1Institute of Food Science and Technology, Bangladesh Council of Scientific
and Industrial Research, Dhaka, Bangladesh.
2Life Sciences Department, Hi-Tech Health Care Limited, Dhaka, Bangladesh.
Corresponding author email: pd-cbirmdp@bcsir.gov.bd
Article Publishing History
Received: 15/10/2021
Accepted After Revision: 11/12/2021
Organochlorine pesticides (OCPs) are chlorinated hydrocarbons that used extensively in the last century for agricultural purposes. Excess use of OCPs results pesticide residues accumulation in the water and fishes and causes various health problems. The concentrations of Organochlorine pesticides (OCPs) residues in water and fish samples of six most contaminated rivers surrounding Dhaka, Bangladesh namely Buriganga, Turag, Balu, Sitalakhya, Bangshai and Dhaleswari were determined and assessed the possible health risks through consumption of accumulated OCPs in fishes from those rivers. .As we know, OCPs are non-biodegradable and thus remain in the environment as pollutants. Therefore, OCPs exposure must be monitored and controlled to reduce the human health risks.
Risk assessment is an important procedure to quantify the potential health risks and provides information the risk managers to control the overuse of OCPs. The concentrations of OCPs residues were determined by Gas Chromatography tandem Mass Spectrometry (GC-MS/MS). After sample collection, sample was extracted and analyzed according to the validated method. Several OCPs residues including Aldrin, p,p-DDE, Eldrine ketone, p,p-DDD, Endrin, α-Endosulfan, Heptachlorepoxide, p,p-DDT, Endosulfan sulfate and β-Endosulfan were detected in river water and residues including Aldrin, p,p-DDE, Heptachlorepoxide, p,p-DDD, Endrin and p,p-DDT were detected in fish samples.
The Health risk index (HI) values of Aldrin, p,p-DDE, Heptachlorepoxide, p,p-DDD, Endrin and p,p-DDT from analyzed three fish species (Acanthobrama microlepis, Barbonymus gonionotus and Batasio tengana) were ranged from 0.036 to 1.696, 0.029 to 2.007, 0.022 to 1.117, 0.0231 to 0.721, 0.019 to 1.597 and 0.019 to 1.205 for Buriganga, Turag, Balu, Sitalakhya, Bangshai and Dhaleswari rivers respectively. OCPs are quantified in river water and fish samples and potential health risks are accessed. This study suggested that, there might have OCPs mediated health risks through long term exposure of OCPs residues from fishes of those polluted rivers. Although, our study provided estimation about the presence of OCPs in water and fishes from six contaminated rivers surrounding Dhaka city but, further studies are suggested to ensure the safety of peoples.
Bangladesh, Fish, Gas Chromatography, Mass Spectrometry, OCPs.
Hasan M.A, Das A.K, Satter M.A.Human Health Risk Surveillance through Organochlorine Pesticides in River Water and Fish from Bangladesh. Biosc.Biotech.Res.Comm. 2021;14(4).
Hasan M.A, Das A.K, Satter M.A.Human Health Risk Surveillance through Organochlorine Pesticides in River Water and Fish from Bangladesh. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/2ZADczE”>https://bit.ly/2ZADczE</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, sources the original author and sources are credited.
INTRODUCTION
Organochlorine pesticides (OCPs) are synthetic chemicals with a strong chemical bond between their chlorine and carbon components. OCPs are toxic to the environment, insoluble in water and fat loving pesticides. OCPs are very dangerous for the environment because, they stay around for a long time after using and may exposure to water, soil and animal bodies. OCPs can contaminate the environment either through direct application or waste disposal and rain water.
Although, OCPS are banned in several developed countries because of their long lasting nature but, still they are used in some of the developing countries. Peoples especially from developing countries where OCPs are used regularly are at a higher risk of OCPs mediated health hazards. Peoples can directly uptake the OCPs residues through breathing and ingest OCPs by taking foods like fish, dairy products and other highly fat containing foods (Lee et al. 2020).
As OCPs maintains very strong bonding in the fatty parts so, they can accumulate in the animal or fish tissues and can pass to the human and other animals after consumption of those animals. When human takes OCPs residues through food for long time, they may suffer from serious health problems such as damage of liver, kidney, thyroid gland, bladder and central nervous system. In agricultural countries, pesticides are used to control of insects, weeds, fungi, bacteria, etc. OCPs are lipophilic in nature with longer half-life. The bioaccumulation and long range transportation ability of OCPs, makes them ideal candidate for contamination of water, air and soil.
There has harmful impact of pesticides in aquatic eco systems (De Lorenzo et al. 2001; Frankart et al. 2003; Gupta 2004; Castillo et al. 2006; Aktar et al. 2009). Although, OCPs are used to prevent malaria and typhus but they are banned in the developed countries because of their harmful effects (Aktar et al. 2009). Several pesticides such as DDT, hexachlorocyclohexane (HCH) and aldrin used in some part of Asia due to cheaper price and activity against some harmful organisms. There have several reports on OCPs mediated toxicity in several fish models (Gupta 2004; Yohannes et al. 2014a; Yohannes et al. 2014b; Zhang et al. 2014; Byrne et al. 2015; Cui et al. 2015; Wang et al. 2015; Lee et al. 2020).
Endosulfan was found to responsible for necrosis of focal liver cells (Capkin et al. 2006). Endosulfan sulphate is a toxic OCPs residue. The toxicity was studied in Zebra fish (Lee et al. 2020). Fishes accumulate and concentrate these hydrophobic compounds in their tissues directly from water and the pesticide residues are transferred to the fish consumers directly by trophic food systems. Humans can be affected by OCPs after long time exposure.
The poisoning of OCPs may cause headache, nausea, dizziness, vomiting, tremor, lack of co-ordination and mental confusion. The use of several OCPs can cause cancer. DDT is reported as a potential cancer-causing agent in humans according to several researchers (Enan and Matsumura 1998; Diel et al. 2002; Han et al. 2010; Ndebele et al. 2010). Reduction of sperm count and damage of spermatogonial cells and sperm morphology can be occurred because of high endosulfan dose (Ennaceur et al. 2008; Wong et al. 2015).
Higher concentrations of DDE and HCB reduced cell proliferation and produced binucleated cells (Ennaceur et al. 2008; Wong et al. 2015). Bangladesh is called land of rivers. Rivers are very important part of Bangladesh. The rivers provide water for cultivation in the vast majority of areas. Rivers are used for transportation in some areas of Bangladesh. There are about 230 rivers currently flowing throughout Bangladesh during summer and winter seasons.
The major river includes Padma, Meghna, Jamuna, Brahmaputra, Karnaphuli etc. All of the rivers are connected to each other and finally falls to the Bay of Bengal. Dhaka, the capital of Bangladesh is surrounded with some rivers. The rivers are: Buriganga River, Turag River, Balu River, Shitalakhya River, Dhaleshwari River and Bangshai River. They supply water to the huge population of Dhaka, Bangladesh. The present condition of these rivers is at very high risk (Li et al. 2020).
The water quality of these six major rivers around Dhaka city is getting polluted day by day and becoming very dangerous for public health. Water from these rivers cannot be used as drinking water. The deteriorating water quality of these six rivers has been a major concern. Rivers are main sources of fish. Bangladeshi peoples prefer fishes on their dishes in almost every day. Fish is one of the importance sources of protein, vitamin, fat and carbohydrate.
Peoples from rural areas get their required protein through consumption of fishes. Fish is considered as one of the most significant indicators of metal pollution in aquatic environment (Rashed 2001). QuEChERS extraction method in combination with GC-MS was developed for the detection of OCPs in shellfish. OCPs analysis method was validated for shellfish and cephalopods (Li et al. 2020; Hwang et al. 2020). A method was developed for the detection of OCPs in potato and sweet potato. A QuEChERS Method with slight modification using N-Doped Graphitized Carbon coupled with GC-MS/MS has been developed for determination of OCPs in tomatoes (Ye et al. 2020; Singh et al. 2020).
In this study, we detected several OCPs residues from water and fish samples from six contaminated rivers (Buriganga River, Turag River, Balu River, Shitalakhya River, Bangshi River and Dhaleshwari River) surrounding Dhaka, the capital of Bangladesh and accessed potential health hazards of peoples who consumed the fishes from those rivers. The limit of detection (LOD) values were appropriate for the detection of OCP residues from both water and fish samples.
Considering the problems in pesticide residues detection, this study conducted on quantitative detection of OCPs by GC-MS/MS. Although, LC-MS/MS is preferred method for the detection of OCPs but, GC-MS/MS is more sensitive method (Pico et al. 2020). Excess use of OCPs in agriculture results pesticide residues accumulation in the water and fishes which in turn causes various health problems. This research will helpful for the assessment of the impact of OCPs on public health, agriculture and environment in Dhaka, Bangladesh.
MATERIAL AND METHODS
In this study, both fish and water samples were collected from 6 (Six) most polluted rivers of Bangladesh namely Buriganga, Turag, Balu, Sitalakhya, Bangshai and Dhaleswari. Theses Rivers are very important as they surrounds Dhaka city. For the sample collection, several sampling points were selected. This study was carried out from November, 2020 to April, 2021. Water samples were collected from the 30-50 cm depth of water surface. Fish samples were collected where the fishing effort is very high.
Three fish species from those rivers were collected for this assay: Acanthobrama microlepis, Barbonymus gonionotus and Batasio tengana. Fishes were purchased from the local fishermen. While sampling, fishes of almost same size and weight were selected. Fish samples were stored in polythene bags and transported to the laboratory. For the analysis, fish muscle tissues were selected. A total of 30 water samples and 60 fish samples of three species were collected randomly. After collection, samples were stored in proper condition to maintain the sample quality. OCPs were extracted from river water samples using liquid-liquid extraction (LLE) method (APHA 2002).
For the extraction of OCPs from fish samples, 2g of fish muscle samples were dissected. The muscle sample was grounded to fine powder using 5 g of Sodium sulfate. After extraction with 50 ml of Acetone (2 times), the extracts were filtered and filtrates were again extracted by 350 ml of deionized water, 15 g of NaCl and 40 ml of of n-hexane/ethyl acetate (3:2). After organic layer separation, the samples were again extracted using 40 ml of n-hexane / ethyl acetate (3:2) and again the organic layer was collected. The extracts were then passed through the anhydrous sodium sulfate, concentrated and added 20 ml of n-hexane.
Finally, the extract was condensed to 1 ml in rotary evaporator (Model: RE100-Pro, DLAB, USA) for GC-MS/MS analysis. In this study, GC-MS system (Model: TRACE 1310, Thermo Fisher Scientific, USA) equipped with Thermo Scientific™ Trace GOLD™ TG-5MS GC Column (0.25 mm X 0.25 µm X 0.25 m) was used. Helium was the carrier gas with a flow rate of 1.2 ml/min. Injection port temperature was 230◦ C and temperature for GC was from 80◦C to 290◦C. 2 µL of sample was injected for analysis. Mass Spectrometer (Model: TSQ DUO, Thermos Scientific, USA) was used for MS analysis.
A stock solution of a mixture of 19 pesticides was used as standard solution. Several dilutions were prepared from Standard solution with a range of 5 ppb to 200 ppb. All of those dilutions were injected and analyzed. Blank solutions method was used to determine LOD and LOQ. Signal and noise ratio 3:1 was considered for LOD determination and 10 times of baseline value of blank samples was considered for LOQ detection.
The accuracy of the method was validated by recovery performance evaluation. For the recovery performance evaluation, both river water and fish samples were spiked with two known concentrations: 5 µg / L and 10 µg/L for river water and 5 µg / kg and 10 µg/Kg for fish samples. Then, the samples were extracted and analyzed with the following equation: Pi= (Si/Ti) × 100Pi= (Si/Ti) × 100………………………… (1); Where, Pi, Si and Ti stands for recovery percentage; results of control from laboratory and percent recovery of the spiked samples respectively.
The estimated daily intake (EDI) of OCPs is based on the OCPs concentration in the fish and the amount of fish consumed. EDI was calculated by the following equation: EDI= (C OCP residues X D Fish intake) / B Average weight …..(2); Here, C OCPs (mg/kg), D fish intake (kg / person) and B average weight (kg / person) are the OCPs residual concentrations in fish, daily intake of fish and average body weight, respectively. HI is defined as health risk assessment of consumers from the consumption of fishes accumulated OCPs in their tissue. HI is calculated from estimated daily intake (EDI) and FAO/WHO prescribed acceptable daily intake (ADI) of OCPs using the following formula:
HI=EDI/ADI………………………………(3); When, calculated HI value is less than 1, the consumers are considered safe from any OCPs mediated health risks (USEP 1986). But, if HI value is higher than 1 then it can be indicated as potential health hazardous.
For data collection, organization and summary preparation, Microsoft Office Excel 2007 and for statistical calculation Statistics 10 software was used.
RESULTS AND DISCUSSION
Method validation: The detection method was validated through recovery evaluation. The spiked river water and fish tissue samples were compared with that of blank samples. The percent recoveries of river water and fish samples were ranged from 87.5% to 99.4% and 87.0% to 100.4% respectively. A linear range was observed from the dilutions of standard solution. The coefficient values (R2) value from this study was 0.95 to 0.99.
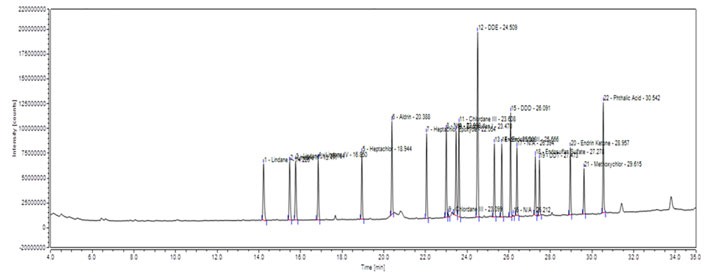
The chromatogram showing the corresponding peaks of OCPs in standard solution is presented in Figure 1. The detected LOD values were ranged from 0.012 to 0.032 µg/L and 0.013 to 0.39 µg /kg for river water and fish tissue respectively. Obtained LOQ values were ranged from 0.42 to 0.87 and 0.39 to 0.88 µg /kg for river water and fish tissue respectively. The retention time, LOD and LOQ values of each pesticide are presented in Table 1.
Table 1. Data obtained from the Chromatography of multi-standards of 19 OCPs. Pesticides,
their retention times, limit of detection (LOD) and Limit of quantification (LOQ).
| Water | Fish | ||||
| OCPs | Retention time (Min) RT | LOQ | LOD | LOQ | LOD |
| µg/L | µg/ kg | ||||
| Aldrin | 20.39 | 0.042 | 0.012 | 0.039 | 0.013 |
| p,p-DDE | 24.51 | 0.067 | 0.023 | 0.056 | 0.029 |
| Eldrine ketone | 28.96 | 0.092 | 0.032 | 0.088 | 0.037 |
| p,p-DDD | 25.8 | 0.032 | 0.021 | 0.043 | 0.038 |
| α-Endosulfan | 23.47 | 0.076 | 0.023 | 0.067 | 0.032 |
| Cis-Chordane | 23.61 | 0.066 | 0.019 | 0.063 | 0.022 |
| Heptachlorepoxide | 22.05 | 0.054 | 0.028 | 0.048 | 0.025 |
| Heptachlor | 18.94 | 0.087 | 0.026 | 0.078 | 0.034 |
| p,p-DDT | 27.2 | 0.053 | 0.013 | 0.064 | 0.039 |
| Methoxychlor | 26.92 | 0.064 | 0.012 | 0.048 | 0.028 |
| Endrin | 25.31 | 0.067 | 0.018 | 0.087 | 0.021 |
| Endosulfan sulfate | 27.28 | 0.054 | 0.024 | 0.059 | 0.036 |
| α-Endosulfan | 23.47 | 0.069 | 0.021 | 0.077 | 0.029 |
| β-Endosulfan | 25.67 | 0.042 | 0.019 | 0.054 | 0.022 |
| Lindane-I | 14.23 | 0.067 | 0.014 | 0.062 | 0.019 |
| Lindane-II | 15.48 | 0.054 | 0.031 | 0.048 | 0.027 |
| Lindane -III | 15.76 | 0.043 | 0.023 | 0.038 | 0.034 |
| Lindane-IV | 16.85 | 0.073 | 0.038 | 0.065 | 0.031 |
| Trans-Chordane | 23 | 0.064 | 0.027 | 0.059 | 0.018 |
| Phthalic Acid | 30.54 | 0.076 | 0.032 | 0.066 | 0.029 |
Figure 1: Chromatogram showing peaks of Organochlorine pesticide standards. A total of 19
pesticides were analyzed with different retention times (RT) for each pesticides.
Analysis of samples: The validated method was applied to analyze the water and fish samples from Buriganga, Turag, Balu, Sitalkhya, Bongshai and Dhaleswari rivers. All the detected OCPs values in water and fish samples are presented in Table 2 and 3 respectively. Several OCPs including Aldrin (0.834±0.056), p,p-DDE (1.782±0.021), Eldrine ketone (0.273±0.032), p,p-DDD (1.421±0.0980, Endrin (1.12±0.068), α-Endosulfan (0.123±0.021), Heptachlorepoxide (0.321± 0.082), p,p-DDT, (1.243±0.043), Endosulfan sulfate (0.023±0.078) and β-Endosulfan (0.242±0.098 ) were detected in Buriganga river water. In Turag river water, OCPs such as Aldrin (0.453± 0.076), p,p-DDE (1.267±0.098), Eldrine ketone (0.123±0.072), p,p-DDD (1.236±0.054), Endrin (1.09±0.032), Heptachlorepoxide (0.231±0.067), p,p-DDT (1.076±0.021) and β-Endosulfan (0.175±0.078) were detected.
OCPs such as Aldrin (0.235± 0.089), p,p-DDE (1.0231±0.078), Eldrine ketone (0.032±0.092), p,p-DDD (1.007±0.033), Endrin (0.989±0.034), α-Endosulfan (0.078±0.032), Heptachlorepoxide (0.165±0.082), p,p-DDT (1.243±0.078) and β-Endosulfan (0.08±0.023) were detected in Balu river water. In Sitalakhya river water, Aldrin (0.482± 0.072), p,p-DDE (0.882±0.065), Eldrine ketone (0.098±0.087), p,p-DDD (0.856±0.075), Endrin (0.776±0.071), α-Endosulfan (0.0945±0.023), Heptachlorepoxide (0.220±0.065) and p,p-DDT (0.605±0.066 ) were detected.
Bangshai river water was contaminated with OCPs such as Aldrin (0.743±0.043), p,p-DDE (0.899±0.034), Eldrine ketone (0.167±0.043), p,p-DDD (0.994±0.033), Endrin (0.907±0.072), α-Endosulfan (0.098±0.032), Heptachlorepoxide (0.182± 0.098) and p,p-DDT (0.882±0.023) while Dhaleswari river water was contaminated with OCPs such as Aldrin (0.241± 0.053), p,p-DDE (0.832±0.045), Eldrine ketone 0.0743±0.032), Endrin (0.997±0.0632), α-Endosulfan (0.0743±0.021), Heptachlorepoxide (0.098±0.034), p,p-DDT (0.993±0.082).
Table 2. OCPs residue levels (μg/L ± SD) detected from Buriganga, Turag, Balu,
Sitalakhya, Bangshai and Dhaleswari river water.
| OCPs | Blank | Buriganga | Turag | Balu | Sitalakhya | Bangshai | Dhaleswari |
| Aldrin | ND | 0.834±0.056 | 0.453± 0.076 | 0.235± 0.089 | 0.482± 0.072 | 0.743±0.043 | 0.241± 0.053 |
| p,p-DDE | ND | 1.782±0.021 | 1.267±0.098 | 1.0231±0.078 | 0.882±0.065 | 0.899±0.034 | 0.832±0.045 |
| Eldrine ketone | ND | 0.273±0.032 | 0.123±0.072 | 0.032±0.092 | 0.098±0.087 | 0.167±0.043 | 0.0743±0.032 |
| p,p-DDD | ND | 1.421±0.098 | 1.236±0.054 | 1.007±0.033 | 0.856±0.075 | 0.994±0.033 | ND |
| Endrin | ND | 1.12±0.068 | 1.09±0.032 | 0.989±0.034 | 0.776±0.071 | 0.907±0.072 | 0.997±0.0632 |
| α-Endosulfan | ND | 0.123±0.021 | ND | 0.078±0.032 | 0.0945±0.023 | 0.098±0.032 | 0.0743±0.021 |
| Cis-Chordane | ND | ND | ND | ND | ND | ND | ND |
| Heptachlorepoxide | ND | 0.321± 0.082 | 0.231±0.067 | 0.165±0.082 | 0.220±0.065 | 0.182± 0.098 | 0.098±0.034 |
| Heptachlor | ND | ND | ND | ND | ND | ND | ND |
| p,p-DDT | ND | 1.243±0.043 | 1.076±0.021 | 1.243±0.078 | 0.605±0.066 | 0.882±0.023 | 0.993±0.082 |
| Methoxychlor | ND | ND | ND | ND | ND | ND | ND |
| Endosulfan sulfate | ND | 0.023±0.078 | ND | ND | ND | ND | ND |
| β-Endosulfan | ND | 0.242±0.098 | 0.175±0.078 | 0.08±0.023 | ND | ND | ND |
| Lindane-I | ND | ND | ND | ND | ND | ND | ND |
| Lindane-II | ND | ND | ND | ND | ND | ND | ND |
| Lindane -III | ND | ND | ND | ND | ND | ND | ND |
| Lindane-IV | ND | ND | ND | ND | ND | ND | ND |
| Trans-Chordane | ND | ND | ND | ND | ND | ND | ND |
| Phthalic Acid | ND | ND | ND | ND | ND | ND | ND |
*ND: Not detected
Table 3. OCPs residue levels (μg/kg ± SD) in fish samples from Buriganga, Turag, Balu,
Sitalakhya, Bangshai and Dhaleswari rivers.
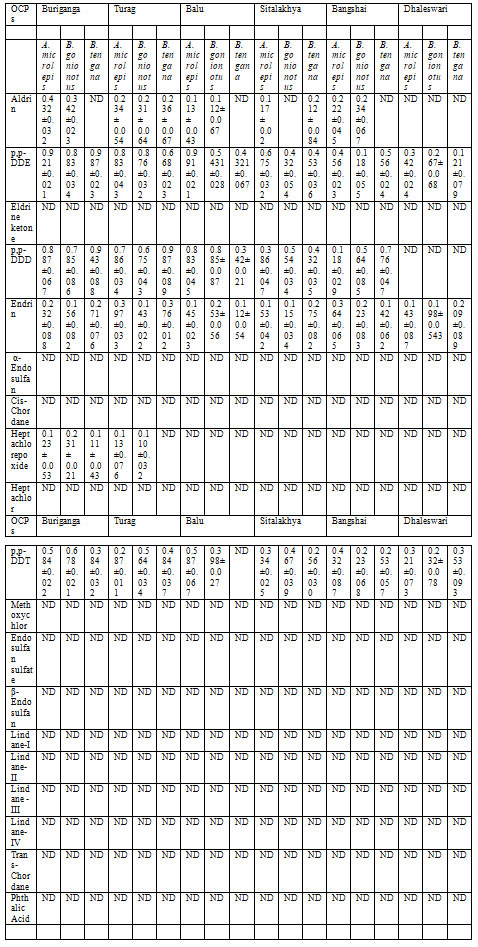
Table 4. Mean values of detected OCPs residues in fish tissues.
| Mean (mg/kg ) | ||||||
| OCPs | Buriganga | Turag | Balu | Sitalakhya | Bangshai | Dhalewari |
| Aldrin | 0.000258 | 0.000233667 | 0.000075 | 0.000109667 | 0.000152 | 0 |
| p,p-DDE | 0.000930333 | 0.000809 | 0.0006554 | 0.00052 | 0.000376667 | 0.000243333 |
| p,p-DDD | 0.000871667 | 0.000816 | 0.000703333 | 0.000457333 | 0.000486 | 0 |
| Endrin | 0.000219667 | 0.000305333 | 0.00017 | 6.26667E-05 | 0.000243 | 0.000183333 |
| Heptachlorepoxide | 0.000155 | 4.95556E-05 | 0 | 0 | 0 | 0 |
| p,p-DDT | 0.000548667 | 0.000445 | 0.000328333 | 0.000352333 | 0.000302667 | 0.000302 |
The ecosystem has been polluted because of use of the OCPs. In African environments Several OCPs including α -HCH, β-HCH dichlorodiphenyltrichloroethane (DDTs), and endosulfans has been detected as most persistent residues (Olisah et al. 2020). Although, the application of OCPs has been reduced worldwide; a recent study in Turkey they have identified OCPs residues in the features of bird species which indicated that, OCPs residues are also present in several wild species (Arikan and Turan 2020). GC-MS has been applied to detect the OCPs residues in Chinese herbal extracts (Hwang and Lee 2000).
Several studies have identified OCPs residues in several food items. In a previous study, Aldrin was detected in several milk samples in very low amount. OCPs were detected in Ginseng roots (Wu et al. 2020). In China, OCPs were detected in vegetable oils samples where higher OCPs concentration was detected in sesame oil and lowest concentration was detected in peanut oil (Cui et al. 2020). In Jordan, several OCPs were identified in honey samples (Tahboub et al. 2020; Lobato et al. 2021).
Health risks assessment through consumption of fishes from these rivers: The EDI (Effective daily intake) values analyzed from this study are presented in the Table 5. The HI (Health risk index) values of three fish species from these six rivers were ranged from 0.036 to 1.696, 0.029 to2.007, 0.022 to1.117, 0.0231 to 0.721, 0.019 to1.597 and 0.019 to 1.205 for Buriganga, Turag, Balu, Sitalakhya, Bangshai and Dhaleswari rivers respectively. The HI values are presented in Table 6. The HI values of Aldrin is higher than 1 in the fishes of Buriganga and Turag rivers. The HI values of Endrin were detected higher than 1 in the fishes of all of the analyzed rivers.
The HI value of Heptachlorepoxide was detected higher than 1 in the fish samples of Buriganga River (Table 6). The analyzed rivers were contaminated with several harmful OCPs and the higher concentrations of OCPs were detected in Buriganga river water while Dhaleswari River was detected with lower OCPs residues. The fish samples from all of those analyzed rivers accumulated OCPs including Aldrin, p,p-DDE, Eldrine ketone, Heptachlorepoxide, p,p-DDD, Endrin and p,p-DDT (Lobato et al. 2021).
The OCPs concentration in fishes from these rivers is presented in Table 3. The average OCPs concentration (mg/kg) in fishes from those rivers is shown in Table 4.Endrin normally stored in the fat tissues and may act as neurotoxin affecting the central nervous system leading to convulsions, seizures, or even death. Although, Endrin is not considered as mutagen and it has no carcinogenic activity but still endrin is a toxic compound and it may cause serious health problems. Aldrin is a neurotoxic pesticide. It can elevate the neurotoxicity. Aldrin can stimulate the central nervous system (CNS) leading to hyperexcitation and seizures. Consumption of fishes from those rivers may be health hazardous as HI values of some of the OCPs are above the safety range.
HI values of some of the OCPs are within the range of safety limit indicated that, consumers may not suffer from health risks from those pesticides but, long time exposure of those OCPs may accumulate in the fat tissues and can be hazardous. In addition, there have several other sources (vegetables, milk, eggs, meat and fruits) from where consumers can exposure OCPs. So, Health conscious peoples should very careful while eating fishes from the rivers contaminated with OCPs. Our current study estimated the level of OCPs in river water and fishes from six contaminated rivers surrounding Dhaka city but, more studies are required to ensure the safety of peoples (Lobato et al. 2021).
Table 5. EDI values calculated from the OCPs from the fish samples.
| EDI | ADI | ||||||
| OCPs | Buriganga | Turag | Balu | Sitalakhya | Bangshai | Dhalewari | |
| Aldrin | 0.00017 | 0.00015 | 4.9E-05 | 7.2E-05 | 1E-04 | 0 | 0.0001 |
| p,p-DDE | 0.00061 | 0.00053 | 0.00043 | 0.00034 | 0.00025 | 0.00016 | 0.001 |
| p,p-DDD | 0.00057 | 0.00054 | 0.00046 | 0.0003 | 0.00032 | 0 | 0.001 |
| Endrin | 0.00014 | 0.0002 | 0.00011 | 4.1E-05 | 0.00016 | 0.000121 | 0.0001 |
| Heptachlor epoxide | 0.0001 | 3.3E-05 | 0 | 0 | 0 | 0 | 0.0001 |
| p,p-DDT | 0.00036 | 0.00029 | 0.00022 | 0.00023 | 0.0002 | 0.000199 | 0.01 |
*ADI= Acceptable daily intake.
Table 6. Health index (HI) values of detected OCPs in the fishes of six contaminated rivers.
| OCPs | HI | |||||
| Buriganga | Turag | Balu | Sitalakhya | Bangshai | Dhalewari | |
| Aldrin | 1.696423714 | 1.536425095 | 0.493146429 | 0.721089667 | 0.999443429 | 0 |
| p,p-DDE | 0.611720748 | 0.531940614 | 0.430944226 | 0.341914857 | 0.247669095 | 0.159998619 |
| p,p-DDD | 0.573145738 | 0.536543314 | 0.462461762 | 0.300709733 | 0.319558886 | 0 |
| Endrin | 1.444371095 | 2.007653905 | 1.117798571 | 0.412051238 | 1.597794429 | 1.205469048 |
| Heptachlor epoxide | 1.019169286 | 0.325841937 | 0 | 0 | 0 | 0 |
| p,p-DDT | 0.036076401 | 0.029260021 | 0.021588855 | 0.023166923 | 0.019901198 | 0.019857363 |
CONCLUSION
The findings of the present study indicated OCPs concentrations in six contaminated river water and fishes. and assessed the possible human health risks through consumption of those fishes. This study will help to figure out the presence of pesticide residues in the contaminated rivers surrounding Dhaka city in very small concentration. Consumers’ health relies on food safety which is ensured by a safe environment. As these rivers are polluting day by day so, the responsible authorities should monitor the presence of pesticide residues on regular basis for public health safety.
Conflict of Interest: Authors declare no conflicts of interests to disclose.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
Ethical Clearance Statement: The Current Research Work Was Ethically Approved by the Institute of Food Science and Technology, Dhaka Bangladesh.
REFERENCES
Aktar, W., Sengupta, D. and Chowdhury, A. (2009). Impact of pesticides use in agriculture: Their benefits and hazards, Interdisciplinary Toxicology, 2(1), pp. 1–12. doi: 10.2478/v10102-009-0001-7.
American Public Health Association (2002). American Water Works Association; Water Environment Federation, Standard Methods for the Examination of Water and Wastewater, 02, pp. 1–541.
Arikan, K. and Turan, S.L. (2020). Organochlorine pesticide residues in feathers of four bird species from western part of Turkey, Turkish Journal of Zoology, 44(5), pp.401-407.
Byrne, S., Miller, P., Waghiyi, V., et al. (2015). Persistent organochlorine pesticide exposure related to a formerly used defense site on St. Lawrence Island, Alaska: data from sentinel fish and human sera, Journal of Toxicology and Environmental Health, Part A, 78(15), pp.976-992. doi: 10.1080/15287394.2015.1037412.
Capkin, E., Altinok, I. and Karahan, S. (2006). Water quality and fish size affect toxicity of endosulfan, an organochlorine pesticide, to rainbow trout, Chemosphere, 64(10), pp. 1793–1800. doi: 10.1016/j.chemosphere.2005.12.050.
Carneiro, R.P., Oliveira, F.A., Madureira, F.D., et al. (2013). Development and method validation for determination of 128 pesticides in bananas by modified QuEChERS and UHPLC–MS/MS analysis, Food Control, 33(2), pp.413-423. doi: 10.1016/j.foodcont.2013.02.027.
Castillo, L.E., Martínez, E., Ruepert, C., et al. (2006) Water quality and macroinvertebrate community response following pesticide applications in a banana plantation, Limon, Costa Rica, Science of the total environment, 367(1), pp.418-432. doi: 10.1016/j.scitotenv.2006.02.052.
Cui, L., Ge, J., Zhu, Y., et al. (2015). Concentrations, bioaccumulation, and human health risk assessment of organochlorine pesticides and heavy metals in edible fish from Wuhan, China, Environmental Science and Pollution Research, 22(20), pp.15866-15879. doi: 10.1007/s11356-015-4752-8.
Cui, Y., Ke, R., Gao, W., et al. (2020). Analysis of Organochlorine Pesticide Residues in Various Vegetable Oils Collected in Chinese Markets, Journal of Agricultural and Food Chemistry, 68(49), pp.14594-14602. https://doi.org/10.1021/acs.jafc.0c05227
DeLorenzo, M. E., Scott, G. I. and Ross, P. E. (2001). Toxicity of pesticides to aquatic microorganisms: A review, Environmental Toxicology and Chemistry, 20(1), pp. 84–98. doi: 10.1002/etc.5620200108.
Diel, P., Olff, S., Schmidt, S. et al. (2002). Effects of the environmental estrogens bisphenol A, o, p′-DDT, p-tert-octylphenol and coumestrol on apoptosis induction, cell proliferation and the expression of estrogen sensitive molecular parameters in the human breast cancer cell line MCF-7, The Journal of steroid biochemistry and molecular biology, 80(1), pp.61-70. doi: 10.1016/S0960-0760(01)00173-X.
Enan, E. and Matsumura, F. (1998). Activation of c-Neu Tyrosine Kinase by o,p′-DDT and β-HCH in Cell-Free and Intact Cell Preparations from MCF-7 Human Breast Cancer Cells, Journal of Biochemical and Molecular Toxicology, 12(2), pp. 83–92. doi: 10.1002/(SICI)1099-0461(1998)12:2<83::AID-JBT3>3.0.CO;2-K.
Ennaceur, S., Ridha, D. and Marcos, R. (2008). Genotoxicity of the organochlorine pesticides 1,1-dichloro-2,2- bis(p-chlorophenyl)ethylene (DDE) and hexachlorobenzene (HCB) in cultured human lymphocytes, Chemosphere, 71(7), pp. 1335–1339. doi: 10.1016/j.chemosphere.2007.11.040.
Forum, R. A. and Agency, U. S. E. P. (1986). Guidelines for the Health Risk Assessment of Chemical Mixtures, Epa, 51(September), pp. 34014–34025.
Frankart, C., Eullaffroy, P. and Vernet, G. (2003). Comparative effects of four herbicides on non-photochemical fluorescence quenching in Lemna minor, Environmental and Experimental Botany, 49(2), pp. 159–168. doi: 10.1016/S0098-8472(02)00067-9.
Fry, D. M. (1995). Reproductive effects in birds exposed to pesticides and industrial chemicals, Environmental Health Perspectives, 103(7), pp. 165–171. doi: 10.1289/ehp.95103s7165.
Gupta, P. K. (2004). Pesticide exposure – Indian scene, Toxicology, 198(1–3), pp. 83–90. doi: 10.1016/j.tox.2004.01.021.
Han, E.H., Kim, H.G., Hwang, Y.P., et al. (2010). The role of cyclooxygenase-2-dependent signaling via cyclic AMP response element activation on aromatase up-regulation by o, p′-DDT in human breast cancer cells, Toxicology letters, 198(3), pp.331-341. doi: 10.1016/j.toxlet.2010.07.015.
Hwang, B.H. and Lee, M.R. (2000). Solid-phase microextraction for organochlorine pesticide residues analysis in Chinese herbal formulations, Journal of Chromatography A, 898(2), pp.245-256. https://doi.org/10.1016/S0021-9673(00)00874-8
Hwang, S.M., Lee, H.U., Kim, J.B. et al. (2020). Validation of analytical methods for organochlorine pesticide detection in shellfish and cephalopods by GC–MS/MS, Food Science and Biotechnology, 29(8), pp.1053-1062. https://doi.org/10.1007/s10068-020-00748-0
Lee, H.K., Kim, K., Lee, J., et al. (2020). Targeted toxicometabolomics of endosulfan sulfate in adult zebrafish (Danio rerio) using GC-MS/MS in multiple reaction monitoring mode, Journal of hazardous materials, 389, p.122056. https://doi.org/10.1016/j.jhazmat.2020.122056
Li, W., Zhang, Z.M., Zhang, R.R., et al. (2020). Effective removal matrix interferences by a modified QuEChERS based on the molecularly imprinted polymers for determination of 84 polychlorinated biphenyls and organochlorine pesticides in shellfish samples, Journal of hazardous materials, 384, p.121241. https://doi.org/10.1016/j.jhazmat.2019.121241
Lobato, A., Fernandes, V.C., Pacheco, J.G., et al. (2021). Organochlorine pesticide analysis in milk by gas-diffusion microextraction with gas chromatography-electron capture detection and confirmation by mass spectrometry, Journal of Chromatography A, 1636, p.461797. https://doi.org/10.1016/j.chroma.2020.461797
Ndebele, K., Graham, B. and Tchounwou, P. B. (2010). Estrogenic activity of coumestrol, DDT, and TCDD in human cervical cancer cells, International Journal of Environmental Research and Public Health, 7(5), pp. 2045–2056. doi: 10.3390/ijerph7052045.
Olisah, C., Okoh, O.O. and Okoh, A.I. (2020). Occurrence of organochlorine pesticide residues in biological and environmental matrices in Africa: A two-decade review, Heliyon, 6(3), p.e03518. https://doi.org/10.1016/j.heliyon.2020.e03518
Pico, Y., Alfarhan, A.H. and Barcelo, D. (2020). How recent innovations in gas chromatography-mass spectrometry have improved pesticide residue determination: An alternative technique to be in your radar, TrAC Trends in Analytical Chemistry, 122, p.115720. https://doi.org/10.1016/j.trac.2019.115720
Rashed, M. N. (2001). Monitoring of environmental heavy metals in fish from nasser lake, Environment International, 27(1), pp. 27–33. doi: 10.1016/S0160-4120(01)00050-2.
Singh, S.P., Sharma, J. and Prakash, P. (2020). Development of a Gas Chromatography Tandem Mass Spectrometry-Based Analytical Method for the Quantitative Determination of Organochlorine Pesticide Residues in Potato Crops, Analytical Chemistry Letters, 10(6), pp.811-826. https://doi.org/10.1080/22297928.2021.1875872
Tahboub, Y.R., Zaater, M.F. and Barri, T.A. (2006). Simultaneous identification and quantitation of selected organochlorine pesticide residues in honey by full-scan gas chromatography–mass spectrometry, Analytica Chimica Acta, 558(1-2), pp.62-68. https://doi.org/10.1016/j.aca.2005.11.004
Wang, J., Liang, W., Henkelmann, B., et al. (2015). Organochlorine pesticides accumulated by SPMD-based virtual organisms and feral fish in Three Gorges Reservoir, China, Environmental pollution, 202, pp.160-167. doi: 10.1016/j.envpol.2015.03.031.
Wong, L.I., Labrecque, M.P., Ibuki, N., et al. (2015). p, p′-Dichlorodiphenyltrichloroethane (p, p′-DDT) and p, p′-dichlorodiphenyldichloroethylene (p, p′-DDE) repress prostate specific antigen levels in human prostate cancer cell lines, Chemico-biological interactions, 230, pp.40-49. doi: 10.1016/j.cbi.2015.02.002.
Wu, P., Gu, M., Wang, Y., et al. (2020). Transfer of organochlorine pesticide residues during household and industrial processing of ginseng, Journal of Food Quality, 2020. https://doi.org/10.1155/2020/5946078
Ye, X., Shao, H., Zhou, T., et al. (2020). Analysis of Organochlorine Pesticides in Tomatoes Using a Modified QuEChERS Method Based on N-Doped Graphitized Carbon Coupled with GC-MS/MS, Food Analytical Methods, 13(3), pp.823-832. https://doi.org/10.1007/s12161-019-01674-6
Yohannes, Y.B., Ikenaka, Y., Nakayama, S.M. et al. (2014). Organochlorine pesticides in bird species and their prey (fish) from the Ethiopian Rift Valley region, Ethiopia, Environmental pollution, 192, pp.121-128. doi: 10.1016/j.envpol.2014.05.007.
Yohannes, Y.B., Ikenaka, Y., Saengtienchai, A., et al. (2014). Concentrations and human health risk assessment of organochlorine pesticides in edible fish species from a Rift Valley lake—Lake Ziway, Ethiopia, Ecotoxicology and environmental safety, 106, pp.95-101. doi: 10.1016/j.ecoenv.2014.04.014.
Zhang, Y., Lin, N., Su, S., et al. (2014). Freeze drying reduces the extractability of organochlorine pesticides in fish muscle tissue by microwave-assisted method, Environmental pollution, 191, pp.250-252. doi: 10.1016/j.envpol.2014.04.018.


