Centre for Drug Discovery and Development, Sathyabama Institute of
Science and Technology, Chennai, Tamil Nadu, India
Corresponding author email: mrkactinos@gmail.com
Article Publishing History
Received: 11/07/2020
Accepted After Revision: 03/09/2020
Lactic Acid Bacteria (LAB) is an important group of microorganism due to their wide application in the food and dairy industries. They have extensively studied for the potential against various bacterial pathogens. The inhibitory activity of LAB is through their production of bacteriocins, organic acids, enzymes, hydrogen peroxide, etc., Bacteriocins are ribosomally synthesized antimicrobial peptides produced by various microorganisms. The incidence of non tuberculous mycobacterial infections increasing worldwide. Mycobacterium fortuitum is one of the rapidly growing non tuberculous mycbacteria which causes skin, bone, joint and pulmonary infections. Their antibiotic resistance and prolonged course of treatment necessitates the development of new candidate to fight against them. In this study, we have partially purified bacteriocins from four LAB strains using solvent extraction method and screened their activity against M. fortuitum MTCC1902 by colony forming unit (CFU) estimation method. The potential strain was identified by 16S rRNA sequencing and their sequences were submitted to Genbank database. The toxicity of potential LAB strain was assessed by both in vitro and in vivo method against Vero cell lines and zebra fish model respectively.The strain BLN48 showed 97.9% reduction in growth of M. fortuitum and 1.89±0.32 log reduction in CFU/ml. The potential strain BLN48 was identified as Enterococcus italicus. E. italicus BLN48 exhibited cytotoxicity against vero cell lines in dose dependent manner whereas under in vivo conditions, 50% of zebrafish larvae survived upto 144 hours post fertilization (hpf) with normal morphological changes. Further purification and characterization of E. italicus BLN48 bacteriocin in future helps in the development of an efficient candidate against M. fortuitum as well as other mycobacterial pathogens.
Partially Purified Bacteriocin, Non Tuberculous Mycobacteria, Mycobacterium fortuitum, Cytotoxicity, Zebrafish
Kalyanasundaram R, Radhakrishnan M, Anbarasu S. Evaluation of Inhibitory Activity of Bacteriocins from Enterococcus italicus BLN48 Against Mycobacterium fortuitum and its Toxicity Profiling. Biosc.Biotech.Res.Comm. 2020;13(3).
Kalyanasundaram R, Radhakrishnan M, Anbarasu S. Evaluation of Inhibitory Activity of Bacteriocins from Enterococcus italicus BLN48 Against Mycobacterium fortuitum and its Toxicity Profiling. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/2WRP77S
INTRODUCTION
Lactic Acid Bacteria (LAB) remains an industrially important group of microorganism due to their wide application in the food and dairy industries (Gomez et al., 2015). LAB are widely distributed in diverse habitats like marine, food products, dairy origin, etc., Usually, fermented foods were screened for bacterial isolates with antimicrobial properties as its microbiota is dominated by LAB. LAB also exists in gastrointestinal tracts, oral cavities of humans as well as animals (Bungenstock et al., 2020; Li et al., 2020). In raw milk, LAB are the predominant microorganisms and they contribute to the fermentation and food preservation process due to their various metabolite production (Rahmeh et al., 2019). LAB has studied extensively for their antagonistic activity against various bacterial pathogens such as food spoiling microorganisms like Listeria monocytogenes, gastrointestinal pathogens and other various gram positive and gram negative bacterial pathogens. Researchers have gained significant attention towards LAB due to their Generally Recognized As Safe (GRAS) status. The inhibitory activity of LAB is mainly through their production of various substances like organic acids, enzymes, bacteriocins, hydrogen peroxide, etc., (Gupta and Garg 2009; Rodrigues et al., 2006 Hussein et al., 2018)
Bacteriocins are ribosomally synthesized antimicrobial peptides produced by various microorganisms. The uses of bacteriocins in functional foods and as an alternative to antibiotics are their emerging application (Quwehand et al., 2004; Messi et al., 2001). Physical stability and non-toxic nature are the major advantages of bacteriocins (Morgan et al., 2005). Different classes of bacteriocins exerts diverse mechanisms against their target like disruption of cell wall, pore formation, inhibition of protein and nucleic acid synthesis, etc., (Cascales et al., 2007; Stevens et al., 1991). LAB produces a diverse nature of bacteriocins in different size, physicochemical properties, spectrum of activity, structures, etc. They secreted in an extracellular space during LAB growth (Venegas et al., 2019; Anbarasu et al., 2020). The crude and purified bacteriocins from LAB have found their potential use as biopreservative agents to enhance the quality and safety of various food products.The potential bacteriocins can be used in both combined and balanced mode as probiotics for human diseases also (Iseppi et al., 2019, Arrioja et al., 2020).
Non Tuberculous Mycobacteria (NTM) are several mycobacterial species other than M. tuberculosis complex and M. leprae. NTM causes opportunistic infections in humans as well as animals and also it transmitted among environment, livestock, wildlife, etc. (Odoi et al., 2020). Mycobacterium fortuitum is one of the rapid growing NTM and is predominantly found in water systems like natural water, tap water, and water used in showers in hospitals and soil. It mainly causes skin, bone, joint infections and pulmonary diseases in immunocompromised and immunosuppressed patients. They also causes surgical site infections (Okamori et al., 2018; Griffith et al., 2007; Goslee and Wolinsky 1976; Wolinsky and Rynearson 1968; Choudhary et al., 2020).
M. fortuitum are often isolated from skin and soft tissues and also from other clinical samples as it causes many types of infection (Garcia et al., 2020). The development of antibiotic resistance and prolonged course of treatment with multiple antibiotics in NTM infection surges the need for the development of new candidates with potential inhibitory substance and less toxicity to fight against these infections. In this study, we have evaluated bacteriocins from four LAB isolates for their anti M. fortuitum activity. The potential LAB isolate was identified through 16S rRNA sequencing and phylogenetic analysis. Toxicity profile of the bacteriocins from the potential LAB strain was also evaluated through in vitro and in vivo method.
MATERIAL AND METHODS
Mycobacterium fortuitumMTCC1902 strain was purchased from Microbial Type Culture Collection (MTCC), Chandigarh, India. de Man Rogosa Sharpe broth (Himedia), Middlebrook 7H9 broth (Himedia), Middlebrook 7H11 Agar (Himedia), Chloroform (SRL), PBS tablets (Sigma) were used in the study. Four lactic acid bacterial strains viz., BLN 34, BLN 36, BLN 39 and BLN 48 previously isolated from different cow milk samples were used in this study. Viability of all the cultures were maintained in MRS agar slants at 4oC (Revathy et al., 2019). Bacteriocin from four selected cultures was produced bysubmerged fermentation process and was partially purified by solvent extraction method using chloroform as described by Burianek et al. 2000 with few modifications. Briefly, 5ml of overnight grown culture in MRS broth was added to 500ml of sterile MRS broth and incubated for 18 hours in shaking incubator at 30˚C. After incubation, the culture was centrifuged at 5000rpm for 10minutes to collect the cell free supernatant. For the extraction of crude bacteriocin, 50% v/v of chloroform has been added to the supernatant and kept in magnetic stirrer at 1000rpm for 20minutes. Then the mixture was subjected to centrifugation at 10000rpm for 30 minutes. After centrifugation, the precipitate in the interphase layer between solvent and aqueous phase was collected carefully and freezed at -20˚C following by lyophilization. The lyophilized form ofpartially purified bacteriocin (PPB) were stored at -20˚C and used for further assays.
Evaluation of PPB against M. fortuitum: Ten mg concentration of partially purified bacteriocin in the form of lyophilized powder was dissolved in 1ml ofPBS buffer in order to get 10mg/ml (w/v)concentration. Desired working concentration of PPB was prepared from the main stock using PBS buffer.Inhibitory activity of PPB prepared from all the four cultures was evaluated against M. fortuitumby colony forming unit (CFU) estimation(Gillespie et al. 2005).Briefly, M. fortuitum MTCC1902 suspension was prepared by inoculating a loopful of M. fortuitum culture into 0.3ml of Middlebrook 7H9 broth followed by vortexing. Then the volume of the suspension was made upto 5ml. using 7H9 broth. In a sterile cryovial, 400µl of 7H9 broth was used as growth control and 350µl of 7H9 broth with 50µl of bacteriocin was used in test vial. All the vials were added with 100µl of M. fortuitum suspension and incubated at 37˚C for 48 hours. After incubation, 100µl of aliquot from each vial was serially diluted in 900µl of PBS buffer upto 10-12 dilution. 50µl of the dilution was spreaded onto Middlebrook 7H11 agar plate. Plating was done in triplicate and all the plates were incubated at 37oC for 48 hours. The plates with individual colonies were taken for the calculation of CFU/ml.
Taxonomy of potential LAB strain:The genomic DNA of LAB strain BLN48 was isolated using solute ready genomic DNA kit. DNA was analyzed by gel electrophoresis and quantified using a spectrophotometer (NanoDrop ND-1000, Thermo Scientific, Gloucester, UK). The 16S rRNA gene sequence of the strain was amplified using the primers: 27F 5´AGAGTTTGATCMTGGCTCAG3´ (forward) and 1492R 5´TACGGYTACCTTGTTACGACTT3´ (reverse) (Kumar Gothwal et al., 2007). The PCR amplified product of the strain was sequenced and analyzed at National Collection of Industrial Microorganisms (NCIM), National Chemical Laboratory (CSIR-NCL), Pune, India. The 16S rRNA gene sequence obtained from the strain BLN48 was aligned with similar sequences available in GenBank using MEGA 7 program. The aligned sequences of the strain BLN48 was used to construct the phylogenetic tree by following neighbor joining algorithm in MEGA 7 program (Saitou and Nei, 1987). The bootstrap estimation (Felsenstein, 1985) was used to determine the confidence of the branches of the phylogenetic tree. The partial 16S rRNA nucleotide sequence of all the four strains has been deposited in GenBank database.
In vitro toxicity analysis of PPB:The cytotoxicity of potential PPB which showed activity against M. fortuitumwere assessed in vitro by adopting MTT (3-(4, 5-dimethyl thiazol-2yl)-2, 5-diphenyl tetrazolium bromide) assay usingVero cell lines. Briefly, in the 96 well plate, 100µl of RMPI 1640 medium was added with 100µl of desired concentration of PPB. Then 200µl of total volume is gently mixed well. 100µl of diluted PPB from the first well was serially diluted in next well till reaching the lowest concentration. The cultured Vero cell lines were harvested by trypsinization and pooled in 50ml vial. Then the cells were plated at a density of 1x100cells/ml. 200µl of vero cells without PPB was used as a control. The cells were incubated at 37˚C in 5% CO2 incubator for 24 hours. After incubation, 20µl of MTT solution was added to all the wells and incubated for 4 hours at 37˚C.The media and MTT was well mixed and the absorbance was measured at 450nm and the percentage of viability was calculated manually,(Vijayarathna and Sasidharan, 2012).
In vivo toxicity analysis of PPB:In vivo toxicity of PPB was evaluated using zebrafish as a model (Sisman et al. 2008). Zero day old zebrafish eggs were purchased from zebrafish aquarium in Kanchipuram district, Tamil Nadu, India. Twenty healthy post hatched zebra fish eggs were transferred to the wells of a 24-well plate along with 1 ml ofembryo water (60 mg of sea salt/ litre of ultrapure water). Different concentrations of PPB of potential LAB strain BLN48 (10, 50 and100 μg/ml) was added to the wells and incubated for 144 hat 28.5◦C. Mortality of the zebra fish was noted after 24, 48, 96 and 144 h. The embryos appeared opaque and white in colour.The dead embryos were degraded soon, whereas the structures of intact embryos were more visible by 48 hours post fertilization (hpf) which allowed a clear distinction between the dead and alive.The mortality rate is calculated. At the end of the incubation period, the embryos were photographed using a light microscope at 10X magnification.
RESULTS AND DISCUSSION
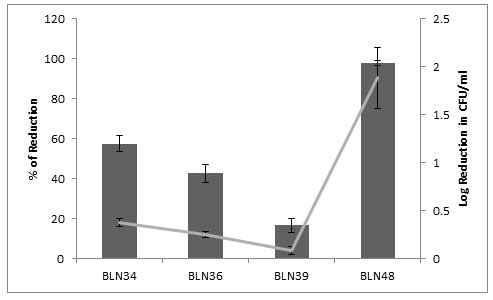
Anti M. fortuitum activity:Among the four isolates tested, BLN48 showed significant inhibitory activity against M. fortuitum MTCC1902 viz., 1.89 log reduction of CFU/ml which corresponds to 97.9% reduction in growth when compared to growth control. Followed by, BLN34 showed slight inhibitory activity by 0.38 log reduction of CFU/ml with 57.56% reduction from growth control (Figure 1).
Figure 1: Anti M. fortuitum activity of Partially purified bacteriocins
Taxonomy of potential LAB strain BLN48:Amplification of 16S rRNA gene from the strain BLN48 resulted in 1435 bp sequences. BLAST analysis showed 99.43% sequence similarity with 16S rRNA gene sequence of Enterococcus italicus DSM 15952. The phylogenetic tree also showed that the strain BLN48 is closely related to Enterococcus italicus(Figure 2). The nucleotide sequence of E. italicus BLN48 was submitted to Genbank with accession number MN880432.
Figure 2: Phylogeny of BLN48
In vitro toxicity analysis of PPB: The cytotoxicity analysis by MTT assay showed that the bacteriocin from BLN48 exhibited the cytotoxicity on vero cell lines in dose dependent manner. At the maximum of 60% of vero cells survived when treated at 10mM concentration of bacteriocin (Table 1).
Table 1. In vitro cytotoxicity analysis of BLN48 by MTT assay against vero cell lines
| PPB | Test concentration | Percentage of Viability |
| BLN48 | 100mM | 16.6 |
| 10mM | 60.4 |
In vivo toxicity analysis of PPB:In the in vivotoxicity analysis of BLN48 done with zebrafish larvae, BLN48 have shown less toxicity viz., around 50% of larvae (compared to control) were survived upto 144 hpf at high concentration (100µg/ml) with healthy morphology under microscopic observation (Table 2). The features like fin movement, swimming nature, tail development are normal to the viable larvae (Figure 3).
Table 2. Mortality rate in in vivo toxicity analysis of BLN48
| Concentration | Control | 10 µg/ml | 50 µg/ml | 100 µg/ml |
| HPF | ||||
| 0 HPF | 20 | 20 | 20 | 20 |
| 24 HPF | 20 | 18 | 19 | 16 |
| 48 HPF | 18 | 17 | 15 | 13 |
| 96 HPF | 16 | 14 | 12 | 9 |
| 144 HPF | 13 | 11 | 9 | 7 |
Figure 3: Morphological changes in zebrafish embryos after treating with BLN48
fortuitum group is responsible for 60-80% of post surgical infections caused by mycobacteria. The successful treatment outcome of M. fortuitum infections often limited by the multi drug resistance, need of combination therapy, prolonged course, etc. (Cynamon et al., 2012).Santos et al (2016) analysed the resistant profile of M. fortuitum isolates and showed their resistance to different classes of antibiotics. Antimicrobial peptides i.e., bacteriocins offers a solution to combat antibiotic resistance of various pathogenic microorganisms. Numerous studies have proved the efficacy of bacteriocins against various drug resistant pathogens (Regmi et al., 2017).
In the present study, the partially purified bacteriocin from Enterococcus italicus BLN48 has showed significant inhibition against M. fortuitum.In the various studies, numerous antibiotics and chemical compounds have been screened against M. fortuitum but there are very less reports on the screening of natural compounds of microbial source against M. fortuitum (Gay et al., 1984; Welch et al., 1979; Bagchi et al., 2007). While there are many other reports focuses on the screening of bacteriocins against other mycobacterium species like M. tuberculosis. A study by Sosunov et al (2007) has assessed the antimycobacterial of five bacteriocins against M. tuberculosis strains. In 2010, Carroll et al., compared the activities of two bacteriocins Lacticin 3147 and nisin against NTM species like M. kansasii, M. avium paratuberculosis.
Their study found that lacticin 3147 showed superior activity than nisin. A study by Aguilar-Pérez et al (2018)have found the inhibitory activity of bacteriocin AS-48 against M. fortuitum and found their MIC as 64µg/ml along with other mycobacterium species. They also found that there is no cytotoxicity obtained against various macrophage cell lines. Our result showed that the E. italicus BLN48 exhibits cytotoxicity on vero cell lines in dose dependent manner. However, the in vivo cytotoxicity assay using zebrafish showed that there is no side effect on the morphology of viable larvae in the presence of E. italicus BLN48 at three different concentrations. A survey by Fortina et al (2008) has described the safety and biotechnological properties of E. italicus of dairy origin. They suggested that E. italicus presence in the cheese lowers the health risk and supports their applications in dairy industry. This study describes the safety profile of E. italicus for their wide application in future.
CONCLUSION
Mycobacterium fortuitum is one of the clinically significant rapidly growing mycobacteria which cause pulmonary, skin and soft tissue infections in immunocompromised and immunosuppressed patients. Partially purified bacteriocin of Enterococcus italicus BLN48 showed significant inhibitory activity against M. fortuitum. Their cytotoxicity assay under both in vitro and in vivo conditions shows that E. italicus BLN48 can be developed as potential candidate against M. fortuitum. Further analysis on their Minimum Inhibitory Concentration (MIC), purification and their characterization and screening them against other mycobacterial species will leads to the efficient use of E. italicus BLN48 for therapeutic purpose.
REFERENCES
Aguilar-Pérez, C., Gracia, B., Rodrigues, L., Vitoria, A., Cebrián, R., Deboosère, N., Song, O.R., Brodin, P., Maqueda, M. & Aínsa, J.A. (2018) Synergy between circular bacteriocin AS-48 and ethambutol against Mycobacterium tuberculosis. Antimicrobial agents and chemotherapy, 62(9).
Anbarasu, S., Kumar, V., Rani, U., Kalyanasundram, R. & Radhakrishnan, M. (2020) Purification and characterization of bacteriocins active against Mycobacterium tuberculosis from lactic acid bacteria. Research Journal of Biotechnology, 15, 4.
Arrioja, D., Mani-López, E., Palou, E., & López-Malo, A. (2020). Antimicrobial activity and storage stability of cell-free supernatants from lactic acid bacteria and their applications with fresh beef. Food Control, 107286.
Bagchi, M. C., Mills, D., & Basak, S. C. (2007). Quantitative structure-activity relationship (QSAR) studies of quinolone antibacterials against M. fortuitum and M. smegmatis using theoretical molecular descriptors. Journal of molecular modeling, 13(1), 111-120.
Bungenstock, L., Abdulmawjood, A., & Reich, F. (2020). Evaluation of antibacterial properties of lactic acid bacteria from traditionally and industrially produced fermented sausages from Germany. Plos one, 15(3), e0230345.
Burianek, L. L., & Yousef, A. E. (2000). Solvent extraction of bacteriocins from liquid cultures. Letters in applied microbiology, 31(3), 193-197.
Carroll, J., & O’Mahony, J. (2011). Anti-mycobacterial peptides: made to order with delivery included. Bioengineered bugs, 2(5), 241-246.
Carroll, J., Draper, L.A., O’Connor, P.M., Coffey, A., Hill, C., Ross, R.P., Cotter, P.D. and O’Mahony, J. (2010) Comparison of the activities of the lantibiotics nisin and lacticin 3147 against clinically significant mycobacteria. International journal of antimicrobial agents, 36(2), 132-136.
Cascales, E., Buchanan, S.K., Duché, D., Kleanthous, C., Lloubes, R., Postle, K., Riley, M., Slatin, S. & Cavard, D. (2007) Colicin biology. Microbiology and molecular biology reviews, 71(1), 158-229.
Choudhary, A., Gopalakrishnan, R., Senthur Nambi, P., Thirunarayan, M.A., Ramasubramanian, V. & Sridharan, S. (2020) Surgical Site Infections Caused by Rapidly Growing Nontuberculous Mycobacteria: an Under-Recognized and Misdiagnosed Entity. Indian Journal of Surgery, 1-6.
Cynamon, M., Jureller, J., Desai, B., Ramachandran, K., Sklaney, M., & Grossman, T. H. (2012). In vitro activity of TP-271 against Mycobacterium abscessus, Mycobacterium fortuitum, and Nocardia species. Antimicrobial agents and chemotherapy, 56(7), 3986-3988.
Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution, 39(4), 783-791.
Fortina, M.G., Ricci, G., Borgo, F., Manachini, P.L., Arends, K., Schiwon, K., Abajy, M.Y. & Grohmann, E. (2008) A survey on biotechnological potential and safety of the novel Enterococcus species of dairy origin, E. italicus. International journal of food microbiology, 123(3), 204-211.
Garcia-Coca, M., Rodríguez-Sevilla, G., Pérez-Domingo, A., Aguilera-Correa, J.J., Esteban, J. & Muñoz-Egea, M.C. (2020) Inhibition of Mycobacterium abscessus, M. chelonae, and M. fortuitum biofilms by Methylobacterium sp. The Journal of Antibiotics, 73(1), 40-47.
Gay, J. D., DeYoung, D. R., & Roberts, G. D. (1984). In vitro activities of norfloxacin and ciprofloxacin against Mycobacterium tuberculosis, M. avium complex, M. chelonei, M. fortuitum, and M. kansasii. Antimicrobial agents and chemotherapy, 26(1), 94-96.
Gillespie, S. H., Basu, S., Dickens, A. L., O’Sullivan, D. M., & McHugh, T. D. (2005). Effect of subinhibitory concentrations of ciprofloxacin on Mycobacterium fortuitum mutation rates. Journal of Antimicrobial Chemotherapy, 56(2), 344-348.
Gómez-Sala, B., Muñoz-Atienza, E., Sánchez, J., Basanta, A., Herranz, C., Hernández, P. E., & Cintas, L. M. (2015). Bacteriocin production by lactic acid bacteria isolated from fish, seafood and fish products. European Food Research and Technology, 241(3), 341-356.
Goslee, S., & Wolinsky, E. (1976). Water as a source of potentially pathogenic mycobacteria. American Review of Respiratory Disease, 113(3), 287-292.
Griffith, D.E., Aksamit, T., Brown-Elliott, B.A., Catanzaro, A., Daley, C., Gordin, F., Holland, S.M., Horsburgh, R., Huitt, G., Iademarco, M.F. & Iseman, M. (2007) An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. American journal of respiratory and critical care medicine, 175(4), 367-416.
Gupta, V., & Garg, R. (2009). Probiotics. Indian journal of medical microbiology, 27(3), 202.
Hussein, A. R., Khalaf, Z. Z., Samir, Z., & Samir, R. (2018). Antibacterial activity of crud Bacteriocin-like substance against food borne bacterial pathogens. Iraqi journal of Science, 59(1A), 16-24.
Iseppi, R., Messi, P., Camellini, S. and Sabia, C. (2019) Bacteriocin activity of Lactobacillus brevis and Lactobacillus paracasei ssp. paracasei. Journal of medical microbiology, 68(9), 1359-1366.
Kumar Gothwal, R., Kumar Nigam, V., Mohan, M. K., Sasmal, D., & Ghosh, P. (2007). Extraction of bulk DNA from Thar Desert soils for optimization of PCR-DGGE based microbial community analysis. Electronic Journal of Biotechnology, 10(3), 400-408.
Li, M., Wang, Y., Cui, H., Li, Y., Sun, Y., & Qiu, H. J. (2020). Characterization of Lactic Acid Bacteria Isolated From the Gastrointestinal Tract of a Wild Boar as Potential Probiotics. Frontiers in veterinary science, 7, 49.
Messi, P., Bondi, M., Sabia, C., Battini, R., & Manicardi, G. (2001). Detection and preliminary characterization of a bacteriocin (plantaricin 35d) produced by a Lactobacillus plantarum strain. International Journal of Food Microbiology, 64(1-2), 193-198.
Morgan, S. M., O’Connor, P. M., Cotter, P. D., Ross, R. P., & Hill, C. (2005). Sequential actions of the two component peptides of the lantibiotic lacticin 3147 explain its antimicrobial activity at nanomolar concentrations. Antimicrobial agents and chemotherapy, 49(7), 2606-2611.
Odoi, J.O., Ohya, K., Moribe, J., Takashima, Y., Sawai, K., Taguchi, K., Fukushi, H., Wada, T., Yoshida, S. & Asai, T. (2020) Isolation and antimicrobial susceptibilities of Nontuberculous Mycobacteria from Wildlife in Japan. Journal of Wildlife Diseases.
Okamori, S., Asakura, T., Nishimura, T., Tamizu, E., Ishii, M., Yoshida, M., Fukano, H., Hayashi, Y., Fujita, M., Hoshino, Y. & Betsuyaku, T. (2018) Natural history of Mycobacterium fortuitum pulmonary infection presenting with migratory infiltrates: a case report with microbiological analysis. BMC Infectious Diseases, 18(1), 1.
Quwehand, A.C. & Vesterlund, S. 2004. Antimicrobial components from lactic acid bacteria. Lactic Acid Bacteria Microbiological and Functional Aspects, Marcel Dekker Inc., New York, USA.
Rahmeh, R., Akbar, A., Kishk, M., Al-Onaizi, T., Al-Azmi, A., Al-Shatti, A., Shajan, A., Al-Mutairi, S. & Akbar, B. (2019) Distribution and antimicrobial activity of lactic acid bacteria from raw camel milk. New microbes and new infections, 30, 100560.
Regmi, S., Choi, Y. H., Choi, Y. S., Kim, M. R., & Yoo, J. C. (2017). Antimicrobial peptide isolated from Bacillus amyloliquefaciens K14 revitalizes its use in combinatorial drug therapy. Folia microbiologica, 62(2), 127-138.
Revathy, K., Manikkam, R., & Anbarasu, S. (2019). Isolation, characterization of lactic acid bacteria from cow and buffalo milk and evaluation for antibacterial and antimycobacterial activity in vitro. Asian Journal of Microbiology Biotechnology and Environmental Sciences, 21(4), 1041-1046.
Rodrigues, L., Banat, I. M., Teixeira, J., & Oliveira, R. (2006). Biosurfactants: potential applications in medicine. Journal of Antimicrobial Chemotherapy, 57(4), 609-618.
Saitou, N., & Nei, M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular biology and evolution, 4(4), 406-425.
Santos, D. R. D. S., Lourenço, M. C. S., Coelho, F. S., Mello, F. C. Q., & Duarte, R. S. (2016). Resistance profile of strains of Mycobacterium fortuitum isolated from clinical specimens. Jornal Brasileiro de Pneumologia, 42(4), 299-301.
Şişman, T., İncekara, Ü., & Yıldız, Y. Ş. (2008). Determination of acute and early life stage toxicity of fat‐plant effluent using zebrafish (Danio rerio). Environmental Toxicology: An International Journal, 23(4), 480-486.
Sosunov, V., Mischenko, V., Eruslanov, B., Svetoch, E., Shakina, Y., Stern, N., Majorov, K., Sorokoumova, G., Selishcheva, A. & Apt, A. (2007) Antimycobacterial activity of bacteriocins and their complexes with liposomes. Journal of antimicrobial chemotherapy, 59(5), 919-925.
Stevens, K. A., Sheldon, B. W., Klapes, N. A., & Klaenhammer, T. R. (1991). Nisin treatment for inactivation of Salmonella species and other gram-negative bacteria. Appl. Environ. Microbiol., 57(12), 3613-3615.
Venegas-Ortega M.G., Flores-Gallegos A.C., Martínez-Hernández, J.L., Aguilar C.N. & Nevárez-Moorillón, G.V. (2019) Production of bioactive peptides from lactic acid bacteria: a sustainable approach for healthier foods. Comprehensive Reviews in Food Science and Food Safety, 18(4), 1039-1051.
Vijayarathna, S., & Sasidharan, S. (2012). Cytotoxicity of methanol extracts of Elaeis guineensis on MCF-7 and Vero cell lines. Asian Pacific Journal of Tropical Biomedicine, 2(10), 826-829.
Welch, D. F., & Kelly, M. T. (1979). Antimicrobial susceptibility testing of Mycobacterium fortuitum complex. Antimicrobial agents and chemotherapy, 15(6), 754-757.
Wolinsky, E., & Rynearson, T. K. (1968). Mycobacteria in soil and their relation to disease-associated strains. American Review of Respiratory Disease, 97(6P1), 1032-1037.