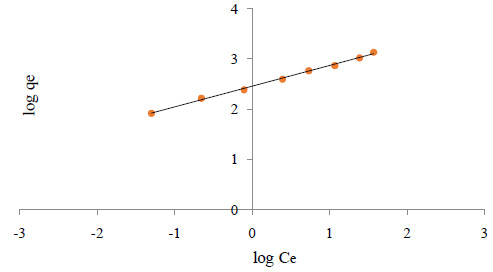 |
Figure 3b |
From figure 3(A), we observed a decrease of incorporated oil with increase of essential oil concentration in each method. Also it can be appreciated that decrease content of cholesterol lead to increase loading of the essential oil (figure 3B).
Image of selected nanoliposome
SEM image of prepared nanoliposomes has been shown in Figure 4. As it can be seen all nanoliposomal particles have spherical structure and system is homogenous in both methods. Both formulations in which used EO: PC: CH 1:4:1. Liposomes prepared by sonication method (right image) were smaller than liposomes treated via extrusion method (left image). It can be due to higher content of essential oil that fluidize liposomal bilayers and increase their susceptivity for aggregation.
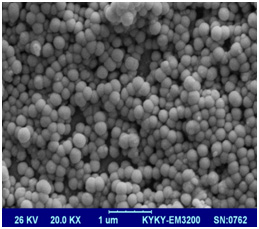 |
Figure 4a: SEM image of Zatariamultifloraessential oil loaded liposomes by sonication (right image) and extrusion (left image) method |
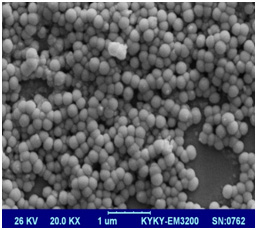 |
Figure 4b |
In vitro essential oil release studies
As showed in Figure 5, the release of EO within 24 hours from nanoliposomes was very inconsiderable and negligible. In the other words, it can be said that EO leakage from liposomes does not occur. The selected formulation was EO: PC: CH 1:4:3 nanoliposomes containing Zataria essential oil.
 |
Figure 5: In vitro essential oil release curve over 24 hours |
Liposome stability
The physical and chemical stability of sonicated formula were evaluated at 5°C, 25°C and
37°C for two months. The sonicated formula (1/4) loaded with Zataria essential oil were stable for at least 8 weeks at room and refrigerator temperatures as the particle size and the EE% of the liposomes did not change significantly during this period (data not shown).
Effect of essential oil concentration on liposomes characteristics
Different methods presented different results in the formation of the nano-sized liposomes. Due to high energy used in probe sonicator, liposomes prepared by this method have, smaller mean size and PDI index compared to extruded ones. Sonication generally produces SUV liposomes with lower size and lower incorporated essential oil than MLVs that are according to Sinico et al findings (Sinico et al., 2005). Comparison the average size of nanoliposomes in different concentration of essential oil in two methods showed significant difference (p<0.05). It has been showed that nanoliposomes size could be affected by EO amount incorporated. Also entrapment of lipophilic compounds in liposome membrane depends on size of liposome (Schwendener & Schott, 2017). Varona et al, also showed an essential oil amount impact on the liposome size (Varona et al., 2011). According to our results, by increasing the concentration of essential oil to total lipid with the same ratio of PC: CH, average size of the nanoparticles have increased. Probably because of higher EO concentration required greater mechanical force in the reduction size process of vesicles. In study of Detoni and colleagues, it was not observed any changes in the size of the nanoparticles containing different ratios of Zanthoxylum tingoassubia oil to the phospholipid, which is inconsistent with our study (Detoni et al., 2009).
PDI (poly dispersity index) usually is considered to be as an indicator of colloidal particle size distribution. The smaller index indicates the particle distribution is more limited and so the system will be homogeneous and more uniform in size (Ruozi et al., 2005). Two methods had low value PDI index although the mean PDI index in sonication method is lower than the extrusion method. In both methods, the mean PDI index changes made no significant difference, in different concentration of EO (0.05
By maintaining the ratio of EO to total lipid (1/3) in the formulations, the effect of different cholesterol concentration on liposome features was also determined. Cholesterol as a usual composition plays an important role in the structure of the liposome. Cholesterol due to its amphiphilic properties, perch into liposome so that a hydroxyl group to the core of water oriented and the hydrophobic tail to the phospholipid bilayer of the liposomes. In previous studies it has been widely reported that the formation and stability of the liposomes is highly dependent on the ratio of cholesterol to phospholipid, so have great impact on the behavior of the carrier in vitro and in vivo conditions (Haeri et al., 2014). Other effects of cholesterol are control and reduce membrane permeability, making of hardness to membrane and layer structural stability (Chan et al., 2004). Results shown in figure 1 part B demonstrated that liposome size increased with the increasing amount of cholesterol in both two methods. Varona and colleagues observed that with lower cholesterol levels, the size of the liposomes reduced which it’s in line with the results of this study (Varona et al., 2011). It is reported that the insertion of cholesterol in liposomal membrane causes liposomes more rigid and resistant to size reduction (Briuglia et al., 2015). Comparison the mean size of two methods, sonication method showed a significant size reduction (p <0.05). Also the mean PDI index in different molar concentrations of cholesterol in two methods showed that changes are significant (p<0.05). Sonication method gave smaller and more homogeneous nanoliposomes than extrude method. Lipophilic molecules, competes with cholesterol molecules for the lipophilic space in the lipid bilayer (Jaafar-Maalej et al., 2010) so, cholesterol might distaste the incorporation of hydrophobic molecules into the liposome bilayer membrane (Fathi Moghaddam et al., 2008; Rezaee et al, 2015; Saffaric et al, 2013 . Ortan et al in their study showed that by increasing the amount of Cholesterol, amount of encapsulated EO (EE%) in liposomes reduced (Ortan et al., 2009). This result was confirmed by Varona et al via essential oil of lavandi (Varona et al., 2011). The same result was obtained in present study with both two methods and encapsulation efficiency in both methods have significantly decreased (p < 0.05). In summary, this study contributes to the understanding effect of preparation method & the different concentration of EO, Cholesteol content on Zataria essential oil loading into liposomes. The study presented here suggested that the physicochemical features of loaded nanoliposomes clearly influenced by EO concentration, cholesterol content and liposome preparation method. It can be deduced by consideration of significance level, sonication method gave smaller nanoliposomes than the extrude method, while higher EO incorporation obtained by extruded method. Acknowledgements
The authors thank Ms Zahra Abbasian & Ms Leila Astarakifor their valuable technical assistance. This research was performed with support from Kashan University of Medical Sciences.
References
- Ali MS, Saleem M, Ali Z, Ahmad VU.(2000) Chemistry of Zataria multiflora (Lamiaceae). Phytochemistry. 55(8):933-6.
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y,(2013) Liposome: classification, preparation, and applications. Nanoscale Res Lett. 8(1):102.
- Bangham A, Standish MM, Watkins J. (1965) Diffusion of univalent ions across the lamellae of swollen phospholipids. Journal of molecular biology. 13(1):238-IN27.
- Belay G, Tariku Y, Kebede T, Hymete A, Mekonnen Y.(2011) Ethnopharmacological investigations of essential oils isolated from five Ethiopian medicinal plants against eleven pathogenic bacterial strains. Phytopharmacology. 1(5):133-43.
- Bhatia A, Kumar R, Katare OP.(2004) Tamoxifen in topical liposomes: development, characterization and in-vitro evaluation. J Pharm Pharm Sci. 7(2):252-9.
- Briuglia M-L, Rotella C, McFarlane A, Lamprou DA.(2015) Influence of cholesterol on liposome stability and on in vitro drug release. Drug delivery and translational research. 5(3):231-42.
- Celia C, Trapasso E, Locatelli M, Navarra M, Ventura CA, Wolfram J.(2013) Anticancer activity of liposomal bergamot essential oil (BEO) on human neuroblastoma cells. Colloids and Surfaces B: Biointerfaces. 112:548-53.
- Chan YH, Chen BH, Chiu CP, Lu YF.(2004) The influence of phytosterols on the encapsulation efficiency of cholesterol liposomes. International journal of food science & technology. 39(9):985-95.
- Chetanachan P, Akarachalanon P, Worawirunwong D, Dararutana P, Bangtrakulnonth A, Bunjop M. (2008) Ultrastructural characterization of liposomes using transmission electron microscope. Advanced Materials Research. 55:709-11.
- Detoni C, Cabral-Albuquerque E, Hohlemweger S, Sampaio C, Barros T, Velozo E. (2009) Essential oil from Zanthoxylum tingoassuiba loaded into multilamellar liposomes useful as antimicrobial agents. Journal of microencapsulation. 26(8):684-91.
- Detoni CB, de Oliveira DM, Santo IE, Pedro AS, El-Bacha R, da Silva Velozo E.(2012) Evaluation of thermal-oxidative stability and antiglioma activity of Zanthoxylum tingoassuiba essential oil entrapped into multi-and unilamellar liposomes. Journal of liposome research.22(1):1-7.
- Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytotherapy Research. 21(4):308-23.
- El-Samaligy M, Afifi N, Mahmoud E. (2006) Increasing bioavailability of silymarin using a buccal liposomal delivery system: preparation and experimental design investigation. International journal of pharmaceutics. 308(1):140-8.
- Fang J-Y, Hong C-T, Chiu W-T, Wang Y-Y. (2001) Effect of liposomes and niosomes on skin permeation of enoxacin. International Journal of Pharmaceutics. 219(1):61-72.
- Fathi Moghaddam H, Shafiee Ardestani M, Saffari M, Navidpour L, Shafiee A, Rahmim A. (2008) Dopaminergic but not glutamatergic neurotransmission is increased in the striatum after selective cyclooxygenase-2 inhibition in normal and hemiparkinsonian rats. Basic & clinical pharmacology & toxicology; 103(4):293-296.
- Haeri A, Alinaghian B, Daeihamed M, Dadashzadeh S.(2014) Preparation and characterization of stable nanoliposomal formulation of fluoxetine as a potential adjuvant therapy for drug-resistant tumors. Iranian Journal of Pharmaceutical Research. 13:3-14.
- Hofheinz R-D, Gnad-Vogt SU, Beyer U, Hochhaus A.(2005) Liposomal encapsulated anti-cancer drugs. Anti-cancer drugs.;16(7):691-707.
- Liolios C, Gortzi O, Lalas S, Tsaknis J, Chinou I.(2009) Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food chemistry. 112(1):77-83.
- Jaafar-Maalej C, Diab R, Andrieu V, Elaissari A, Fessi H. (2010)Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. Journal of liposome research 20(3):228-43.
- Khatibi SA, Misaghi A, Moosavy M-H, Amoabediny G, Basti AA.(2015) Effect of Preparation Methods on the Properties of Zataria multiflora Boiss. Essential Oil Loaded Nanoliposomes: Characterization of Size, Encapsulation Efficiency and Stability. Pharmaceutical Sciences. 20:141.
- Martin A, Varona S, Navarrete A, Cocero MJ. (2010)Encapsulation and co-precipitation processes with supercritical fluids: applications with essential oils. Open Chemical Engineering Journal. 4(1):31-41.
- Meure LA, Foster NR, Dehghani F. (2008)Conventional and dense gas techniques for the production of liposomes: a review. Aaps Pharmscitech. 9(3):798-809.
- Misaghi A, Basti AA. (2007)Effects of Zataria multiflora Boiss. essential oil and nisin on Bacillus cereus ATCC 11778. Food control. 18(9):1043-9.
- Ortan A, Câmpeanu G, Dinu-Pirvu C, Popescu L.(2009) Studies concerning the entrapment of Anethum graveolens essential oil in liposomes. Roum Biotechnol Lett. 14:4411-7.
- Rezaee S, Khalaj A, Adibpour N, Saffary M.(2015) Correlation between lipophilicity and antimicrobial activity of some 2-(4-substituted phenyl)-3 (2H)-isothiazolones. DARU Journal of Pharmaceutical Sciences.;17(4):256-263.
- Riaz M. (1996) Liposomes preparation methods. Pak J Pharm Sci. 9(1):65-77.
- Saedi Dezaki E, Mahmoudvand H, Sharififar F, Fallahi S, Monzote L, Ezatkhah F. (2016) Chemical composition along with anti-leishmanial and cytotoxic activity of Zataria multiflora. Pharm Biol. 2016;54(5):752-8.
- Saffari Ma, Hoseini Shirazi F, Moghimi HR(2016) Terpene-loaded Liposomes and Isopropyl Myristate as Chemical Permeation Enhancers Toward Liposomal Gene Delivery in Lung Cancer cells; A Comparative Study. Iran J Pharm Res. 15(3):261-267.
- Saffari M, Moghimi HR, Dass CR. (2016) Barriers to Liposomal Gene Delivery: from Application Site to the Target. Iran J Pharm Res. 15(Suppl):3-17.
- Saffaric M, Tamaddon AM, Shirazi FH, Oghabian MA, Moghimi HR.(2013) Improving cellular uptake and in vivo tumor suppression efficacy of liposomal oligonucleotides by urea as a chemical penetration enhancer.; The journal of gene medicine. 15(1):12-19.
- Schwendener RA, Schott H. (2017) Liposome Formulations of Hydrophobic Drugs. Methods Mol Biol.;1522:73-8.
- Shahsavari N, Barzegar M, Sahari MA, Naghdibadi H. (2008)Antioxidant activity and chemical characterization of essential oil of Bunium persicum. Plant foods for human nutrition. 63(4):183-8.
- Shashi K, Satinder K, Bharat P. (2012) A complete review on: liposomes. International Research Journal of Pharmacy.;3(7):10-16.
- Sinico C, De Logu A, Lai F, Valenti D, Manconi M, Loy G.(2005) Liposomal incorporation of Artemisia arborescens L. essential oil and in vitro antiviral activity. European Journal of Pharmaceutics and Biopharmaceutics.;59(1):161-8.
- Sunar S, Aksakal O, Yildirim N, Agar G, Gulluce M, Sahin F. (2009) Genetic diversity and relationships detected by FAME and RAPD analysis among Thymus species growing in eastern Anatolia region of Turkey. Romanian Biotechnological Letters.;14(2):4313-8.
- Ruozi B, Tosi G, Forni F, Fresta M, Vandelli MA.(2005) Atomic force microscopy and photon correlation spectroscopy: two techniques for rapid characterization of liposomes. European Journal of Pharmaceutical Sciences. 25(1):81-9.
- Valenti D, De Logu A, Loy G, Sinico C, Bonsignore L, Cottiglia F.(2001) Liposome-incorporated Santolina insularis essential oil: preparation, characterization and in vitro antiviral activity. Journal of liposome research. 11(1):73-90.
- Varona S, Martín Á, Cocero MaJ.(2011) Liposomal incorporation of lavandin essential oil by a thin-film hydration method and by particles from gas-saturated solutions. Industrial & Engineering Chemistry Research.;50(4):2088-97.
- Wen Z, Liu B, Zheng Z, You X, Pu Y, Li Q. (2010)Preparation of liposomes entrapping essential oil from Atractylodes macrocephala Koidz by modified RESS technique. Chemical Engineering Research and Design. 88(8):1102-7.
- Yoshida P, Yokota D, Foglio M, Rodrigues RF, Pinho S. (2010)Liposomes incorporating essential oil of Brazilian cherry (Eugenia uniflora L.): characterization of aqueous dispersions and lyophilized formulations. Journal of microencapsulation.;27(5):416-25.


