Department of Pharmacy, School of Medical and Allied Sciences, Yamuna Expressway Gautam Buddh Nagar India
Corresponding author email: rvnimiet@gmail.com
Article Publishing History
Received: 10/04/2020
Accepted After Revision: 26/05/2020
Natural medicines from plants are one of the options to avoid drug resistance and toxic effects of synthetic drugs. The objective of this study was to find out the new resources of bioactive phytochemical constituents. Dried crude powder of Salvadora persica bark was used for extraction in different solvents. Modified maceration method was used in extraction of Salvadora persica bark and highest % yield was found to be 1.96 in methanol. Extracts were found to be soluble in different pH buffer system i.e. PBS 6.4, PBS 7.4 and 0.1N HCl. pH value of extract was found to be near 7 so no irritation effect will cause at application site. Heavy metal and microbial load was not found to be on extract. Phytochemical screening of extract was reported the presence of various phytochemical constituents such as alkaloids, glycosides, tannins, Flavonoids, proteins, carbohydrates, sterol and terpenoids. H2O2 and DPPH scavenging methods were performed and confirmed antioxidant activity of extract.Various in-vitro methods were used to evaluate the bioactivity of extract. DPPH and H2O2 scavenging methods were confirmed the antioxidant activity of extract while disc diffusion method was confirmed for antimicrobial activity of the Salvadora persica bark extract. Outcomes of the present study confirmed that the antioxidant and antimicrobial potential of extract is very high. Antioxidant activity was found to be significant and more than standard drug (ascorbic acid) while antimicrobial activity was found to be less than the standard drug (ciprofloxacin). In future the Salvadora persica bark extract may prove as new milestone in the formulation of natural antioxidants and antimicrobial agents.
Salvadora persica Bark; Natural Antioxidant, Natural Antimicrobial; λ Max Validation, Microbial load
Kumar D, Sharma P. K. Extraction and Evaluation of Salvadora persica Bark Extract for its Antioxidant and Antimicrobial Activity. Biosc.Biotech.Res.Comm. 2020;13(2).
Kumar D, Sharma P. K. Extraction and Evaluation of Salvadora persica Bark Extract for its Antioxidant and Antimicrobial Activity. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/2W8r5nX
Copyright © Kumar and Sharma et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Plants are the friend of humans and have helped them to fulfill their daily needs. Herbals from nature have served us as an important source of various phytochemical constituents, particularly from the rich Indian treasure of medicinal plants for curing and healing of many diseases. Phytochemical constituents have been obtained from different parts of plants as leaves, stems, bark, root, flower, fruits and seeds. Different parts of plant are used as cosmetics, food, flavor, fuel and medicines (Bargah., 2015). The toothbrush tree, Salvadora persica L belongs to Salvadoraceae family and is also known as ‘Miswak’ or Pilu. It is evergreen shrub about 400-600 cm in height with a short trunk. It has green leaves, red to brick red fruits and brown bark. Leaves are sub-succulent, curvaceous, elliptic, 1-10cm long and 1-3cm wide. Flowers are small, greenish white and about 10cm long. Petals are 1-3 mm long, (Chabane et al., 2017 Malviya et al 2019).
The bark of Salvadora persica is slightly rough, grayish brown on main stem, paler elsewhere. Research studies have revealed many phytochemical constituents to be present in its bark such as caffeine, theobromine, trigonelline, ursolic acid, oleanolic acid and trimethylamine (Anthoney et al., 2015; Abeer et al., 2011). Various studies have reported several activities of Salvadora persica bark including anti-gonorrhea (Shokeen et al., 2009), tonic, and stimulant in low fever, ascarifuge, gastric troubles (Verma et al., 2009). Other studies have reported its bark as vesicant and stimulant, to relieve cough and used as purgative, (Mathur et al., 2015). Bark was used to treat sores (Anthoney et al., 2015). Ursolic acid and oleanolic acid was extracted from Salvadora persica bark. Ursolic acid inhibited tumorigenesis, tumor promotion, invasion, metastasis, angiogenesis and induction of tumor cell differentiation. Oleanolic acid has hepato-protective, antistomach ulcer, hypoglycemic, anti-hyperlipidemic, anti-hypertensive, cardio-tonic, anti-dysrhythmic, anti-aggregation of blood platelet, anti-cancer, protection of renal toxicity, anti-inflammatory, anti-microbial and anti-fertility activities with low toxicity (Abeer et al., 2011, Malviya et al 2019).
MATERIAL AND METHODS
Material: Salvadora persica bark was collected from Shiddhbaba Ashram Ghanghauli Aligarh, India. Dried bark was powdered using electric granulating machine. Plant was authenticated by school of biotechnology, Gautam Buddha University (state government university) Greater Noida, India. Chemicals; Chloroform, Ethyl Acetate, Methanol, Ethanol was provided by research lab of SMAS Department, Galgotias University.
Method of extraction of plant material: Modified fractional maceration method was used to extract the plant material. Dried coarse powder of stem was placed in round bottom flask (RBF). Required amount of selected solvent were poured in RBF. RBF kept for 24 hours with occasionally shaking. Strained off the liquid part and repeated it three times. Liquid part was filter and concentrate. Remaining part of powder was treated with different solvent and repeats the process (Kumar et al., 2014).
Determination of % yield of extract: % Yield = Practical Yield * 100 / Theoretical YieldSolubility studies of extract:Salvadora persica bark extracts were shaken in different solvent and observed their solubility (Malviya., 2011).
Determination of pH of extract:1 gram of Salvadora persica bark extract was dissolved in 100ml distilled water and determined pH using digital pH meter (Malviya., 2011).
Phytochemical studies of the extract:Phytochemical assays were done to reveal the pharmacological active substances in extract using test methods described previously (Peach et al., 1955). Phytochemical substances were evaluated using various methods such as Dragendorff test, Wagner test, Hager’s test, Legal test, Ferric chloride test, Froth formation test, Zinc Hydrochloride reduction test, Biuret test, Molish’s test, and Libermann-Burchard test.
Determination of microbial load in extract: Microbial load of extract was determined using modified spread method previously described (Mamun et al., 2014). 1000µg/ml of extract solutions was prepared. Sterile plates of nutrients agar were prepared and extract solution spread on them. Prepared plates were incubated in incubator for 48 hours. Microbial colonies formed on agar plates were counted using colony counter in CFU (colony forming unit).
Limit test for heavy metals of extract:Limit test for heavy metals (lead, arsenic) were performed as ‘Indian Pharmacopoeial’ procedure (Indian Pharmacopoeia., 1996).
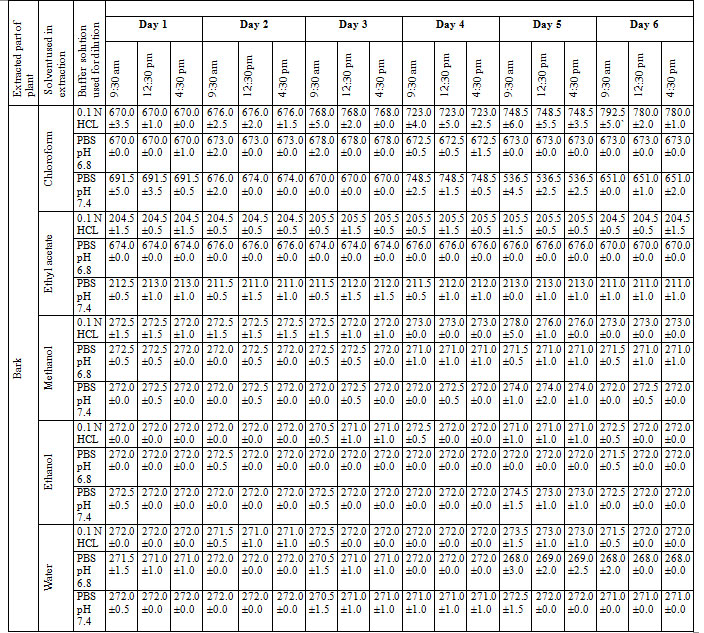
Determination and validation of λ max of extract: The solutions of different concentration of extract (SPBEC, SPBEEA, SPBEM, SPBEE and SPBEW) were prepared using buffer solution of 0.1 N HCL, phosphate buffer pH 6.8, phosphate buffer pH 7.4. Wavelengths of extract were recorded at absorbance less than one at the range of 200-800nm TID (Three times a day) for six days, (Malviya et al., 2019).
DPPH radicals scavenging activity of extract: DPPH radical scavenging assay (Verma et al., 2012) was used to evaluate the antioxidant potential of extract. 0.1mM DPPH solution was prepared in methanol and kept in dark place. Extract solution of different concentration (20, 40, 60, 80, 100µg/ml) were prepared in methanol and sonicated to obtained complete solubility. Ascorbic acid solutions were used as standard drug solution. Extract and ascorbic acid solutions were mixed in DPPH solution separately and allowed to stand for 30 minutes. Absorbance was recorded at 517nm using Shimadzu spectrophotometer. Methanol was used as control solution. Percentage DPPH scavenging was calculated using following equation.
DPPH scavenged (%) = [(Abs control – Abs extract) / Abs control] x 100
Abs control – is the absorbance of the control reaction
Abs extract – is the absorbance in the presence of the sample of the extracts
H2O2 scavenging activity of extract: H2O2 scavenging assay (Jayaprakash et al., 2004) was used to evaluate the antioxidant activity of extract. Extract of different concentration (20, 40, 60, 80, 100 µg/ml) were prepared. 40mM solution of H2O2 was prepared in phosphate buffer solution pH 7.4. Extract of different concentration (5ml) and H2O2 solution (5ml) was mixed in 10 ml volumetric flask. Absorbance was recorded at 230nm using Shimadzu spectrophotometer. Phosphate buffer solution pH 7.4 was used as control solution. Percentage H2O2 scavenging was calculated using following equation.
% H2O2 scavenging ability of extract = [(Abs control – Abs extract)/Abs control] x 100
Abs control – is the absorbance of the blank solution
Abs extract – is the absorbance in the presence of the sample of the extracts.
Antimicrobial activity of extract: Disc diffusion method was used to determine antimicrobial potential of extract. Test microbes gram positive (Bacillus subtilis) and gram negative (Escherichia coli) was obtained from Department of Medical Lab Technology, School of Medical and Allied Sciences, Galgotias University, India. in this method, Petri dishes were washed and sterile using autoclave. These sterile petri dishes were used to prepare MH agar plates. Microbial solution was spread on surface of agar plate using cotton. Extract and ciprofloxacin solution contain Disc were applied on microbial surface of MH agar plate. Agar plates were incubated in incubator for 24 hours. Antimicrobial potential of extract was inhibited the growth of microbes. Microbial free area was measured in millimeter (Shubha et al., 2010).
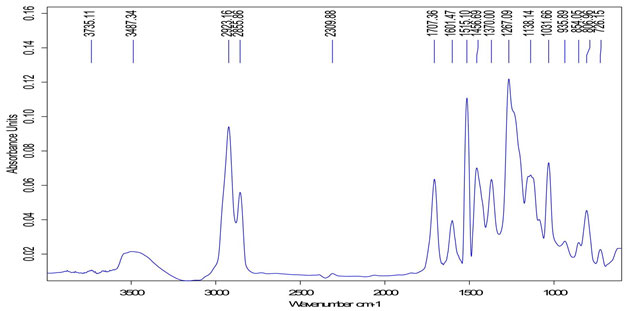
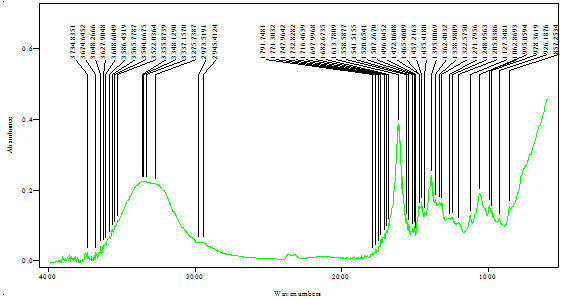
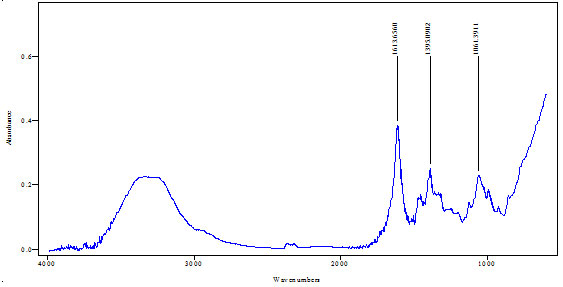
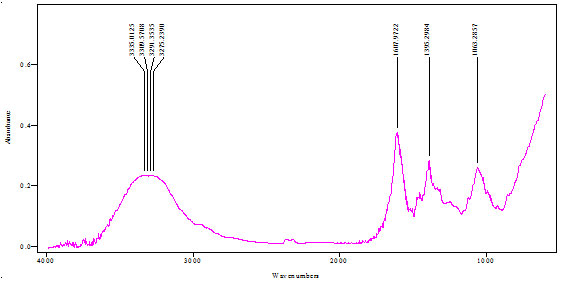
FTIR analysis of extract:FTIR analysis (Kumar et al., 2015) is considered as most important tool for identifying the types of functional group present in sample. The wavelength absorbed light is recorded in spectrum. By interpreting the data of wavelength, chemical bond or possible functional group can be determined. Dried powders of different solvent extract of Salvadora persica bark was used to FTIR analysis. 10mg of dried extract was encapsulated in 90mg of KBr pellet to prepared sample discs. Sample of extract was loaded to Shimadzu FTIR spectroscope with the range of 400-4000cm-1 with resolution of 4cm-1.
RESULTS AND DISCUSSION
Percentage yield of extract was affected by various factors such as solvent, temperature, pH, tome and composition of plant material. In this research, Salvadora persica bark extract was obtained using different solvent such as chloroform, ethyl acetate, methanol, ethanol and water. The obtained comparative results were reported in table 1.
Table 1: % yield of extract
| Extract | % yield |
| SPBEC | 0.50 |
| SPBEEA | 0.20 |
| SPBEM | 1.96 |
| SPBEE | 1.81 |
| SPBEW | 1.05 |
Solubility is a most important factor that affects the therapeutic effect of drugs. Solubility of different extract of Salvadora persica bark were observed in cool and hot water, 0.1N HCl, phosphate buffer solution pH 6.8 and 7.4. SPBEC and SPBEEA were found to be insoluble in cool water and partial soluble in hot water. All extracts were found to be soluble in 0.1N HCl, phosphate buffer solution pH 6.8 and 7.4. Obtained results were listed in table 2.
Table 2: Solubility study of Salvadora persica leaves extract
| SPBEC | SPBEEA | SPBEM | SPBEE | SPBEW | |
| Cool water (25oC) | IN | IN | S | S | S |
| Hot water (40oC) | PS | PS | S | S | S |
| 0.1N HCL | S | S | S | S | S |
| Phosphate buffer pH 6.8 | S | S | S | S | S |
| Phosphate buffer pH 7.4 | S | S | S | S | S |
S: Soluble, PS: Partial soluble, IN: insoluble
Prepared 1% solution of extracts were used to determine pH and found to be near neutral as listed in table 3. Based on these results, it may claim that when extract will use in formulation shows no irritation effect.
Table 3: pH of extract
| 1% solution of extract | pH |
| SPBEC | 7.0±0.2 |
| SPBEEA | 7.0±0.1 |
| SPBEM | 6.8±0.2 |
| SPBEE | 6.6±0.1 |
| SPBEW | 6.9±0.2 |
Phytochemical assay revealed many bioactive substances present in the extract as listed in table 4.
Table 4: Phytochemical Screening of Salvadora persica bark extract
| Test performed | SPBEC | SPBEEA | SPBEM | SPBEE | SPBEW | |
| Alkaloids | Dragendorff test, Wagner test, Hager’s test | – | – | + | + | + |
| Glycosides | Legal test | – | – | + | + | + |
| Tannins | Ferric chloride test | – | – | + | + | + |
| Saponins | Froth formation test | – | – | + | + | + |
| Flavonoids | Zinc Hydrochloride reduction test | + | – | + | + | + |
| Proteins | Biuret test | – | – | + | + | + |
| Carbohydrates | Molish’s test | – | – | + | + | + |
| Sterols and Terpenoids | Libermann-burchard test | + | – | + | + | + |
No microbial colony was found on agar plates. Absence of microbes in extract might be due to use of chloroform, ethyl acetate, methanol and ethanol during the fractional maceration process. Limit tests were confirmed the absence of heavy metal (lead, arsenic) in extract.
Procedure discussed in the present manuscript provides an accurate and convenient method to determine and validate the λ max of unknown sample. The different concentrations of extract in different buffer solution were obtained and recorded in table 5.
Table 5: Determination and validation of λ max of extract
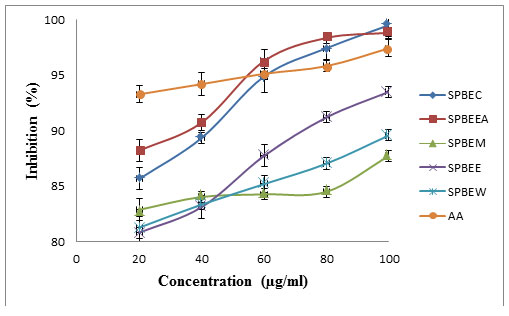
DPPH scavenging method was confirmed the antioxidant potential of extract. Percentage inhibitions of DPPH and comparative potential of extract and ascorbic acid were presented in figure 1 and calculated IC50 values were listed in table 6.
Figure 1: Comparative assessment of antioxidant potential (using DPPH scavenging model) of SPBEC: Salvadora persica bark extract in chloroform, SPBEEA: Salvadora persica bark extract in ethyl acetate, SPBEM: Salvadora persica bark extract in methanol, SPBEE: Salvadora persica bark extract in ethanol, SPBEW: Salvadora persica bark extract in water, AA: Ascorbic acid.
Table 6: IC50 of S. persica bark
| Sample | DPPH assay IC-50(µg/ml) |
| SPBEC | 192.05 |
| SPBEEA | 248.88 |
| SPBEM | 633.00 |
| SPBEE | 163.17 |
| SPBEW | 289.20 |
| AA | 861.42 |
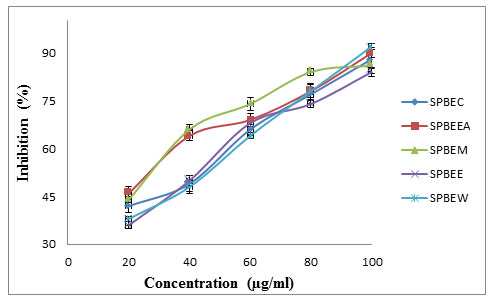
H2O2 scavenging method was used to determined antioxidant activity of Salvadora persica stem extracts (SPBEC, SPBEEA, SPBEM, SPBEE and SPBEW). Obtained percentage inhibition and comparative assessment was showed in figure 2 and calculated IC50 presented in table 7.
Figure 2: Comparative assessment of antioxidant potential of extract using H2O2 scavenging model.
Table 7: IC50 of S. persica stem
| Extract | H2O2 assay IC50 (µg/ml) |
| SPBEC | 36.00 |
| SPBEEA | 21.96 |
| SPBEM | 19.21 |
| SPBEE | 39.33 |
| SPBEW | 39.71 |
Obtained result were confirmed the antimicrobial activity of extract. Comparative of result of extract and ciprofloxacin was presented in table 8.
Table 8: Antimicrobial activity of extract
| Bacteria type | Zone of inhibition (mm) | |||||||||||
| SPBEC
(µg/ml) |
SPBEEA
(µg/ml) |
SPBEM
(µg/ml) |
SPBEE
(µg/ml) |
SPBEW
(µg/ml) |
Ciprofloxacin
(µg/ml) |
|||||||
| 100 | 200 | 100 | 200 | 100 | 200 | 100 | 200 | 100 | 200 | 100 | 200 | |
| E. Coli | 3 | 6 | 2 | 5 | 3 | 7 | 3 | 6 | 4 | 6 | 6 | 18 |
| B. Subtilis | 3 | 5 | 3 | 6 | 4 | 6 | 4 | 7 | 3 | 4 | 5 | 16 |
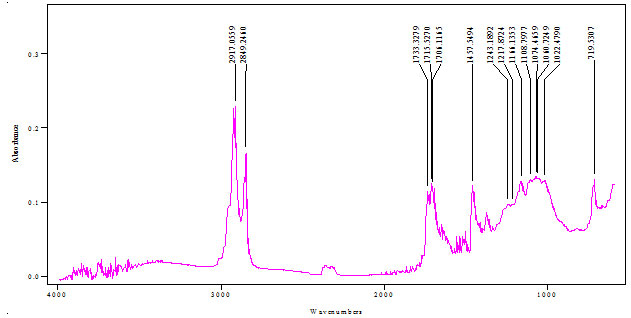
FTIR spectra of Salvadora persica bark extracts (SPBEC, SPBEEA, SPBEM, SPBEE and SPBEW) were measured and presented in figures 3 to 7. Recorded wavelengths and the probable functional groups (obtained by FTIR analysis) were obtained in the extracts were reported. By interpreting the data and find possible functional groups were listed in table 9,10,11,12,13.
Figure 3: FTIR of SPBEC
Figure 4: FTIR of SPBEEA
Figure 5: FTIR of SPBEM
Figure 6: FTIR of SPBEE
Figure 7: FTIR of SPBEW
Table 9: Functional group in SPBEC
| Peak value (cm-1) | Group presence |
| 2917 | C─H |
| 1733 | C=O |
| 1166 | O-H |
Table 10: Functional group in SPBEEA
| Peak value (cm-1) | Group presence |
| 2923 | C-H |
| 1707 | C=O, C=N |
| 1456 | C=C |
| 1370 | N-O |
| 1267 | C-O |
| 1138 | O-H |
| 935 | C-H |
Table 11: Functional group in SPBEM
| Peak value (cm-1) | Group presence |
| 3337 | O-H |
| 1613 | C≡C |
| 1472 | C=C |
| 1395 | C-H |
| 1248 | C-O |
| 1062 | O-H |
| 995 | C-H |
Table 12: Functional group in SPBEE
| Peak value (cm-1) | Group presence |
| 1613 | C-C, C=O |
| 1395 | N-O, C-H |
| 1061 | N-H |
Table 13: Functional group in SPBEW
| Peak value (cm-1) | Group presence |
| 3335 | O-H |
| 1607 | C=O |
| 1395 | N-O |
| 1063 | N-H |
CONCLUSION
Modified maceration method was used in extraction of Salvadora persica bark and highest % yield was found to be 1.96 in methanol. Extracts were found to be soluble in different pH buffer system i.e. PBS 6.4, PBS 7.4 and 0.1N HCl. pH value of extract was found to be near 7 so no irritation effect will cause at application site. Heavy metal and microbial load was not found to be on extract. Phytochemical screening of extract was reported the presence of various phytochemical constituents such as alkaloids, glycosides, tannins, Flavonoids, proteins, carbohydrates, sterol and terpenoids. H2O2 and DPPH scavenging methods were performed and confirmed antioxidant activity of extract. Disc diffusion method was confirmed antimicrobial response of extract. FTIR analysis of extract was used to detect characteristic peak range and the presence of possible functional groups.
ACKNOWLEDGEMENT
Authors would like to thank Department of Pharmacy, School of Medical and Allied Sciences, Galgotias University, for provide lab facility during research work.
REFERENCES
Abeer, YI., El-Gengaihi, SE., Motawe, HM. (2011). Phytochemical and cytotoxicity investigations of Salvadora persica bark extracts, Journal of The Arab Society for Medical Researches, 6(2):127-133.
Anthoney, ST., Lasity, TT. (2015). Phytochemical and Antibacterial Evaluation of Ethanolic extract of Salvadora persica Root Extract against Selected microorganisms. International Journal of Bioassays, 4.12: 4658-4666.
Bargah, RK. (2015). Preliminary test of phytochemical screening of crude ethanolic and aqueous extract of Moringa pterygosperma Gaertn, Journal of pharmacognosy and phytochemistry, 4(1): 7-9.
Chabane, OA., Saada, DA., Bekada, AMA., Selselet-Attou G.(2017). In vitro study of the antimicrobial effects of phenolic extract of the Salvadora persica (Miswak) on the growth of certain microorganisms responsible for oral infections. Res. J. Microbiol., 12: 58-73.
HOI. (1996). Indian Pharmacopoeia, 7th ed. New Delhi, India: Government of India, Controller of Publications; volume II. pp. A-42(arsenic), A-44(lead).
Jayaprakasha, GK., Jaganmohan, RL., Sakariah, KK. (2004). Antioxidant activities of flavidin in different in vitro model systems. Bioorg. Med. Chem. 12:5141–5146.
Kumar, D., Singhal, A., Bansal, S., Gupta, SK. (2015). Extraction, isolation and evaluation Trigonella foenumgraecum as mucoadhesive agent for nasal gel drug delivery. J Nepal Pharm Asso. 27:40–45.
Kumar, RA., Swamy, MR. (2014). Phytochemical screening by FTIR spectroscopic analysis of leaf extracts of selected Indian Medicinal plants, Int. J. Curr. Microbiol. App. Sci, 3(1): 395-406.
Malviya R. (2011). Extraction characterization and evaluation of selected muclilage as pharmaceutical excipients. Polim. Med. 41(3): 39–44.
Malviya, R., Sharma, P.K., Dubey, S.K. (2019). Microwave Facilitated Green Synthesis and Characterization of Acrylamide Grafted Copolymer of Kheri (Acacia chundra) Gum Polysaccharide, The Natural Products Journal, 9: 1.
Mamun, AA., Shaha, TK., Khan, MM., Kabir, MS. (2014). Determination of Microbial Load in Multivitamin and Cough Syrups Sold in Dhaka City, International Journal of Pharmaceutical Sciences and Drug Research, 6(3): 235-238.
Mathur, S. (2015). Medicinally Potent and Highly Salt Tolerant Plant of Arid Zone – Salvadora persica L. (Meswak): A Review. Journal of Plant Sciences. 3:1 (1): 45-49.
Peach, K., Tracy, MV. (1955). Modern Methods of Plant Analysis. Vol III and IV Springer, Heidelberg; pp. 258-61.
Shokeen P, Bala M, Tandon V.(2009) Evaluation of the activity of 16 medicinal plants against Neisseria gonorrhoeae, International Journal of Antimicrobial agents, Elsevier 33: 86-91.
Shubha HS, Hiremath RS. Evaluation of antimicrobial activity of Rasaka Bhasma, Ayu., 2010; 31(2): 260-262.
Verma R, Purohit S, Bhandari A, Kumar B, Priyanka P (2009). Salvadora persica L (Tooth Brush Tree): A Review, Journal of Pharmacy Research 2(12): 1809-1812.
Verma S, Gupta A, Kushwaha P, Khare V, Srivastava S, Rawat, A.K.S.(2012) Phytochemical Evaluation and Antioxidant Study of Jatropha curcas Seeds. Phcog. J. 2012; 4(29): 5-54.