Department of Microbiology, Government Institute of Science, Aurangabad
Article Publishing History
Received: 09/11/2016
Accepted After Revision: 21/12/2016
Cotton stalk is one of the abundant feedstock and has been selected for producing ethanol at economically feasible manner. In the present investigation a comparative account of ethanol production has been developed from acid and enzymatically hydrolyzed cotton stalks. For this cotton stalk was subjected to series of treatment including acid hydrolysis followed by detoxification in one set; and alkaline pretreatment followed by enzyme hydrolysis in second set. The sugars released during acid and enzyme hydrolysis was obtained as 11g/L and 24.5 g/L respectively. Both the sets were separately fermented for ethanol production. During fermentation, test organisms in association utilized 93.84% and 97.81% of total available sugars and produced an ethanol concentration of 4.96 g/L and 9.56 g/L with corresponding yield of 0.179 g/g and 0.191 g/g of biomass (native cotton stalk) respectively.
Fermentation, Bioethanol, Cotton Stalk, Saccharomyces Cerevisiae, Pachysolen Tannophilus
Baig M. Z, Smita M. D. Comparative Study of Bioethanol Production From Acid and Enzymatically Hydrolyzed Cotton Stalk Using Co Culture of Saccharomyces Cerevisiae and Pachysolen Tannophilus. Biosc.Biotech.Res.Comm. 2016;9(4).
Baig M. Z, Smita M. D. Comparative Study of Bioethanol Production From Acid and Enzymatically Hydrolyzed Cotton Stalk Using Co Culture of Saccharomyces Cerevisiae and Pachysolen Tannophilus. Biosc.Biotech.Res.Comm. 2016;9(4). Available from: https://bit.ly/2KUgIiO
INTRODUCTION
The increasing need for ethanol as energy source has stimulated worldwide investigations in search of cheaper substrate for bulk ethanol production. As a substrate, conventional crop such as corn and sugarcane are unable to meet the global demand of bioethanol production due to their primary value of food and feed therefore, lignocellulosic substance such as agricultural wastes are attractive feedstock for bioethanol production (Behera
et al., 2010). In the present investigation cotton stalk was used as substrate. According to United State Department of Agriculture (USDA), India is expected to emerge as largest cotton producer in the world, estimated cotton area in country in 2015-16 is 11.26 million hectors and cotton production is estimated as 6.3 million metric tons. The lignocellulosic nature and potential availability of cotton stalk open its way as renewable raw material for various commercial applications including ethanol production (Kaur et al., 2012). Prior to ethanol fermentation by organisms, the feedstock needs to be process by scarification technology in order to retain fermentable sugars. Acid hydrolysis is simple and easy method to perform and is prominently used for depolymerization of biomass into fermentable sugar. Acid hydrolysis was carried out in two stages including concentrated acid decrystallization followed by dilute acid hydrolysis with steam and heat treatment (Liao et al., 2006). It is the most widely used method for saccharification of lignocellulosic material, due to its relatively low cost, ease of use and high efficiency. The important drawback of this treatment is the formation of toxic compound (furfural and hydroxymethyl furfural) released during hydrolysis. These inhibitors decrease the fermentation yield by retarding microbial activity, which must be removed by applying proper detoxification process (Chandel et al., 2007).
Another method is alkaline pretreatment and enzymatic hydrolysis of lignocellulosic biomass. The major effect of alkali pretreatment is the saponification of intermolecular ester bonds which crosslink lignin and carbohydrates, thus increasing porosity and internal surface of the biomass matrix as well as decreasing the degree of crystallinity of cellulose, resulting in improved susceptibility of remaining polysaccharides to enzyme attach during hydrolysis (Sun and Cheng, 2002). Alkaline pretreatment process utilizes lower temperature and pressure compare to other pretreatment technologies (Balat et al., 2008). However, unlike acid pretreatment, it is much more time consuming and some of the alkali is converted to irrecoverable salt or incorporated as salt into the biomass by the pretreatment reaction (Mosier et al., 2005).
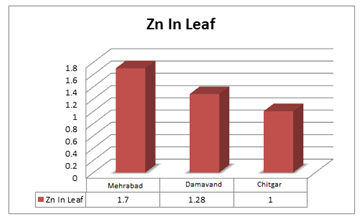 |
Figure 1: Comparative analysis of ethanol concentration obtained from acid and enzymatically hydrolyzed cotton stalk using co culture of Saccharomyces cerevisiae and Pachysolen tannophilus. |
Enzyme hydrolysis is another method of degrading pretreated cellulose to mono sugars with the help of complex of enzyme known as cellulases. Cellulasee is described in terms of three major classes. The endoglucanases (EC 3.2.1.4, EG) act randomly on soluble and insoluble cellulose chain. The exoglucanases, which include cellobiohydrolases (EC 3.2.1.91, CBHs), acts processively to preferentially liberate cellobiose (and glucose in some cases) from the reducing and non-reducing ends of the cellulose chain. The â-glucosidase (EC 3.2.1.21) liberates D-glucose from cellobiose and exoglucosidases. Among the studied microorganism, fungi are most active against natural polymers, being capable of producing different amounts of each type of cellulases, which act synergistically. Almost all commercial cellulases obtained by submerged fermentation are produced by the fungi Trichoderma, Humicola, Aspergillus and Penicillium (Sohail et al., 2009; Tolan and Foody, 1999).
The present study is the extension of our previous work carried out to produce ethanol from acid and enzymatically hydrolyzed cotton stalk using co culture of Saccharomyces cerevisiae (hexose fermenting yeast) and Pachysolen tannophilus (pentose fermenting yeast). In this study a comparison has been made to focus on pros and cons of each saccharification and fermentation process based on sugar and ethanol yield respectively.
MATERIALS AND METHODS
Biomass
The cotton stalks was collected from the farmers field and were shredded, sundried, debarked, bailed and ground to 1mm particle size. It contains approximately 42.40% glucan and 23.20% xylan (carbohydrate content was determined by the method of Laboratory Analytical Procedure (LAP # 002) of National Renewable Energy Laboratory (NREL) using HPLC, Zodiac. Ltd). Klason lignin was found to be 24.18%, determined by method adopted by Teramoto et al., (2008).
Yeast Cultures
The cultures of Saccharomyces cerevisiae MTCC 36 and Pachysolen tannophilus MTCC 1077 were procured from Microbial Type Culture Collection, IMTECH-Chandigarh, India.
Saccharification Process
In this process two separate sets of biomass were prepared for hydrolysis, one set was hydrolyzed by using acid and another set was hydrolyzed by using the enzyme.
Acid Hydrolysis
Cotton stalk was subjected to dual stage sulfuric acid treatment. During its first stage 75% H2SO4 was used to decrystallize the biomass under specific sample acid ratio of 1:2 (by weight) followed by diluting this decrystallized biomass to make it 1N in second stage, then employing steam under pressure at 121oC in an autoclave for 30 minutes and four hour heat treatment at 90oC in water bath respectively (Baig, 2014). The obtained acid hydrolysate was detoxified by addition of dried lime up to pH 10 for an hour and then filtered and readjusted of pH up to 6 with acid. The obtained over limed hydrolysate was treated with 4% (w/v) charcoal treatment for half an hour with stirring and then filtered (Baig and Dharmadhikari, 2014). The obtained filtrate solution was used as sole carbon source for fermentation.
Enzyme Hydrolysis
Alkaline pretreatment and enzymatic hydrolysis was carried out in second set of experiment. In this regard, 2.0 % (w/v) concentration of alkaline solution has been prepared from NaOH pellets (Qualigens. Ltd) in aqueous medium. 5 gram of cotton stalk powder was treated with alkaline solution at a substrate loading of 10% (w/v). The flask was steam treated at 121oC for 60 minutes. After steam treatment, the biomass has been separated from lignified liquor by centrifugation at 10000 rpm for 10 minutes and supernatant (black liquor) was separately collected for quantitative detection of lignin content. The delignified biomass was repeatedly washed with distilled water till to become neutral pH and dried in hot air oven at 60oC till constant weight. Enzymatic hydrolysis of pretreated biomass was carried out using commercial cellulases purchased from Sisco Research Laboratories Pvt. Ltd. Mumbai, India. Pre-treated cotton stalk was incubated with 5% solid loading in 50mM acetate buffer (pH 4.8) with 100 CMC (carboxymethyl cellulose) unit of enzyme per gram of biomass and was incubated at 50oC with 150 rpm for 72 hours (Baig and Dharmadhikari, 2012). After incubation, the sample was centrifuged in chilled condition at 5000 rpm for 10 minutes and supernatant was collected as sugar solution for fermentation process.
Fermentation Studies
The sugar solutions obtained from both the hydrolysate were separately fermented for analyzing the potential of bioethanol production and develop a comparative account in between them. The reliability of results was checked statistically by passing through ANOVA (analysis of variance).
Inoculum Development
The culture maintained on Yeast and Malt Extract Agar (YM medium: 0.3% yeast extract, 0.3% malt extract, 0.5% peptone and 1% glucose, pH 6.5). Cell mass required for inoculum development was obtained by growing each culture separately on YM medium in Erlenmeyer flask aerobically at 30 ºC on rotary shaker incubator with 150 rpm for 48 h. After incubation, completely activated yeast cells were harvested by centrifugation at 4000 rpm at 4ºC for 10 min, repeatedly washed with distilled water and used as cell mass for inoculum development. Inoculum was prepared in cotton stalk hydrolysate, supplemented with 0.5 % yeast extract, 1% peptone and pH was adjusted to 5.5 %. The yeast cells, harvested by centrifugation were added in inoculum and incubated on rotary shaker incubator with 150 rpm at 30 ºC for 24 h and grown aerobically to promote healthy growth of yeast cells in hydrolysate and used as inoculum for fermentation studies. Quantification of cell mass was perform by spread-plated method to ensure that each time the inoculation stayed at approximately 6.0 × 107 cfu/mL corresponding to 10 g dry w/L (Yadav et al., 2011).
Fermentation
The obtained hydrolysate was supplemented with 0.1% yeast extract, peptone, NH4Cl, KH2PO4 and 0.05% of MgSO4.7H2O, MnSO4, CaCl2.2H2O, FeCl3.2H2O and ZnSO4 in 250 mL flasks, adjusting the pH 5.5 and autoclaved at 110 ºC for 20 min (Pasha et al., 2007). Fermentation performed in semi aerobic mode of aeration (250 mL Erlenmeyer flask containing 150 mL of fermentation medium), and was initiated by transferring separately developed 10 % (v/v) co culture inoculum. Proportion of Saccharomyces cerevisiae and Pachysolen tannophilus in each inoculum was in the ratio of 60:40 respectively. Flasks were sealed with aluminum foil and were allowed to agitate with 120 rpm for first 24 hours and then kept in static mode at 30oC for 72 hours. Samples were withdrawn at every 12 hours interval from separate flask for the estimation of product formation, substrate utilization and growth of cell mass (Baig, 2014).
Analytical Methods
Sample obtained during fermentation was transferred to pre weighted centrifuged tube and was centrifuged at 10000 rpm for 10 min at 4 ºC. The supernatant was collected and analyzed for concentration of ethanol and residual sugars in broth while pellet was repeatedly washed with distilled water and dried in hot air oven at 60 ºC till constant weight. The difference between initial and final weight was recorded as cell biomass and expressed in g/L (Oberoi et al., 2010). The DNSA method of Miller, (1959) was adopted to quantify the amount of reducing sugars. Glucose oxidase method was used for glucose estimation (Bergmeyer et al., 1974). Total content of phenolic was determined by Folin-Ciocalteus (FC) method (Singleton and Rossi, 1965). Furans were estimated by Martinez et al., (2000). Ethanol estimation was carried out by Gas Chromatography (Shimadzu Japan). GC was carried out according to NREL procedure LAP # 011, using ZB-Wax column (30mm × 0.25mm) with Flame Ionization Detector (FID). Cell density was measured turbidometrically at 600 nm by using UV-VIS spectrophotometer.
Fermentation Efficiency
Fermentation efficiency was calculated as
Practical yield of ethanol
Fermentation efficiency = x 100
Theoretical yield of ethanol
Theoretical yield is 0.511 gram per gram of sugar consumed.
RESULTS AND DISCUSSION
Acid Hydrolysis
The hydrolysis process yielded maximum fermentable sugar and specifically D-glucose of 0.49 g/g and 0.36 g/g of biomass (native cotton stalk) respectively (Baig, 2014). The obtained results were in agreement with those of Liao et al., (2006). Byproducts of hydrolysis such as furans and phenolics were also formed with a concentration of 1.971 mg/L and 4.909 g/L respectively. To overcome these inhibitors, detoxification with over liming followed by charcoal treatment was applied on hydrolysate. It gives maximum reduction in inhibitors including 92.69% furans and 88.89% phenolics while 19.84% sugar losses were also reported during process (Baig and Dharmadhikari, 2014). The detoxified hydrolysate achieved having sugar concentration of 11 g/L, corresponds to a yield of 0.396 g/g of biomass was then exposed to fermentation for ethanol production.
| Table 1: Comparative performance of sugar yield and ethanol fermentation obtained from acid and enzymatically hydrolyzed cotton stalk by co-culture of Saccharomyces cerevisiae and Pachysolen tannophilus | ||
| Comparative account | Acid hydrolysate | Enzyme hydrolysate |
| Initial sugar conc. (g/L) | 11.00 | 24.50 |
| Initial sugar yield (g/g of biomass)
Ethanol concentration (g/L) |
0.396
04.96 |
0.490
09.56 |
| Ethanol yield (g/g of biomass) | 0.179 | 0.191 |
| Ethanol yield (g/g of holocelluloses) | 0.278 | 0.298 |
| Ethanol yield (g/g of fermentable sugar) | 0.446 | 0.392 |
| Fermentation efficiency (%) | 87.52 | 76.85 |
| Sugar consumed (%) | 93.84 | 97.81 |
| Cell mass concentration (g/L) | 08.06 | 12.20 |
Enzyme Hydrolysis
The second set comprised of alkaline pretreatment and enzymatic hydrolysis. Upon alkaline pretreatment, lignin extraction from debarked cotton stalk was significantly achieved up to 80% (0.201 gram of lignin per gram of biomass). Following pretreatment of cotton stalk, the delignified solid residue was enzymatically hydrolyzed via 100 CMC units of enzyme at substrate loading of 10% (w/v); yielded total sugar of 0.49 g/g of biomass, corresponds to a concentration of 24.5 g/L; as was optimized in previous studies (Baig and Dharmadhikari, 2012). Similar findings were also reported from Silverstein et al., (2007).
Comparative Account Of Ethanol Production From Acid And Enzyme Hydrolysate
The sugar concentration obtained after acid and enzyme hydrolysis of cotton stalk was 11 g/L and 24.5 g/L respectively, which were kept constant in fermentation broth and fermented separately. As the fermentation started, during first 6 hours of inoculum addition no ethanol production could be detected in both the sets, while it commenced from 12 hours onwards and steadily increased up to 48 hours. It was found maximum at this stage, where co-culture of Saccharomyces cerevisiae and Pachysolen tannophilus in association utilized 93.84% from acid hydrolysate and 97.81% from enzyme hydrolysate of total available sugars and produced ethanol of 4.96 g/L and 9.56 g/L in acid and enzyme hydrolysate respectively.
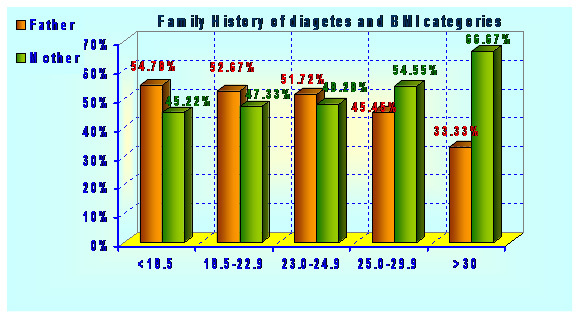 |
Figure 2: Comparative analysis of ethanol yield calculated from total biomass, holocelluloses and fermentable sugar available for fermentation respectively |
The obtained yield from the fermentation containing acid hydrolysate was recorded as 0.179 g/g of biomass (native cotton stalk), 0.278 g/g of holocelluloses and 0.446 g/g of sugar available for fermentation. While using enzyme hydrolysate; it was recorded as 0.191 g/g of biomass (native cotton stalk), 0.298 g/g of holocelluloses and 0.392 g/g of sugar available for fermentation. The efficiency of fermentation containing acid hydrolysate as carbon source was recorded as 87.52% while with enzyme hydrolysate it shows 76.85%.
Comparative analysis showed that yield calculated from total biomass (native cotton stalk) and holocelluloses found higher in fermentation of enzyme hydrolysate as compare to acid hydrolysate. In contrast to that, Fermentation efficiency and ethanol yield obtained from available sugar for fermentation is significantly higher in acid hydrolysate as compare to enzyme hydrolysate. This might be due to high sugar concentration of enzymatically treated biomass compare to acid hydrolysate, and traces of inhibitors (i.e. furans and phenolics) still present in acid hydrolysate (even after detoxification), which favors respiration mode (increased in cell mass concentration) in enzyme hydrolysate over fermentation (ethanol production).
In both the cases, sugars were effectively consumed by yeast cultures but consumption rate was slightly higher in enzyme hydrolysate as compared to acid hydrolysate, the possible reason might be the presence of traces of inhibitors even after detoxification. Such inhibitors were not observed in enzyme hydrolysis; as it separately delignified by alkaline treatment and no harsh condition developed during hydrolysis as was in acid hydrolysis, as discussed earlier. As for sugar consumption pattern is concern, no diauxy was observed in both the cases, as both contained same types of sugar molecules. Simultaneously cell mass concentration was also increased up to 36 hours of incubation and after that no significant change was observed. It was found greater in enzyme hydrolysate (12.20 g/L) compare to acid hydrolysate (8.06 g/L), as sugar concentration was found to be higher in fermentation of enzyme treated cotton stalk. Our results are harmony with results reported earlier by Gupta et al., (2009), who reported that fermentation of both acid and enzymatic hydrolysates of prosopis juliflora, containing 18.24 g/L and 37.47 g/L sugars, with Pichia stipitis and Saccharomyces cerevisiae produced 7.13 g/L and 18.52 g/L of ethanol with corresponding yield of 0.39 g/g and 0.49 g/g, respectively.
CONCLUSION
This study could establish a successful comparison in between acid and enzyme hydrolysis of cotton stalk in order to achieve maximum sugar and ethanol yield. Fermentation of enzyme hydrolysate was found to dominate over acid hydrolysate. The difference in the ethanol yield is due to initial sugar concentration, which influenced the fermentation efficiency. The fermentation process gave maximum theoretical yield, but pretreatment and saccharification process needs scientific efforts to make it more feasible and cost effective.
REFERENCES
Baig, M.Z., (2014). Studies on production of bioethanol from cotton stalk (Ph.D. thesis), Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, India.
Baig, M.Z., Dharmadhikari S. M., (2012). Optimization of pre-treatment and enzymatic hydrolysis of cotton stalk. J of Pure Appl Microbiol. 6(03): 1437-1441.
Baig, M.Z., Dharmadhikari, S.M., (2014). Optimization of detoxification with over liming and charcoal treatment for increasing the fermentability of cotton stalk hydrolysate. Ind J of Appl Res. 4(07): 08-10.
Balat, M., Balat, H., Oz, C., (2008). Progress in bioethanol processing. Prog Energ Combust. 34: 551-573.
Baptista, C.M.S.G., Colas, J.M.A., Oliveira, A.C.M., Oliveira, N.M.C., Roche, J.M.C., Dempsey, M.J., Lannigan, K.C., Benson, P.S., (2006). Natural immobilization of microorganism for continuous ethanol production. Enzyme Microb Technol. 40: 127-131.
Behera, S., Kar, S., Mohanty, R.C., Ray, R.C., (2010). Comparative study of bioethanol production from mahula (Madhuca latifolia L.) flowers by Saccharomyces cerevisiae cells immobilized in agar agar and Ca-alginate matrices. Appl Energy. 87:96-100.
Bregmeyer, H.U., Gawehn, K., Grassl, M., (1974). Methods of enzymatic analysis (Bregmeyer, H.U., ed) academic press Inc., New York. 1(2): 457-458.
Chandel, A.K., Kapoor, R.K., Singh, A., Kuhad, R.C., (2007). Detoxification of sugarcane bagasse hydrolyzate improves ethanol production by Candida shehatae NCIM 3501. Bioresour Technol. 98: 1947-1950.
Gupta, R., Sharma, K.K., Kuhad, R.C., (2009). Separate hydrolysis and fermentation (SHF) of Prosopis juliflora, a woody substrate, for the production of cellulosic ethanol by Saccharomyces cerevisiae and Pichia stipitis-NCIM 3498. Bioresour Technol. 100: 1214-1220.
Kaur, U., Oberoi, H.S., Bhargav, V.K., Sharma-Shivappa, R.R., Dhaliwal, S.S. Ethanol production from alkali and ozone treated cotton stalk using thermo tolerant Pichia kudriavzevii HOP-1. Ind. Crop. Prod., 2012; 37: 219-226.
Liao, W., Liu, Y., Liu, C., Wen, Z., Chen, S., (2006). Acid hydrolysis of fiber from dairy manure. Bioresour Technol. 97: 1687-1695.
Martinez, A., Rodriguez, M.E., York, S.W., Preston, J.E., Ingram, L.O., (2000). Effect of Ca(OH)2 treatments (“overliming”) on the composition and toxicity and bagasse of hemicellulose hydrolysate. Biotechnol Bioeng. 69: 526-536.
Miller, G.L., (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal chem. 31: 426-428.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y.Y., Holtzapple, M., Ladish, M., (2005). Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol. 96 (6): 673-686.
Oberoi, H.S., Vadlani, P.V., Madl, R.L., Saida, L. Abeykoon. J.P., (2010). Ethanol production from orange peels: two-stage hydrolysis and fermentation studies using optimized parameters through experimental design. J Agric Food Chem. 58: 3422-3429.
Pasha, C., Kuhad, R.C., Rao, L.V., (2007). Strain improvement of thermo tolerant Saccharomyces cerevisiae VS3 strain for better utilization of lignocellulosic substrates. J Appl Micro biol. 103: 1480-1489.
Sharma, N., Kalra, K.L., Oberoi, H.S., Bansal, S., (2007). Optimization of fermentation parameters for production of ethanol from kinnow wastes and banana peels by simultaneous saccharification and fermentation. Ind J Microbiol. 47: 310-316.
Silverstein, R.A., Chen, Y., Sharma-Shivappa, R.R., Boyette, M.D., Osborn, J.A., (2007). A comparison of chemical pre-treatment methods for improving saccharification of cotton stalks. Bioresour Technol. 98: 3000-3011.
Singleton, V.L., Rossi, J.A., (1965). Colorimetric of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 16: 144-158.
Sun, Y., Cheng, J., (2002). Hydrolysis of lignocellulosic material for ethanol production: a review. Bioresour Technol. 83: 1-11.
Sohail, M., Siddiqi, R., Ahmad, A., and Khan, S.A., (2009). Cellulases production from Aspergillus niger MS82: effect of temperature and pH. N Biotechnol. 25: 437-441.
Yadav S., K., Naseeruddin, S., Prashanthi, G.S., Sateesh, S., Rao, L.V., (2011). Bioethanol fermentation of concentrated rice straw hydrolyzate using co-culture of Saccharomyces cerevisiae and Pichia stipites. Bioresour Technol. 102(11): 6473-6478.
Teramoto, Y., Lee, S-H, Endo, T., (2008). Pretreatment of woody and herbaceous biomass for enzymatic saccharification using sulfuric acid-free ethanol cooking. Bioresour Technol. 99: 8856-8863.
Tolan, J.S., Foody, B., (1999). Cellulases from submerged fermentation. Adv Biochem Eng Biotechnol. 65: 41-67.
USDA. United State Department of Agriculture, World Agricultural Supply and Demand Estimate, October 9, 2015.