1Department. of Zoology, Jagadamba Mahavidyalaya, Achalpur city(MS), India.
2Department. of Zoology, Govt. Vidarbha Institute of Science and Humanities, Amravati (MS), India.
3Department. of Zoology, Nabira Mahavidyalaya, Katol 441302, Dist. Nagpur (MS), India.
4Department. of Zoology, Arts Commerce and Science College Maregaon District Yavatmal (MS), India.
Corresponding author email: truptikhedkar9@gmail.com
Article Publishing History
Received: 25/04/2023
Accepted After Revision: 15/06/2023
The purpose of the present study was to investigate the immunocytochemical aspects of the intestine of the teleost fish Notopterus notopterus. The distribution and relative frequency of the endocrine cells in the intestine were studied immunocytochemically using Streptavidin biotin peroxidase complex method. The aim was analyse the distribution and localization of immunoreactivity in the intestine of Notopterus notopterus against the antisera NPY. The samples were taken from the proximal and distal intestine. The NPY immunoreactive endocrine cells were found in the distal intestine at low frequencies than proximal intestine. These immunoreactive cells were distributed among more in mucosa epithelium and very low in the lamina propria and submucosa of intestine of N.notopterus.
Immunocytochemistry , Intestine, Teleost, Notopterus Notopterus
Khadse T. A, Gadhikar Y. A, Khedkar T. S, Chirde S. G. Localization of NPY Immunoreactivity in the Proximal and Distal Intestinal Region of Teleost Fish, Notopterus. Biosc.Biotech.Res.Comm. 2023;16(2).
Khadse T.A, Gadhikar Y.A, Khedkar T.S, Chirde S.G. Localization of NPY Immunoreactivity in the Proximal and Distal Intestinal Region of Teleost Fish, Notopterus. Biosc.Biotech.Res.Comm. 2023;16(2). Available from: <ahref=”https://bit.ly/2U8EBeg“>https://bit.ly/2U8EBeg</a>
INTRODUCTION
The intestine in the carnivorous fish is shorter than that of omnivores and herbivores. The intestine of the fish plays an important role in digestion absorption of dietary nutrients and also involved in immunological functions (Cao et al.2011; Salianas et al .2011).Morphometric features are important in identifying the fish species and their interaction in various habitats like freshwater, rivers and seas (Akombo et al.2011). Gastrointestinal tract (GI) of fishes can be divided into four topographical regions headgut, foregut, midgut and hindgut. Although in some cases there is no clear morphological distinction between the midgut and hindgut , morphometric characteristic of intestine of Notopterus notopterus was performed.
The endocrine cells secreted the gastrointestinal hormones which are distributed throughout the mucosa of gastrointestinal tract(GI). A great variety of endocrine type secretions are generated in the mucosa of the gastrointestinal tract of fish. Peptides are the name given to these secretions. For the last 15 years more than 45 gastrointestinal peptides have been determined in studies on fish GI tract. NPY was isolated and sequenced for the first time from swine brain (Tatemoto, 1982, Bjorgen and Koppang 2021).
Table 1. Morphometric characteristic of Notopterus notopterus
| Notopterus notopterus | Fish total body length(cm) | Fish standard length(cm) | Digestive tract
length(cm) |
Intestine length
(cm) |
Weight(gm) |
| Mean ± SD | 20 ± 1 | 19.5 ± 1 | 6.333 ± 0.3 | 5 ± 0.5 | 90 ± 10 |
It has been characterized as one of the most highly conserved neuroendocrine peptide through out the evolution .NPY has been involved in the regulation of a wide range of physiological central effects like the control of appetite, body weight homeostasis, the modulation of reproductive processes. This paper examines the distribution and localization of endocrine cells in the proximal and distal intestine of teleost fish N.notopterus using immunocytochemical study.
Table. 2. Morphological different cells in proximal and distal intestine of Notopterus notopterus
| Types of cells | Proximal per villi/per sec | Distal per villi/per sec |
| Round | ++ | ++ |
| Tadpole | +++ | ++ |
| Ovale | ++ | + |
| Triangular | ++ | + |
| Sac like | + | + |
| Rod like | + | + |
| Elongated | + | + |
| Cucurbit like | + | + |
+ — average , ++ –moderate, +++ — maximum
Table 3. Meannumber of endocrine cells per intestinal folds (Mean ± SD) in N.notopterus (120 intestinal fold were examine for NPY antisera)
|
NPY like immunoreactivitiy from 120 folds divided into 3 groups |
||
| Intestinal region (N.notopterus) | Proximal | Distal |
| Mean ± SD | 68.66 ± 1.52752 | 35.6 ± 6.4291005 |
The Asian Knifefish (Notopterus notopterus) is a carnivorous and predatory fish. Intestine of the carnivorous fishes is short or more or less straight, because meaty foods can be digested more readily than vegetable ones. This fish accepts most kind of live frozen food. Some specimen accepts pellets and dry food. It is available in India, Pakistan, Bangladesh, Nepal, Thailand, Malaysia, and Indonesia.. Body is highly compressed, dorsal and ventral profile almost equally convex. Hence in the present investigation, immunocytochemical were carried out.
MATERIAL AND METHODS
Sample collection :Twelve live adult Notopterus notopterus of either sex with body length ranging from 19-22 cm , weighing 80-100 gm were collected from fresh water pond called as Chatri Talab, Amravati, Maharashtra, India ( 200 56’ N, 770 47’ E). Fishes were anaesthetized with 2-phenoxy ethanol (1:2000) for 10-15 mins in the anaesthetic tank. After being anaesthetized, the fish was scarified by transcardial perfusion by ice cold Phosphate Buffer Saline (PBS, pH 7.45) solution and processed for immunocytochemical studies.
Immunocytochemical Study: For immunocytochemical study, the intestine was excised, separated into proximal and distal region and fixed in Bouins fluid for 24 hours. After fixation, fixative was removed by using cold sucrose solution prepared in PBS. The tissue were cryoprotected in 10% sucrose for 2 hours, 20% sucrose for 2 hours and 30% sucrose for overnight at 40 C .Cryoprotected tissues were embedded in 15% Polyvinyl Pyrrolidone Phosphate (PVP) on cryoprotected peg.12-20 micron thick sections were obtained by cryostat -29 0 C and spread on the slides subbed with poly L-lysine (sigma).The slides were preserved in deep freezer and proceed for immunocytochemical staining. Immunocytochemical staining was carried out by using Streptavidin –biotin peroxidase complex method- The slides were transferred to humidity chamber and sections were washed in PBS (3×5 min) and then washing with Triton X 0.5 % (20 min).
The sections were treated with blocking solution 1% BSA (30 min).3)The sections were incubated with primary antibody (NPY Sigma ,Cat # N-9528) at dilution of 1:2000 with PBS containing 0.3 % Triton X-100 1% BSA and then washed with PBS(3×5min).4) The sections were incubated with secondary antibody (Biotin IgG complex, Sigma, Cat #BA2) for 2 hours and then washed in PBS (5 min).4) The sections were incubated with Streptavidin peroxidase conjugate at dilution (1:100) for 2 hours and then washed in PBS(3×5 min) and then double distilled water (2×5 min).5) For visualization of reaction 3-Amino -9-ethylcarbazole (AEC) solution were applied on sections for 5-10 min until reddish brown colour appear .6)Then slides were washed in double distilled water (2×5 min) and mounted with glycerol gelatin.7) The sections were examined with light microscope and photograph were taken.
Figure 1: Transverse section through proximal intestine showing different layers Mucosa (MU), Sub mucosa (SM),
Muscularis (MU), Longitudinal Muscle Layer (LML),Circular Muscle Layer (CML), Lamina propria (Lp) and Lumen(Lu) (X 100).
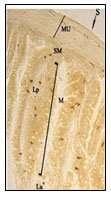
Figure 2: Magnified view of open type tadpole like
and rod like NPY IR endocrine cells.
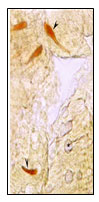
Figure 3: Magnified view of open type sac like and
pyramid like NPY IR endocrine cells.
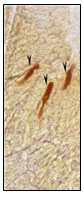
Figure 4:Open type triangular and tadpole
like NPY IR endocrine cells.
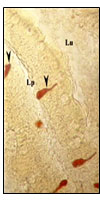
Figure 5:Magnified view of open type triangular and
elongated NPY IR endocrine cells (X400).
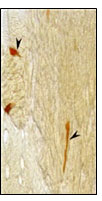
Figure 6: Magnified view of open type pyramid like and spindle
shaped open type NPY IR endocrine cell (X1000).
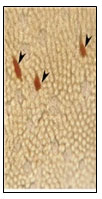
Figure 7: Magnified view of closed type
round NPY IR endocrine cells.

Figure 8: Open type tadpole like, piriform, trianglular,
spindle shaped NPY IR endocrine cell (X400).
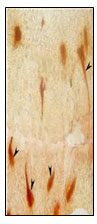
Figure 9: Open type cucurbit like NPY IR endocrine cell (X400).

Figure 10: Magnified view of sac like NPY IR endocrine cell (X1000).
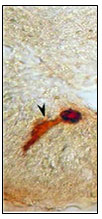
Figure 11: Magnified view of elongated NPY IR endocrine
cell with two long cytoplasmic processes (X1000).

Figure 12: Closed type round NPY IR endocrine cell (X400).
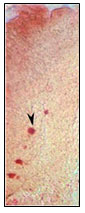
RESULTS AND DISCUSSION
The sections obtained from the proximal and distal intestinal tissue of Notopterus notopterus were formed by mucosa, submucosa , muscularis and serosa layer which is similar to the basic organization of intestinal wall of other teleost fishes according to the histological studies (Fig.1).
In the present investigation, essentially NPY immunoreactive (IR) endocrine cells were observed in the epithelium of intestinal mucosa and few existed in the lamina propria and submucosa of the Notopterus notopterus. Density of NPY IR endocrine cells were more pronounced in the proximal intestine than the distal intestine. All NPY IR cells were stained dark brown colour. Two types of NPY IR endocrine cells were observed in both proximal and distal intestine –open type and closed type. Numerous open type tadpole like NPY IR endocrine cells having long cytoplasmic processes extending to the lumen and basal process extending to the lamina propria were observed in the mucosal epithelium (Fig.2,4).
Open type rod like,( Fig 2),Spindle shaped(Fig.6),Pyramid like with short cytoplasmic process extending to the lumen and basal broad process extending to the lamina propria,(Fig.,6),elongated (Fig.5), Piriform like(Fig.6),Triangular like (Fig.6).NPY IR endocrine cells were observed in the intestinal mucosal epithelium. Open type Sac like NPY IR endocrine cells with apical cytoplasmic processes extending to the lumen, the basal part narrow extending to the lamina propria & middle part of the cell body to be broader are observed in the apical, middle and basal part of the intestinal mucosal epithelium.(Fig.3)Closed type oval like and round like(Fig.7) NPY IR endocrine cells were observed in the intestinal mucosal epithelium
Tadpole like open type NPY IR endocrine cells with one long cytoplasmic processes (fig.8), two long cytoplasmic processes(fig.11) extended to the lumen and basal process extending to the lamina propria are observed in the intestinal mucosal epithelium.Open type elongated, Spindle shaped and Piriform like (fig.8), Cucurbit like(fig.9) NPY IR endocrine cells were observed in the intestinal mucosal epithelium Open type triangle like NPY IR endocrine cells with long cytoplasmic process extending to the lumen and basal process to the lamina propria in the intestinal mucosal epithelium.(fig.8).
Sac like open type IR endocrine cells were observed in the intestinal mucosal epithelium (fig.10) and in sub-mucosa. Closed type oval like NPY IR endocrine cells were observed in the lamina propria. Closed type round like NPY IR endocrine cells were observed in the intestinal mucosal epithelium (fig.12).
Studies concerning regulatory peptides in fish intestine have been carried out since last several years. But intestinal NPY like endocrine cells have not been previously documented in species of N.notopterus. In the present, we have studied the distribution and localization of NPY regulatory peptide from intestine of the fish N.notopterus. In the present study immunoreactive endocrine cells were detected more in the mucosal epithelium of proximal intestine than distal intestine. Similar results of NPY were reported in Anguilla anguilla (Domeneghini et al.2000 ; Youson et al.2001) and with variation to above results less amount of NPY immunoreactive cells were observed in the intestine of Salmotrutta (Dezfuli et al. 2000) and Stizostedianlucio perca (Ozen et al.2010).
Open and closed types of endocrine cells were observed in the intestine of N.notopterus in the present study. In open type, hormones were carried to the gut lumen (lumen endocrine)by long apical cytoplasmic processes (Liu et al. 2003; Pan and Fang 1995) sand in closed type cells it is covered by lumen epithelia (Liu et al.2003 ;Talatar and Simsek 1993).The endocrine cells of the gastrointestinal tract were different in the relative frequency of regional distribution and cell types between species (Leet al.2004; Pan et al. 2000;Reverter et al.2001; Salianas et al.2011 Bjorgen and Koppang 2021) ).
CONCLUSION
The distribution and relative frequency of immunoreactive endocrine cells in Notopterus notopterus are similar to that of other teleost fishes. However few difference in morphology of immunoreactive endocrine cells were observed. From morphometric analysis of the intestine of Notopterus notopterus it was confirmed that intestine is relatively shorter in length as compared to other teleost fishes. NPY may involved in many physiological functions like regulation of food intake and modulation of reproductive processes. These studies could be extended to study for the immunology of this fish.
Authors’ Contribution: YG, TK and SC, were responsible for conceptualization and design of the research. TUK, YG were responsible for specimen collection. SC and TK were responsible for result analysis and interpretation. TUK and YG were responsible for laboratory techniques. All authors contributed equally in literature research, manuscript preparation, editing and review.
Statement of Ethics: The study was approved by the Institutional Animal Ethics Committee ((IAEC) Of Government Vidharbha Institute of Science and Humanities, Amravati.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors did not receive any financial support for the research, authorship and publication of this article.
REFERENCES
Akombo, P.M., Atile, J.I., Adikwu, I.A., &Araoye, P.A. (2011). Morphometric measurements and growth patterns of four species of the genus Synodontis (Cuvier, 1816) from lower Benue river, Markurdi, Nigeria. International Journal of Fisheries and Aquaculture, 3(15), 263-270.
Bjorgen H and Koppang EO ( 2021). Anatomy of teleost fish immune structures and organs Immunogenetics. 2021; 73(1): 53–63. Published online 2021 Jan 11. doi: 10.1007/s00251-020-01196-0
Cao, X.J., Wang, W.M., & Song, F. (2011). Anatomical and histological characteristics of the intestine of Topmouth culture (Culture alburnus). Anat. Histol. Embryol, 40, 292-298.
Dezfuli, B.S., Arrighi, S., Domeneghini, C., &Bosi, G. (2000). Immunohistochemical detection of neuromodulators in the intestine of Salmotrutta L. naturally infected with Cyathocephalustruncatus Pallas (Cestoda). Journal of Fish Diseases, 23, 265-273.
Domeneghini, C., Radaelli, G., Arrighi, S., Macarello, F., &Veggetti, A. (2000). Neurotransmitters and putative neuromodulators in the gut of Anguilla anguilla (L.). Localizations in the enteric nervous and endocrine system. European Journal of Histochemistry, 44(3), 295-306.
Lee, J.H., Ku, S.K., Park, K.D., & Lee, H.S. (2004). Immunohistochemical study of the gastrointestinal endocrine cells in the Korean aurchaperch. Journal of Fish Biology, 65, 170-181.
Liu, Y., Tytgat, G.N.G., Xiao, S.D., & Ten-kate, F.J.W. (2003). Gastric endocrine cells. Chinese Journal of Digestive Diseases, 4, 160-167.
Ozen, R.M., Kenan, Cinar., &Nurgul, Senol. (2010). Endocrine cells in the gastrointestinal tract mucosa of Zader (Stizostedionlucioperca). Kafkas University Veterinary Faculty Journal, 16(Suppl-B), S339-S345.
Pan, Q.S., & Fang, Z.P. (1995). Present progress in the study of the APUD cells in gastro-entero-pancreatic endocrine system of the fishes. Acta Hydrobiologica Sinica, 19, 275-282.
Pan, Q.S., Fang, Z.P., & Zhao, Y.X. (2000). Immunocytochemical identification and localization of APUD cells in the gut of seven stomachless teleost fishes. World Journal of Gastroenterology, 6(1), 96-101.
Reverter, J.M.C., Rodriguez, G.M., Zanuy, S., Carrilo, M., &Larhammar, D. (2001). Molecular evolution of the neuropeptide Y (NPY) family of peptides: Cloning of three NPY-related peptides from the sea bass (Dicentrarchuslabrax). Regulatory Peptides, 95, 25-34.
Salianas, I., Yang, A. Z., &Sunyer, O. (2011). Mucosal immunoglobulins and B cells of the teleost fish, Dve. Comparative Immunology, 35, 1346-1365.
Talatar, H., &Simsek, H. (1993). Hacettepeuniversitesi tip fakultesiichastaliklari ABD gastroenteroloji:unitesiHekimlerYayinBirligi, Ankara, pp. Gastroenteroloji, 551.
Tatemoto, K. (1982a). Neuropeptide Y: Complete amino-acid sequence of the brain peptide. Proceedings of the National Academy of Sciences of the United States of America, 79, 5485-5489.
Youson, J. H., Al-Mahrouki, A. A., Naumovski, D., & Conlon, J. M. (2001). The endocrine cells in the gastro entero pancreatic System of the Bowfin, Amia calva L. An immunohistochemical, Ultrastructural and immunocytochemical analysis. Journal of Morphology, 250, 208-224.


