1Department of Botany, Bahauddin Zakariya University, Multan.Pakistan
2Institute of Biotechnology and Genetic Engineering (IBGE) University of Sindh, Jamshoro. Pakistan.
3Department of Botany, University of Gujrat, Gujrat. Pakistan
4Department of Botany, Government College University, Faisalabad. Pakistan
Corresponding author email: yasingmn_bzu@yahoo.com
Article Publishing History
Received: 25/03/2020
Accepted After Revision: 15/05/2020
The occurrence of soil’s heavy metals may be beneficial and / or toxic to the environment as these elements may be among the essentials required for the plant growth (like Fe, Cu, Zn, Cr or Mo) in trace quantities, but at higher concentrations they may also be poisonous. An experiment was conducted on four mash bean (Vigna mungo (L Hepper) genotypes to evaluate the toxic or beneficial effects of chromium (III) applied in rhizospheric environment of the plant. Photosynthetic phytochemicals in the form of chlorophyll a, chlorophyll b, total carotenoids, plant nitrate reductase activity and physiological growth as leaf area index (LAI) were recorded against 10.0, 20.0, 30.0, 40.0, 50.0 and 60.0 mg kg-1 of chromium (III) concentrations. Seeds of four genotypes sterilized with 10% (V/V) hydrogen peroxide were sown in earthen pots filled with homogenized loamy soil.
Chromium was added in soil as CrCl3 solution after twenty days of germination. Data were collected on expiry of twenty five days after chromium addition. Increasing amount of chromium (Cr) appeared to be responsible for gradual reduction in Nitrate Reductase Activity (NRA), photosynthetic pigments and physiological growth of leaf in term of Leaf Area Index (LAI). The lowest significantly effective) dose was 20 mg kg-1 in this regard. While the most effective proven dose was 60 mg kg-1 for each attribute. The observations were excluded from the ongoing trend when 10 mg kg-1 chromium (Cr) reflected an increase in studied characteristics, the most being 13.59% for Nitrate Reductase Activity (NRA) at this level. Of the genotypes, MASH 80 was the most sensitive while MASH 88 was the least sensitive to chromium stress.
Chromium, Mash, Nitrate Reductase, Chlorophyll, Carotenoid, Leaf Area Index
Ghulam Yasin, Mumtaz S, Sameen S, ul Haq I, Tabassum I, Hussain K, Nawaz K, Noman A. Escalating Rhizospheric Chromium Pollution Grades as Plant Foes or Friends?-Evaluation by Enzyme Assay, Physiological Growth and Photosynthetic Phytochemicals of the Mash Bean, Vigna mungo. Biosc.Biotech.Res.Comm. 2020;13(2).
Ghulam Yasin, Mumtaz S, Sameen S, ul Haq I, Tabassum I, Hussain K, Nawaz K, Noman A. Escalating Rhizospheric Chromium Pollution Grades as Plant Foes or Friends?-Evaluation by Enzyme Assay, Physiological Growth and Photosynthetic Phytochemicals of the Mash Bean, Vigna mungo. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/3hz5wGR
Copyright © Ghulam et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Chromium (Cr) is a natural element mostly found in rocky and volcanic areas. The IARC (International Agency for Research on Cancer) has classified this element as a carcinogenic agent. Therefore, its proper management and very accurate investigations of soil–plant systems are required. This hazardous Cr has high solubility rate in water, so it contaminates the ground water and, in this way, it is transferred to food chain through soil plant interaction system. The Cr also has negatively impacted on the plant-growth also by the impairment with essential physiological-processes of cells (Sharma et al. 2020).
Many industries like dyeing, electroplating, leather, tanning and steel discharge effluents of chromium causing significant rise in environmental Cr contents. Chromium high solubility contaminates groundwater and enters into food chain through soil plant interconnection (Joutey et al. 2015; Kumar et al. 2019). The Agency for Toxic Substances and Disease Registry,,(CERCLA, 2019). Chromium is ranked 17th among the most hazardous metals (Agency for Toxic Substances and Disease Registry, 2019).
Its high redox potential enables chromium to change oxidation state easily (Prado et al. 2016; Shahid et al. 2017). It is most common and has two stable [hexavalent Cr (VI) & trivalent Cr (III)] oxidation states (Ashraf et al. 2017)..These two oxidation forms differ in respect of their toxicity, bioavailability as well as translocation in plants organs (Shahid et al. 2017). Eventually, the stable form is Cr (III), whereas for plants the Cr (VI) is most noxious, as when they enter the cell, these oxidation states attack the proteins, lipids and DNA (Tchounwou et al. 2012;Stambulska et al. 2018).
In plants excess chromium concentration disrupts many physio-biochemical attributes, (Ud Din et al. 2015;Kamran et al. 2017). Toxicity of metal relies upon its complex interactions with signal transduction, genetics and macromolecules (Santos et al. 2012; Eleftheriou et al. 2015; Kumari et al. 2016). The Cr toxicity could affect growth of plant by many ways including changes in structure of cell membrane, chloroplast, pigments, disturbing water balance, mineral nutrition, enzymes and the assimilation processes, (Reale et al. 2016; Ali et al. 2015; Farooq et al. 2016;Cervantes and Campos-Garcia, 2007;Anjum et al. 2017). The major toxic effects of chromium are due to its role in ROS (reactive oxygen species) production, which alters the redox-balance in among plants tissues (Anjum et al. 2017).
In view of above consideration of the literature provided by different plant research groups, it was found that they have addressed the aspects of uptake to sub-cellular distribution of Cr in plants, seriously, as they also affect many plant photosynthetic parameters (pigmentation), enzymatic and other antioxidative systems including different endogenous plant hormone levels. Roots of some plants secrete organic molecules such as acids which act as ligands and can modify solubility of many metals in the soil (Khanna et al. 2019;Kaur et al. 2017;Kaur et al. 2018; Kohli et al. 2018).
Plant uptake Cr through active and passive mechanisms have been studied by many workers, (Shanker et al. 2005; Cervantes 2001;Appenroth et al. 2008 and De Oliveira et al. 2013). Due to its structural similarity with sulphate and phosphate, uptake of chromium by root cells is through sulphate or phosphate (De Oliveira et al. 2016). Cr (VI) when it enters into the cells of plants, gets converted to Cr (III) which finally gets bonded to cell walls and blocks further Cr transport to the plants, (Kabata and Szteke, 2015).
Mash bean (Vigna mungo (L.) Hepper) is a member of Papillionaceae family and is one of the most important world’s pulse crops and in Pakistan, it is paradoxically the least researched crop in spite of its high nutritive and economic value. On the basis of chemical analysis, the seeds of mash bean have been found to contain oil, fats, carbohydrates, protein, with traced vitamin A and B amounts (James, 1981). It fixes free atmospheric N2 for its consumption and enriches with N for next the crops (Sen, 1996). Considering the importance of mash bean and in particular the ever increasing toxicity of chromium in environment, the present experiment was devised to find out the extent of chromium concentration which could be toxic to the plant under study.
MATERIAL AND METHODS
To find out whether chromium metal is toxic or beneficial element for plant, an experiment was devised to evaluate the photosynthetic pigments, Nitrate Reductase (EC 1.6.6.1) Activity (NRA) and leaf area index of four mash bean (Vigna mungo (L Hepper) genotypes under various chromium applied concentrations.
Materials: After the initial survey, effluent hazard-free sandy loam soil was selected, which was air-dried, grounded, passed through a 2 mm sieve and mixed well. Seeds of four mash genotypes i.e., MASH 80, MASH 88, MASH 97 and MASH ES-1 were used in the experiment. The genotypes have their origin in Ayub Agricultural Research Institute (AARI), Faisalabad (Pakistan) and National Agricultural Research Centre (NARC) Islamabad (Pakistan). These were obtained from Pulse Section, Ayub Agricultural Research Institute (AARI), Faisalabad (Pakistan). For imposing metal pollution in soil, chloride of chromium of Sigma Aldrich, Japan was used.
Experimental design and methodologies: Experiment was designed with complete randomization of treatments and genotypes to avoid unequal exposure of environmental factors. Each treatment was repeated four times by pots and plants. Pots of 30 cm diameter were filled with 10 kg sandy loam soils and lined with polyethylene bags ensuring seepage prevention. These were arranged in completely randomized design. Seeds sterilized with 0.1% (V/V) HgCl2, similar in size and weight, were germinated and thinning was performed after germination to maintain one seedling in each arranged pot to avoid the imbalanced uptake of soil’s nutrients by plants. Weeds were uprooted from time to time by hand weeding and hoeing in order to avoid weed crop competition. Insects and pests were control by foliar spray of Thiodon insecticides of Hoechst (Pvt) Ltd, Pakistan. Plants were irrigated with normal irrigation water.
Chromium treatment: Quantified amounts of chromium chloride were added in soil accordingly to raise the chromium levels of 10.0, 20.0, 30.0, 40.0, 50.0 and 60.0 mg kg-1 soil. Metals salts were applied in soil as a water solution of CrCl3, method similar to that used by (Stoeva and Bineva, 2003) after twenty days of sowing while the pots without the addition of metals salts acted as control.
Photosynthetic phytochemical quantification: Photosynthetic phytochemicals in the form of pigments contents were measured by using the formula of Arnon, 1949) after twenty five days of metal imposition. The leaves were extracted with 80% acetone. By using spectrophotometer (Hitachi Model-U 2001 Japan), the absorbances were measured at 645nm and 663nm for Chl a and b contents respectively and at 480 nm for carotenoids. Carotenoids contents were calculated after (Goodwin, 1965) and chlorophylls were calculated according to the (Lichtenthaler, 1987) formulae Carotenoids (mg g-1 leaf fresh weight) = (Acar/EM) × 1000.
Chl a in leaf fresh weight (mg g-1)=(12.7(OD663)-2.69(OD645))×V/1000×W. Chl b in leaf fresh weight(mg g-1)=(22.9(OD645)-4.68(OD663))×V/1000×W. Where Acar = OD480+0.114(OD663)-0.638(OD645); EM (100%) =2500; OD =Optical density;
V is volume of sample and W is weight of sample.
Estimation of Nitrate Reductase activity: Nitrate reductase activity was determined on the expiry of twenty five days after metal imposition using the method of (Sym, 1984).
Leaf Area Index: The leaf area index (LAI) was calculated by using the following formula given by Puttaswamy et al., (1976).
LAI = L × W × N × K Where, L= length of the leaf (cm);W= maximum width of the leaf in cm;N= number of leaves per plant;K= constant (0.65 for legume crops).
Statistical analysis: The data collected were analyzed for analysis of variance (ANOVA) with computer based COSTAT package (CoHort Software, Berkeley, CA) software. Duncan’s New Multiple Range (DNMR) test performed at 5% (Duncan, 1955) to compare means. The F values (significant) were tested for mean differences by LSD tests at significance level(0.05%), by using MSTAT-C Computer Statistical Programme,(MSTAT Development Team, 1989).
RESULTS AND DISCUSSION
Chlorophyll a contents (mg g-1 leaf F. wt): According to Duncan’s Multiple Range Test (Table: 1), increasing intensity of metal stress by its escalating levels appeared to be responsible for gradual reduction in chlorophyll a contents. Index of variability in chlorophyll a revealed that chromium (III) application above the limit of 10mg kg-1 caused statistically marked reduction in the pigment concentration. Maximum effect in all the genotypes was predominantly observed by 60 mg kg-1. A deviation from the ongoing role of metal was noted when 10 mg kg-1 metal was supplemented to soil medium of MASH 88 and MASH 97 plants which exhibited an increase in chlorophyll a content by 17.156% and 9.295% respectively from the control. Among the genotypes, MASH 88 revealed maximum (0.645) and MASH ES-1 revealed minimum (0.439) values. MASH 97 differed from MASH 80 by a value of 6.837%.
Chlorophyll b Contents (mg g-1 leaf F. wt): Chromium (III) stress imposition induced a reduction in chlorophyll b content and established an inverse correlation between the two (Table: 2). A remarkable and statistically non significant effect of chromium (III) in decreasing pigment concentration was when supplied above 10mg kg-1 concentration. Maximum effect in all the genotypes was by 60 mg kg-1 but in MASH 88 was by 50mg kg-1.
Metal level of 10mg kg-1 in MASH 88 and MASH 97 provided an opposite index of its action and increased the chlorophyll b contents by 11.122% and 10.163% from untreated control plants. Among the genotypes, MASH 88 revealed maximum (0.553) and MASH 80 revealed minimum (0.590) values. Differences of 72.758% and 90.689% were statistically obvious for MASH 97 and MASH 88 respectively from MASH 80.
Total Carotenoids Contents (mg g-1 leaf F. wt): Higher concentrations of chromium (III) induced reduction in carotenoids contents corresponded to its levels of application (Table: 7). Carotenoids were affected significantly by chromium (III) concentrations of not less than 20mg kg-1. Maximum effect in all the genotypes was by 60mg kg-1.
Though irregularly, chromium (III) at lower level of its concentration, exhibited enhancement effects as 10mg kg-1 revealed an increase of 16.760 % and 10.606% for plants of MASH 88 and MASH 97 respectively. Similarly 20mg kg-1 chromium (III) increased the carotenoids contents of MASH 88 by 21.092%. Differences of 69.587% and 78.350% were statistically obvious for MASH 97 and MASH 88 respectively from MASH 80.Genotypes, MASH 88 revealed maximum (0.346) and MASH 80 revealed minimum (0.194) values for total carotenoids contents.
Nitrate Reductase (EC 1.6.6.1) Activity (NRA): From the data for mean values, it could be inferred that the chromium (III) supply impaired the activity of nitrate reductase for its reduction potential. The decrease in Nitrate Reductase Activity (NRA) established an inverse correlation with metal concentration (Table: 4). Nitrate Reductase Activity (NRA) value, when measured under the influence of more than 10mg kg-1 chromium (III), was found to be statistically lower than that of untreated control. Maximum effect (42.382%) was conceived by metal toxicity of 60mg kg-1.
A similar pattern of metal stress was extendable to all the genotypes. However, the application of 10 mg kg-1 chromium (III) to the plants of MASH 97 and MASH ES-1 stimulated the Nitrate Reductase Activity (NRA) by 1.433 % and 13.593% respectively. Different sensitivity range for chromium (III) was found in genotypes. Among the genotypes, MASH 88 revealed maximum (0.732) and MASH 80 revealed minimum (0.626) values.
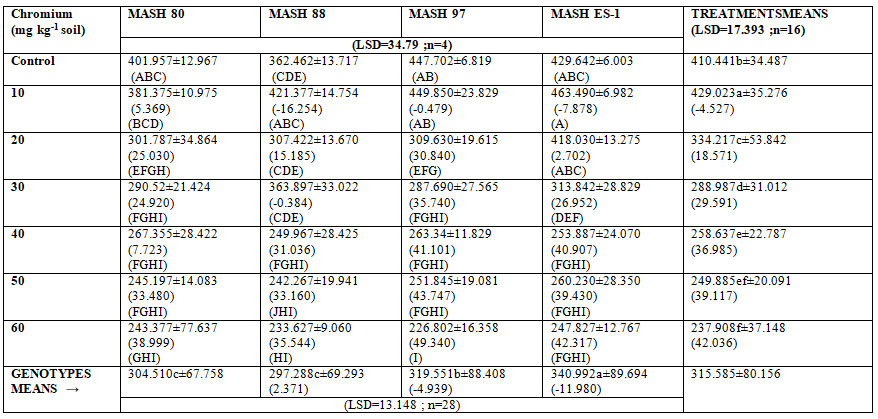
Leaf area index (LAI): From the data for mean values, it could be inferred that the chromium (III) supply reduced the leaf area index (Table: 8). The decrease in leaf area index (LAI) established an inverse correlation with metal concentration. Leaf area index (LAI) value, when measured under the influence of more than 10mg kg-1 chromium (III), was found to be statistically lower than that of untreated control. Maximum effect (42.036%) was conceived by metal toxicity of 60mg kg-1.
A similar pattern of metal stress was extendable to all the genotypes. However, the application of 10mg kg-1 chromium (III) stimulated the leaf area index (LAI) in all genotypes Different sensitivity range for chromium (III) was found in genotypes. Among the genotypes, MASH ES-1 revealed maximum (340.992) leaf area index (LAI) and MASH 88 revealed minimum (297.288) values.
Table 1: Chlorophyll a contents (mg g-1 leaf F. wt) of 45 days old mash (Vigna mungo (L.) Hepper) grown in chromium (III) supplemented soil (0, 10, 20, 30, 40 50 and 60mg/kg soil) (Values represent. means ± SE). These in parentheses represent the %age of increase (+)/decrease (-) over untreated of row#1 or over MASH 80 for genotypes means. These values followed by letters of dissimilar, which are different at P = 0.05 among means of treatments and genotypes (lower case letter) as well as among interactions (upper case letters).
| Chromium (mg kg-1 soil) |
MASH 80
|
MASH 88
|
MASH 97
|
MASH ES-1
|
TREATMENTSMEANS (LSD=0.044 ;n=16) |
| (LSD=0.088 ;n=4) | |||||
| Control
|
1.204±0.002 (A) | 1.055±0.054 (BC) | 0.979±0.031 (CD) | 0.995±0.085 (BC) | 1.058a±0.103 |
| 10
|
1.192±0.115 (0.996) (A) | 1.236±0.129 (-17.156) (A) | 1.070±0.053 (-9.295) (B) | 0.903±0.068 (9.246) (DE) | 1.100a±0.159 (-3.969) |
| 20 | 0.827±0.057 (31.312) (EF) | 0.992±0.089 (5.971) (BC) | 0.782±0.120 (20.127) (F) | 0.508±0.100 (48.944) (GH) | 0.777b±0.198 (26.559) |
| 30 | 0.436±0.061
(63.787) (H) |
0.586±0.027
(44.450) (G) |
0.449±0.090
(54.136) (H) |
0.342±0.035
(65.628) (I) |
0.453c±0.104
(57.183) |
| 40 | 0.241±0.016
(79.983) (JK) |
0.331±0.010
(68.625) (I) |
0.296±0.091
(69.765) (IJ) |
0.167±0.030
(83.216) (KL ) |
0.259d±0.077
(75.519) |
| 50 | 0.113±0.028
(90.614) (LM) |
0.161±0.02
(84.739) (KLM) |
0.153±0.040
(84.371) (LM) |
0.087±0.016
(91.250) (LM) |
0.129e±0.040
(87.807) |
| 60
|
0.078±0.013
(93.521) (M) |
0.157±0.032
(85.118) (KLM) |
0.084±0.004
(91.419) (M) |
0.075±0.009
(92.462) (M) |
0.098e±0.038
(90.737) |
| GENOTYPES
MEANS → |
0.585b±0.462 | 0.645a±0.427
(-10.256) |
0.545c±0.382
(6.837) |
0.439d±0.362
(24.957) |
0.553±0.412 |
| (LSD=0.033 ; n=28) | |||||
Our results revealed that chlorophyll concentration was decreased by chromium application in a concentration dependent manner (Table 1&2). A significant reduction of chlorophyll in chromium treated plant is reported by many researchers (Rai et al. 2014; Rajendran et al. 2019;Sinha et al. 2004;Balal et al. 2017;Islam et al. 2016;Zou et al. 2009; Amin et al. 2013;Tang et al. 2012;Amin et al. 2014). Chlorophyll reduction might be either due to inhibiting biosynthesis of chlorophyll (Lushchak, 2010; Sharma et al. 2019; Chandra and Kulshreshtha, 2004) or destruction of chlorophyll molecule in Cr treated plants (Valko et al. 2006).
Table 2. Chlorophyll b contents (mg g-1 leaf F. wt) of 45 days old mash (Vigna mungo (L.) Hepper) grown in chromium (III) supplemented soil (0, 10, 20, 30, 40 50 and 60mg/kg soil) (Values represent means ± SE). Values in parentheses are percentage to increase(+)/decrease(-) over untreated of row#1 or over MASH 80 for genotypes means. These followed by dissimilar letters if different at P=0.05 among means of treatments and genotypes (lower case letter) as well as among interactions (upper case letters).
| Chromium
(mg kg-1 soil) |
MASH 80
|
MASH 88
|
MASH 97
|
MASH ES-1
|
TREATMENTSMEANS (LSD=0.049 ;n=16) |
| (LSD=0.089 ;n=4) | |||||
| Control
|
0.639±0.043
(F) |
0.935±0.135
(BC) |
0.915±0.126
(C) |
0.860±0.170
(CD) |
0.837a±0.166 |
| 10
|
0.624±0.101
(2.347) (F) |
1.039±0.061
(-11.122) (A) |
1.008±0.050
(-10.163) (AB) |
0.830±0.063
(3.488) (D) |
0.875a±0.182
(-4.540) |
| 20 | 0.402±0.0289
(37.089) (H) |
0.896±0.082
(3.660) (CD) |
0.733±0.114
(19.890) (E) |
0.461±0.093
(46.395) (GH) |
0.623b±0.221
(25.567) |
| 30 | 0.205±0.031
(67.918) (JK) |
0.520±0.024
(44.385) (G) |
0.416±0.086
(54.535) (H) |
0.305±0.032
(64.534) (I) |
0.361c±0.129
(56.869) |
| 40 | 0.107±0.007
(83.255) (LM) |
0.290±0.009
(68.983) (IJ) |
0.269±0.087
(70.601) (IJ) |
0.142±0.028
(83.488) (KL) |
0.202d±0.091
(75.866) |
| 50 | 0.042±0.014
(93.427) (MN) |
0.134±0.022
(85.668) (KL) |
0.133±0.039
(85.464) (KL) |
0.063±0.023
(91.970) (LMN) |
0.093e±0.048
(88.888) |
| 60
|
0.012±0.013
(98.122) (N) |
0.060±0.029
(35.187) (LMN) |
0.032±0.014
(96.502) (MN) |
0.027±0.009
(96.860) (MN) |
0.033f±0.024
(96.057) |
| GENOTYPES
MEANS → |
0.290d±0.254 | 0.553a±0.387
(-90.689) |
0.501b±0.372
(-72.758) |
0.384 c±0.335
(-32.413) |
0.432±0.359 |
| (LSD=0.037 ; n=28) | |||||
Lowering in chlorophylls could occurs due to increase in activities of chlorophyllase enzyme and nutrients deficiency because of higher concentration of metal translocation toward shoots (Shakoor et al. 2014;Khan et al. 2016). On exposure of plants to Cr stress, chlorophyll contents are depleted and it could cause disruption of the chlorophyll biosynthesis of plants, (Chandra and Kulshreshtha, 2004). Cr reduced chlorophyll content by inhibiting activity of δ-aminolevulinic acid dehydratase (ALAD) enzyme which is involved in chlorophyll synthesis as reported by Hayat et al. (2012).
Metal stress reduced carotenoid content (Table 3), which might be attributed to activation of osmotic stress leading to biosynthesis of abscisic acid (ABA) in tissues by damaging the carotenoids by the NCED (9-cis epoxycarotenoid dioxygenase).Another possible reason for reduction in carotenoid content might be biosynthesis of anthocyanins. The anthocyanins are synthesized during stress and interfere with carotenoids (Burger and Edwards, 1996). The experimental results revealed, as a general trend, reduction in nitrate reductase activity by metal stress (Table 4). Inhibition of NRA by metal might be caused either by reduction of biosynthesis enzyme or by suppression of activity of existing enzyme.
Depolarization of NR thiol or SH groups by metal also been found to reduce enzyme activity, (Jones and Mhuimhneachain 1995). Reduced NRA may be attributed to decreased N content availability to plants, either due to shortage in soil or consumption by the plant itself, (Campbell 1999). Stress mediated decreased cytokinin levels might cause a reduction in nitrate reductase activity (Bueno et al 1994), or through phosphorus limitation (Gniazdowska and Rychter 2000). Another reason for NRA might be due to reduced chlorophyll content or reduced rate of photosynthesis (Rai et al 1992; Li et al. 2012; Zhang et al 2018).
Table 3. Total Carotenoids contents (mg g-1 leaf F. wt) of 45 days old mash (Vigna mungo (L.) Hepper) grown in chromium (III) supplemented soil (0, 10, 20, 30, 40 50 and 60mg/kg soil) (Values represent means ±SE). These values in parentheses represent % age increase (+) / decrease (-) over untreated of row#1 or over MASH 80 for genotypes means. These are followed with dissimilar letters, are different @ P =0.05 among means of treatments and genotypes (lower case letter) as well as among interactions (upper case letters).
| Chromium
(mg kg-1 soil) |
MASH 80
|
MASH 88
|
MASH 97
|
MASH ES-1
|
TREATMENTSMEANS (LSD=0.041 ;n=16) |
| (LSD=0.077 ;n=4) | |||||
| Control
|
0.432±0.57
(FG) |
0.531±0.119
(DE) |
0.594±0.017
(CD) |
0.773±0.164
(A) |
0.582a±0.159 |
| 10
|
0.403±0.102
(6.712) (G) |
0.672±0.044
(-16.760) (B) |
0.657±0.067
(-10.606) (BC) |
0.545±0.039
(29.495) (DE) |
0.569a±0.126
(2.233) |
| 20 | 0.197±0.033
(54.398) (I) |
0.643±0.076
(-21.092) (BC) |
0.480±0.059
(19.191) (EF) |
0.275±0.056
(64.424) (H) |
0.399 b±0.188
(31.443) |
| 30 | 0.130±0.023
(69.907) (IJ) |
0.285±0.052
(46.327) (H) |
0.298±0.062
(49.831) (H) |
0.177±0.024
(77.102) (I) |
0.222 c±0.083
(61.855) |
| 40 | 0.059±0.008
(86.342) (JK) |
0.186±0.007
(64.971) (I) |
0.180±0.068
(69.696) (I) |
0.072±0.018
(90.685) (JK) |
0.125d±0.069
(78.522) |
| 50 | 0.069±0.056
(84.027) (JK) |
0.079±0.014
(85.122) (JK) |
0.081±0.017
(86.363) (JK) |
0.036±0.010
(95.342) (K) |
0.066 e±0.033
(88.659) |
| 60
|
0.068±0.064
(84.259) (JK) |
0.027±0.017
(94.915) (K) |
0.016±0.005
(97.306) (K) |
0.019±0.004
(97.542) (K) |
0.032 e±0.037
(94.501) |
| GENOTYPES
MEANS → |
0.194 c±0.159 | 0.346 a±0.258
(-78.350) |
0.329 a±0.242
(-69.587) |
0.271 b±0.277
(-39.690) |
0.285±0.242 |
| (LSD=0.031 ; n=28) | |||||
It has been noted from the results of NR activities which depends on rate of photosynthesis or its products which are required by photosynthetically generated reductant (NADH) and free energy for functions (Raghuram and Sopory 1995). The experimental results revealed a gradual reduction in leaf area index with increasing concentration of metal (Table 5). Leaf area reduction can be due to growth inhibition in metal treated plants (Ouariti and Ghorbal, 1997). Leaf growth reduction might be the result of low water potential due to very negative solute potential in the soil solution (Hayward and Spurr, 1944).
Table 4. Nitrate Reductase (EC 1.6.6.1) Activity (NRA) of 45 days old mash (Vigna mungo (L.) Hepper) grown in chromium (III) supplemented soil (0, 10, 20, 30, 40 50 and 60mg/kg soil) (Values represent means±SE). These values in parentheses..represent %age increase(+)/decrease(-) over untreated of row#1 or over MASH 80 for genotypes means. These are followed with dissimilar letters, are different @ P =0.05 among means of treatments and genotypes (lower case letter) as well as among interactions (upper case letters).
| Chromium
(mg kg-1 soil) |
MASH 80
|
MASH 88
|
MASH 97
|
MASH ES-1
|
TREATMENTSMEANS (LSD=0.026 ;n=16) |
| (LSD=0.0444 ;n=4) | |||||
| Control
|
0.743±0.023
(E) |
0.963±0.039
(A) |
0.837±0.030
(D) |
0.951±0.037
(AB) |
0.873a±0.097 |
| 10
|
0.844±0.029
(-13.593) (D) |
0.902±0.026
(6.334) (C) |
0.849±0.036
(-1.433) (D) |
0.910±0.025
(4.3112) (BC) |
0.876a±0.040
(-0.343) |
| 20 | 0.609±0.012
(18.034) (JK) |
0.837±0.030
(13.084) (D) |
0.763±0.010
(8.841) (E) |
0.721±0.052
(24.185) (EF) |
0.733b±0.089
(16.036) |
| 30 | 0.583±0.013
(21.534) (KL) |
0.658±0.043
(31.671) (GHI) |
0.726±0.007
(13.261) (EF) |
0.616±0.014
(35.226) (IJK) |
0.646c±0.059
(26.002) |
| 40 | 0.551±0.014
(25.841) (LM) |
0.670±0.017
(30.425) (GH) |
0.692±0.024
(17.323) (FG) |
0.579±0.009
(42.481) (KL) |
0.623c±0.063
(28.636) |
| 50 | 0.522±0.002
(29.744) (MIN) |
0.634±0.018
(34.164) ( HIJ) |
0.640±0.011
(23.536) (HIJ) |
0.547±0.022
(43.112) (LM) |
0.586d±0.055
(32.875) |
| 60
|
0.533±0.027
(28.263) (MN) |
0.462±0.060
(52.024) (O) |
0.490±0.032
(42.457) (NO) |
0.528±0.135
(44.479) (MIN) |
0.503e±0.0753
(42.382) |
| GENOTYPES
MEANS → |
0.626c±0.115
(9.668) |
0.732a±0.168
(-5.627) |
0.714a±0.118
(-3.030) |
0.693b±0.171 | 0.691±0.149 |
| (LSD=0.020 ; n=28) | |||||
Reduced cytokinin contents by metal might be responsible for growth reduction by inhibition of cell division and cell elongation. The results of the experiment indicated that plants have potential to withstand up to 10 mg/kg soil chromium concentration as no toxicity has observed at this level of concentration. These effects of chromium may simply be due to the response raised by dose concentrations of the seedlings where the plant growth is stimulated under low dose while it suppressed under high doses (Shah et al. 2008). The absence of effect at low concentration could be attributed to the fact that low dose of Cr in roots than in shoot has restricted effect on the root, while higher in the shoots have also been observed, (Selvam and Wong, 2008).
Table 5: Leaf Area Index (LAI) of 45 days old mash (Vigna mungo (L.) Hepper) grown in chromium (III) supplemented soil (0, 10, 20, 30, 40 50 and 60mg/kg soil) (Values represent means ± SE). These values in parentheses..represent %age increase(+)/decrease(-) over untreated of row#1 or over MASH 80 for genotypes means. These are followed with dissimilar letters, are different @ P =0.05 among means of treatments and genotypes (lower case letter) as well as among interactions (upper case letters).
REFERENCES
Ali, S., Bharwana, S.A., Rizwan, M., Farid, M., Kanwal, S., Ali, Q., Ibrahim, M., Gill, R.A., Khan, M.D. (2015). Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ Sci Pollut Res 22:10601–10609.
Amin, H., Arain, B.A., Amin, F., Surhio, M.A. (2013). Phytotoxicity of chromium on germination, growth and biochemical attributes of Hibiscus esculentus L. Am J Plant Sci 4:720–726.
Amin, H., Arain, B.A., Amin, F., Surhio, M.A. (2014). Analysis of growth response and tolerance index of Glycine max (L.) Merr. under hexavalent chromium stress. Adv Life Sci 1:231–241.
Anjum, S.A., Ashraf, U., Khan, I., Tanveer, M., Shahid, M., Shakoor, A., Wang, L. (2017). Phyto-toxicity of chromium in maize: Oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere 27:262–273.
Appenroth, K.J., Luther, A., Jetschke, G., Gabrys, H. (2008). Modification of chromate toxicity by sulphate in duckweeds (Lemnaceae). Aquat Toxicol 89:167–171.
Arnon, D.I. (1949). Copper enzymes in isolated chloroplast. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24: 1-15.
Ashraf, A., Bibi, I., Niazi, N.K., Ok, Y.S., Murtaza, G., Shahid, M., Kunhikrishnan, A., Li, D., Mahmood, T. (2017). Chromium (VI) sorption efficiency of acid-activated banana peel over organo-montmorillonite in aqueous solutions. Int J Phytorem 19:605–613.
Balal, R.M., Shahid, M.A., Vincent, C., Zotarelli, L., Liu, G., Mattson, N.S., Rathinasabapathi, B., Martínez-Nicolas, J.J., Garcia-Sanchez, F. (2017). Kinnow mandarin plants grafted on tetraploid rootstocks are more tolerant to Cr-toxicity than those grafted on its diploids one. Enviro Exp Bot 140:8–18.
Bueno, M.S., Alonso, A., Villalobos, N. (1994). Nitrate reduction in cotyledons of Cicer arietinum L., regulatory role of cytokinins. Plant Sci 95: 117–124.
Burger, J., Edwards, G.E. (1996). Photosynthetic efficiency, and photodamage by UV and visible radiation, in red versus green leaf coleus varieties. Plant Cell Physiol 37: 395-399.
Campbell, W.H. (1999). Nitrate reductase structure, function and regulation: bridging the gap between biochemistry and physiology. Ann Rev Plant Physiol Plant Mol Biol 50: 277–303.
CERCLA (2019). Priority List of Hazardous Substances Agency for Toxic Substances and Disease Registry, USA. [(accessed on 20 September 2019)]; Available online: https://www.atsdr.cdc.gov/spl/
Cervantes, C., Campos-Garcia, J. (2007). Molecular Microbiology of Heavy Metals. Springer; Berlin/Heidelberg, Germany: Reduction and efflux of chromate by bacteria; pp. 407–419.
Cervantes, C., Campos-Garcia, J., Devars, S., Gutierrez-Corona, F., Loza-Tavera, H., Torres-Guzman, J.C., Moreno-Sanchez, R. (2001). Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev 25:335–347.
Chandra, P., Kulshreshtha, K. (2004). Chromium accumulation and toxicity in aquatic vascular plants. Bot Rev 70:313–327.
De Oliveira, L.M., Gress, J., De, J., Rathinasabapathi, B., Marchi, G., Chen, Y., Ma, L.Q. (2016). Sulfate and chromate increased each other’s uptake and translocation in As-hyperaccumulator Pteris vittata. Chemosphere 147:36–43.
De Oliveira, L.M., Ma, L.Q., Santos, J.A., Guilherme, L.R., Lessl, J.T. (2014). Effects of arsenate, chromate, and sulfate on arsenic and chromium uptake and translocation by arsenic hyperaccumulator Pteris vittata L. Environ Pollut 184:187–192.
Duncan, D. B. (1955). Multiple Range and Multiple F-Test. Biometrics 11: 1-42.
Eleftheriou, E., Adamakis, I.D., Panteris, E., Fatsiou, M. (2015). Chromium-induced ultrastructural changes and oxidative stress in roots of Arabidopsis thaliana. Int J Mol Sci 16:15852–15871.
Farooq, M., Ali, S., Hameed, A., Bharwana, S., Rizwan, M., Ishaque, W., Farid, M., Mahmood, K., Iqbal, Z. (2016). Cadmium stress in cotton seedlings: Physiological, photosynthesis and oxidative damages alleviated by glycinebetaine. S. Afr J Bot 104:61–68.
Gniazdowska, A., Rychter, A.M. (2000). Nitrate uptake by bean (Phaseolus vulgaris L.) roots under phosphate deficiency. Plant Soil 226: 79–85.
Hayat, S., Khalique, G., Irfan, M., Wani, A.S., Tripathi, B.N., Ahmad, A. (2012). Physiological changes induced by chromium stress in plants: An overview. Protoplasma 249:599–611.
Hayward, H.E., Spurr, W.B. (1944). Effect of isosmotic concentrations of inorganic and organic substrates on entry of water into corn roots. Bot Gaz 106: 131-139.
Islam, F., Yasmeen, T., Arif, M.S., Riaz, M., Shahzad, S.M., Imran, Q., Ali, I. (2016). Combined ability of chromium (Cr) tolerant plant growth promoting bacteria (PGPB) and salicylic acid (SA) in attenuation of chromium stress in maize plants. Plant Physiol Biochem 108:456–467.
Jackson, M.L. (1962). Soil Chemical analysis. Constable and company, Ltd. England.
James, A.D. (1981). Legumes in United States. Department of Agriculture, Beltsville, Maryland Plenum press New York.
Jones, P., Mhuimhneachain, M.N. (1995). The activity and stability of wheat nitrate reductase in vitro. Phytochem 24: 385-392.
Joutey, N.T., Sayel, H., Bahafid, W., El Ghachtouli, N. (2015). Reviews of Environmental Contamination and Toxicology. Volume 233. Springer; Berlin/Heidelberg, Germany: 2015. Mechanisms of hexavalent chromium resistance and removal by microorganisms. pp. 45–69.
Kabata-Pendias, A., Szteke, B. (2015). Trace Elements in Abiotic and Biotic Environments. CRC Press; Boca Raton, FL, USA:
Kamran, M., Eqani, S., Katsoyiannis, A., Xu, R., Bibi, S., Benizri, E., Chaudhary, H. (2017). Phytoextraction of chromium (Cr) and influence of Pseudomonas putida on Eruca sativa growth. J Geochem Explor 182:269–274.
Kaur, R., Yadav, P., Sharma, A., Thukral, A.K., Kumar, V., Kohli, S.K., Bhardwaj, R. (2017). Castasterone and citric acid treatment restores photosynthetic attributes in Brassica juncea L. under Cd (II) toxicity. Ecotoxicol Environ Saf 145:466–475.
Kaur, R., Yadav, P., Thukral, A.K., Sharma, A., Bhardwaj, R., Alyemeni, M.N., Wijaya, L., Ahmad, P. (2018). Castasterone and citric acid supplementation alleviates cadmium toxicity by modifying antioxidants and organic acids in Brassica juncea. J Plant Growth Regul 37:286–299.
Khan, I., Iqbal, M., Ashraf, M.Y., Ashraf, M., Ali, S. (2016). Organic chelants-mediated enhanced lead (Pb) uptake and accumulation is associated with higher activity of enzymatic antioxidants in spinach (Spinacea oleracea L.). J Hazard Mater 317:352–361.
Khanna, K., Jamwal, V.L., Sharma, A., Gandhi, S.G., Ohri, P., Bhardwaj, R., Al-Huqail, A.A., Siddiqui, M.H., Ali, H.M., Ahmad, P. (2019). Supplementation with plant growth promoting rhizobacteria (PGPR) alleviates cadmium toxicity in Solanum lycopersicum by modulating the expression of secondary metabolites. Chemosphere 230:628–639.
Kohli, S.K., Handa, N., Sharma, A., Gautam, V., Arora, S., Bhardwaj, R., Alyemeni, M.N., Wijaya, L., Ahmad, P. (2018). Combined effect of 24-Epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma 255:11–24.
Kumar, V., Parihar, R.D., Sharma, A., Bakshi, P., Sidhu, G.P.S., Bali, A.S., Karaouzas, I., Bhardwaj, R., Thukral, A.K., Gyasi-Agyei, Y. (2019). Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 236:124364.
Kumar, V., Sharma, A., Kaur, P., Sidhu, G.P.S., Bali, A.S., Bhardwaj, R., Thukral, A.K., Cerda, A. (2019). Pollution assessment of heavy metals in soils of India and ecological risk assessment: A state-of-the-art. Chemosphere 216:449–462.
Kumari, V., Yadav, A., Haq, I., Kumar, S., Bharagava, R.N., Singh, S.K., Raj, A. (2016). Genotoxicity evaluation of tannery effluent treated with newly isolated hexavalent chromium reducing Bacillus cereus. J Environ Manag 183:204–211.
Li, Y.C., Liang, L.L., Wang, Q.C. (2012). Influence of Pb on photosynthesis and chlorophyll fluorescence characteristics in Pyrus ussuriensis and Malus baccata. J Northwest Forest Univ 27: 21–25.
Lichtenthaler, H.K. (1987). Chlorophyll and carotenoids; Pigments of photosynthetic biomembranes. Methods in Enzymol 148: 350 – 385.
Lushchak, V.I. (2011). Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol 153:175–190.
Ouariti, H. , Ghorbal, M.H. (1997). Responses of bean and tomato plants to cadmium, growth, mineral nutrition and nitrate reduction. Plant Physiol Biochem 35: 347 354.
Prado, C., Chocobar, Ponce, S., Pagano, E., Prado, F.E., Rosa, M. (2016). Differential physiological responses of two Salvinia species to hexavalent chromium at a glance. Aquat Toxicol 175:213–221.
Puttaswamy, S.S., Timmagowda, S., Krishnamurthy, K. (1976). Determination of leaf area in pulses. Current Research 5: 47
Raghuram, N., Sopory, S.K. (1995). Light regulation of nitrate reductase gene expression mechanism and signal response coupling. Physiol Mol Biol Plants 1: 103–104.
Rai, V., Tandon, P.K., Khatoon, S. (2014). Effect of chromium on antioxidant potential of Catharanthus roseus varieties and production of their anticancer alkaloids: Vincristine and vinblastine. BioMed Res Int pp:1–10.
Rai, U.N., Tripathi, R.D., Kumar, K. (1992). Bioaccumulation of chromium and toxicity on growth, photosynthetic pigments, photosynthesis in vivo nitrate reductase activity and protein content in a chlorococcalean green alga Glaucocystis ostochinearum. Chemosphere 25, 721–732.
Rajendran, M., An, W.H., Li, W.C., Perumal, V., Wu, C., Sahi, S.V., Sarkar, S.K. (2019). Chromium detoxification mechanism induced growth and antioxidant responses in vetiver (Chrysopogon zizanioides (L.) Roberty). J Cent South Univ 26:489–500.
Reale, L., Ferranti, F., Mantilacci, S., Corboli, M., Aversa, S., Landucci, F., Baldisserotto, C., Ferroni, L., Pancaldi, S., Venanzoni, R. (2016). Cyto-histological and morpho-physiological responses of common duckweed (Lemna minor L.) to chromium. Chemosphere 145:98–105.
Richards, L.A. (1954). Diagnosis and improvements of saline and alkali soils.USDA Hand book No 60, US Govt. Printing Office, Washington, DC. p, 160.
Santos, C., Rodriguez, E. Botany. (2012). In-Tech; Rijeka, Croatia Review on some emerging endpoints of chromium (VI) and lead phytotoxicity; pp. 61–82.
Selvam, A., Wong, J.W.C. (2008). Phytochelation and synthesis and Cadmium uptake by Brassica napus. Environ Technol 29:765-773.
Sen, S., (1996). Economic Botany. New Central Book Agency (Pvt.) Ltd. Calcutta, India. pp, 42-43.
Shah, F.R., Ahmad, N., Masood, K.R., Zahid, D.M., (2008). The influence of Cd and Cr on the biomass production of Shisham (Dalbergia sissoo Roxb.) seedlings. Pak J Bot 40:1341-1348.
Shahid, M., Shamshad, S., Rafiq, M., Khalid, S., Bibi, I., Niazi, N.K., Dumat, C., Rashid, M.I. (2017). Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 178:513–533.
Shakoor, M.B., Ali, S., Hameed, A., Farid, M., Hussain, S., Yasmeen, T., Najeeb, U., Bharwana, S.A., Abbasi, G.H. (2014). Citric acid improves lead (Pb) phytoextraction in Brassica napus L. by mitigating Pb-induced morphological and biochemical damages. Ecotoxicol Environ Safety 109:38–47.
Shanker, A.K., Cervantes, C., Loza-Tavera, H., Avudainayagam, S. (2005). Chromium toxicity in plants. Environ Int 31:739–753.
Sharma, A., Kumar, V., Shahzad, B., Ramakrishnan, M., Sidhu, G.P.S., Bali, A.S., Handa, N., Kapoor, D., Yadav, P., Khanna, K. (2019). Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J Plant Growth Regul 38:1–23.
Sharma, A., Kapoor, D., Wang, J., Shahzad, B., Kumar, V., Bali, AD., Jasrotia, S., B.,
Sinha, S., Saxena, R., Singh, S. (2005). Chromium induced lipid peroxidation in the plants of Pistia stratiotes L.: Role of antioxidants and antioxidant enzymes. Chemosphere 58:595–604.
Stambulska, U.Y., Bayliak, M.M., Lushchak, V.I. (2018). Chromium (VI) toxicity in legume plants: Modulation effects of rhizobial symbiosis. BioMed Res Int pp:1–13.
Stoeva, N., Bineva, T. (2003). Oxidative changes and photosynthesis in oat plants grown in contaminated soil. Bulg J Plant Physiol 29: 87–95.
Sym, G.J. (1983). Optimization of the in vivo assay conditions for nitrate reductase in barley. J Sci Food Agric 35: 725-730.
Tang, J., Xu, J., Wu, Y., Li, Y., Tang, Q. (2012). Effects of high concentration of chromium stress on physiological and biochemical characters and accumulation of chromium in tea plant (Camellia sinensis. L.) Afr J Biotechnol 11:2248–2255.
Tchounwou, P.B., Yedjou, C.G., Patlolla, A.K., Sutton, D.J. (2012). Molecular, Clinical and Environmental Toxicology. Springer; Berlin/Heidelberg, Germany: Heavy metal toxicity and the environment; pp. 133–164.
UdDin, I., Bano, A., Masood, S. (2015). Chromium toxicity tolerance of Solanum nigrum L. and Parthenium hysterophorus L. plants with reference to ion pattern, antioxidation activity and root exudation. Ecotoxicol Environ Saf 113:271–278.
Valko, M., Rhodes, C., Moncol, J., Izakovic, M., Mazur, M. (2006). Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40.
Yuan, H., Yan, D. (2020). Chromium Bioaccumulation and Its Impacts on Plants: An Overview. Plants (Basel) 9: 100
Zou, J., Yu, K., Zhang, Z., Jiang, W., Liu, D. (2009). Antioxidant response system and chlorophyll fluorescence in chromium (VI) treated Zea mays L. seedlings. Acta Biol Crac Ser Bot 51:23–33.


