Green Catalysis and Process Technology Research laboratory, Department of Chemical Engineering, Maulana Azad National Institute of Technology, Bhopal-462 051 and SERB Indo-US Postdoctoral Research Fellow,
Corresponding author Email: sureshs@manit.ac.in
Article Publishing History
Received: 27/11/2016
Accepted After Revision: 26/06/2017
In this research work wheat husk has been used as silica source for synthesis of SiO2 nanocatalyst by sol-gel method. High concentration of COD is found in refinery wastewater as pollutant. The size of synthesized SiO2 nanoparticles found from 5 to 30 nm range. AFM, FEG-SEM and TEM analysis confired that synthesiszed nanocatalyst are in nano range. FTIR, XRD, and EDAX analysis confirmed that catalyst is SiO2.The COD was removed by photocatalytic reaction in aluminium UV-lamp photoreactor. The optimum percent removal of COD is found to be 85% at 8 hour reaction time and 250C temperature and at 9.3 pH.BET surface area is found to be 300m2/g.
Sio2, Nanocatalyst, Wheat Husk, Cod, Refinery Wastewater
Bharati R, Suresh S. COD Reduction from Refinery Wastewater Using Sio2 Photocatalyst Synthesized by Wheat Husk. Biosc.Biotech.Res.Comm. 2017;10(1).
Bharati R, Suresh S. COD Reduction from Refinery Wastewater Using Sio2 Photocatalyst Synthesized by Wheat Husk. Biosc.Biotech.Res.Comm. 2017;10(1). Available from: https://bit.ly/2XNIwhd
Introduction
Wheat husk contain silica in abundant quantity. Wheat husk has been used as agriculture waste to synthesis SiO2 nanocatalyst. Refinery wastewater contains COD, BOD, Total hydrocarbon (TOH), and phenolic compound as pollutants (Uddeen et al., 2011, Yu et al., 2016). Industrial wastewater is big challenge for environment (Oubrayame et al., 2015). Wheat husk has been used as agriculture waste for synthesis of SiO2 so it is green synthesis method.
In this research work COD has been treated from refinery waster and effect of various parameters like pH, and time have been analyzed. In this work UV-lam aluminum photocatalyst has been used for 8 hour reaction time at 6 h optimum percent removal has been found. By aluminum photocatalytic reactor intensity of light has been controlled to get maximum percent removal. Various characterization like FEG-SEM, TEM, XRD, FTIR, AFM have been carried out.
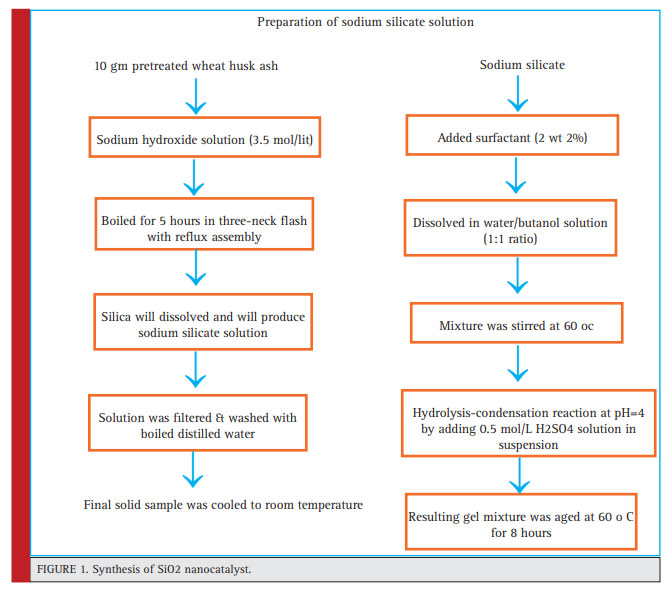 |
Figure 1: Synthesis of SiO2 nanocatalyst |
 |
Figure 2: Shows FEG-SEM Image EDX Image |
Materials and Methods
Materials
All chemicals used in this experiment were used to analytical grade (AR). Orthophosphoric acid (85% purity) acid to make 1 M solution, NaOH to make a 1N solution to maintained pH, double distilled water, refinery wastewater from Northern refinery industry.
 |
Figure 3: TEM Image |
 |
Figure 4: XRD analysis |
 |
Figure 5: AFM analysis |
Methods
Synthesis of SiO2 nanocatalyst by sol-gel method
Wheat husk was collected as agriculture waste from agriculture land , then it was washed and dried in oven at 60oC.Silicat was extracted from wheat husk and nanocatalys of SiO2 was made bt sol-gel method the procedure has been shown in flow diagram 1 (Gayen et al., 2011).10 gm wheat husk was activated by orthophosphoric acid.
Characterization
FEG-SEM, TEM, XRD, FTIR , AFM, EDAX had been carried out to know size, shape and morphology of nanocatalyst.FEG-SEM, TEM and AFM confirmed that particles are in nano range. XRD confirmed phase identification of SiO2 nanocatalyst (Mourhly et al., 2015). FTIR test shows Si-O-Si stretching for nanocatalyst and for other functional groups present. TEM and FEG-SEM FTIR were carried out at SAIF, IIT Mumbai. AFM test was carried out at North Maharashtra university, Jalgaon. AFM was performed at MANI, Bhopal.
 |
Figure 6: FTIR analysis |
 |
Figure 7: Reduction with respect to time |
Treatment Method
For treatment of refinery wastewater aluminum photocatalytic reactor was sed. The reaction was carried out in three neck flask. The light source was taken UV-lamp. There are Six UV-lamp are there in aluminum reactor. By this arrangement intensity of light can change. The photocatalytic reaction was performed for 8 hours. The samples were analyzed at different time internal 1h,2h,3h,4h,5h,6h,7h,8h .The reaction was performed at different catalyst load 0.5g/L, 1.0g/L and 1.5g/L. The optimum percentage removal is 85% at 0.5g/L catalyst load for refinery wastewater and 9.3 pH.
Results and Discussion
Characterization: Results shown by characterization found that synthesized catalyst are in nano range
Treatment of refinery wastewater:
Synthesized nanocatalyst by wheat husk shows good results to treat refinery wastewater by photocatalytic reaction (Choquette et al., 2014).
 |
Figure 8: COD redction with respect to pH |
Conclusion
From characterization of nanocatalyst it has been concluded that synthesized nanocatalyst are irregular in shape in shape. The size of nanocatalyst is from 5 to 25 nm. Synthesized nanocatalyst are crystalline in nature. The optimum percent removal of COD from refinery wastewater is 85% at 6 hour reaction time. The optimum pH for COD removal is 9.0.The results show that synthesized nanocatalysts are good photocatalyst for COD removal form refinery wastewater.
Acknowledgement
The authors wish to thank Ministry of Human Resource Development, Government of India (MHRD) and Maulana Azad National Institute of Technology (MANIT) Bhopal, India to provide fund and facilities to carry out experimental work.
References
- Mourhly, M. Khachani1, A. El Hamidi1, M. Kacimi, M. Halim and S. Arsalan, (2015) The Synthesis and Characterization of Low-cost Mesoporous Silica SiO2from Local Pumice Rock, Nanomater Nanotechnol 35(5) 4-6 .
- Choquette, M.L., Shewa, W. A., Lalman J. A., Shanmugam, S. (2014), Photocatalytic degradation of phenol and phenol derivatives using a nano-TiO2 Catalyst: Integrating quantitative and qualitative factor using response surface methodology, Water, 6(1),1785-1806.
- Oubrayame H., Souabi S., Bouhria M., Alami S.Y., Albizane A. (2015), Performance of wastewater treatment in petrochemical Refinery plant SAMIR,International Journal of engineering and Innovative Technology, 5(2), 74-81.
- R.N. Gayen, S. K. Hussain R. (2011) A. K. Bhar, ZnO films prepared by modified Sol-gel technique, Indian j. pure appl. phy. 49(1) 470-477.
- Uddeen, B.H.D., Daud, W.M.A.W., Aziz, A.R.A., (2011) Treatment technologies for petroleum refinery effluents: A review, Process safety and environmental protection, 89(1), 95-105.
- Yu, L., Han, M., HeF., (2016) A review of treating oily waste water Arabian Journal of chemistry, 5(1), 1-10.


