Shri M. M. Patel Institute of Sciences and Research, Kadi Sarva Vishwavidyalaya, Gandhinagar – 382015, India
Corresponding author email: chaudharykomal98@yahoo.com
Article Publishing History
Received: 11/07/2020
Accepted After Revision: 10/09/2020
Microbial cellulase find applications in many industries and constitute a significant share of the world’s industrial enzyme market. In order to improve the cost function of the cellulase producing processes with enhanced yield and novel activities, superior bioprocesses are formulated these days. The current study was designed to isolate and identify superior cellulose-degrading fungi from cellulosic waste and selection of different cellulosic waste like paper waste, cotton ginning, wheat Bran and sugarcane bagasse for cellulase production under solid state fermentation. Various physical parameters such as temperature, pH and incubation time were optimized for induced cellulase production from Trichoderma sp. in SSF. Among 14 fungal isolates, four isolates were selected by primary screening of CMCase method. Solid state fermentation was carried out with four Agro wastes such as waste paper, cotton ginning waste wheat bran and sugarcane bagasse.
Among 4 fungal strains, A11 isolated which was identified as Trichoderma sp. were selected by secondary screening showing higher cellulase activity on solid state fermentation and wheat bran was found to be the best substrate for cellulase production. In the present study the maximum enzyme production by fungal isolate A11 using Wheat bran as substrate showed 8.49 U/g and 2.23 U/g activities of CMCase and FPase respectively recorded at 35ºC on 120 hrs of incubation period at 5 pH. Isolation of cellulase producing fungal strain will help in bio-conversion of cellulosic materials to glucose and other fermentable sugars which can be further used for the production of bio-ethanol.
Agro Wastes, Cmcase, Fpase, Solid State Fermentation
Chaudhary K, Padhiar A. Cellulase Production by Fungi from Agro Wastes under Solid State Fermentation. Biosc.Biotech.Res.Comm. 2020;13(3).
Chaudhary K, Padhiar A. Cellulase Production by Fungi from Agro Wastes under Solid State Fermentation. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/3bUCS0C
Copyright © Chaudhary and Padhiar This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
All over the world, there is a growing concern about the over dependence on fossil fuels as well as their possible role in global warming. Because of this, there is a huge search for a biofuel to use as an alternative source of energy, utilizing the existing lignocellulosic biomass as a substrate for bioethanol production. (Cai et al., 2019). Exploiting the full chemical potential of cellulosic waste for the energy industry can be sustainably mediated by enzymes called cellulase. Till date fungi are the main cellulase producing microorganisms, though a few filamentous fungi of Trichoderma sp. and Aspergillus sp. are the main source for cellulase production (Xue et al., 2017; Ezeilo et al., 2017). This group of multi-enzyme complex of the glycosyl hydrolase family consist of mainly endo-b-1,4-glucanase, exo-b-1,4- glucanase (cellobiohydrolase) and b-glucosidase. The complete hydrolysis of cellulose to fermentable sugar showing that the process is completed by the cellulase enzymes synergistically breakdown the homogenous polysaccharide (Srivastava et al., 2018; Waill et al., 2019). Although traditionally, the enzymes of industrial importance have traditionally been produced in submerged fermentation (SmF) because of the ease of handling and good control of environmental factors such as temperature, aeration, agitation and pH (Singh et al., 2007). However, solid state fermentation (SSF) techniques have to be found to be better adapted to increase the yield and additionally due to the ability of filamentous fungi to grow well on solid substrates which reduces the cost of enzyme production. (Hui et al., 2010; Waill et al., 2019).
The use of low cost agri- cultural waste adds value to SSF process as it not only makes it cost effective but also promotes a substantial reduction in environmental pollution by agro-industrial residues A part of this it is has been reported that SSF is to be most appropriate process for developing countries due to other benefits such as maximum productivity, simple technique, low investment, energy requirement low and less water requirement, good product recovery and lack of foam build up (Zeng and Chen, 2009; Souza and Magalhaes, 2010; Ezeilo et al., 2020). Thus, Cellulases enzymes are allowed for application in various industries; pulp and paper industry, textile and bioethanol industries, wine and brewery industry, food industries, animal feed industry, agricultural and detergent industries (Darwesh et al. 2020).
The current study is concerned with the hyper-production of cellulase using low-cost agro-industrial residue as a substrate for fungal co-culture using SSF. Selection of agro waste as a carbon source and optimization of various physical parameters for production of cellulase from the co-culture of Trichoderma sp. which was isolated from wheat bran in solid state fermentation. Further, the use of whole fermented broth for saccharification of lignocellulose was compared with the free enzyme in order to minimize the unit operations intended for economic feasibility. The applicability of cellulase was analysed for saccharification of various lignocellulosic substrates.
MATERIAL AND METHODS
Isolation of Cellulose-Degrading Fungi: For isolation of culture samples were collected from paper industry waste, wheat bran waste, soil sample and wood furnishing were collected from different site of Gandhinagar and Kadi in sterile polythene bags. Isolation and primary screening of cellulolytic fungal species was done on Carboxyl agar medium (CMC). The cellulose degradation by fungal were tested using the media proposed by Hart et al. (2002).
Streak plate method: Streak plate method is best method for isolation of fungi. First streak is made with the mix colonies then other are continue of the previous strike using separate sterile tooth peck for isolation of pure culture from mix colonies.
Sprinkled method: Sprinkled method is useful for the soil samples and sawdust. Soil samples were sprinkled on the potato dextrose plate. Finally, petri dishes were incubated at 300C for fungal growth. Pure colonies were separated from mix colonies after 72 hr of incubation at 300C.
Serial dilution method: Serial dilution method is use to reduce dense culture to a usable concentration. 0.5 gm of samples were taken and add in 9 ml of sterile distill water and mix well with the help of vortex for 10 min. From this stock solution, 10-1 to 10-5 serial dilutions were prepared. 1 ml from dilution medium inoculated into Potato dextrose agar medium. The plats were incubated for 4 to 5 days at 300C for observation of the fungi.
Primary screening of cellulose degrading fungi: The individual microorganism was grown on basal salt media supplemented with 1% Carboxy methylcellulose (CMC) medium. The pure cultures were inoculated in the center with almost equal amounts and incubated at 30ºC until substantial growth was recorded. The petri plates were incubated at 50ºC for 30 min. Plates were flooded with 1% Iodine solution and allowed it to stand for 5-10 minutes. The clear zone was observed around the colony due to hydrolysis of cellulose by cellulase which is produced by fungi.
Characterization and Identification of fungal Isolates: Fungal colonies were isolated form different samples for cellulase producing microorganisms by serial dilution method. The isolates were inoculated on sterile PDA plates and incubated at 30°C for 48 hours in order to obtain a pure culture. The various isolates were determined based on Morphological characteristics and microscopic examinations and the reproductive and vegetative structures were also studied (Devanathan et al., 2007).
Culture Maintenance: Isolated fungi strain was maintained on Potato dextrose agar (PDA) slants. The pH of the medium was maintained as neutral and culture was incubated at 300C for 5-6 days and stored in refrigerator at 40C. Sub culturing was carried out once in every 2-3 months.
Solid state fermentation (SSF) and Substrate preparation for cellulase production: Inoculum preparation was carried out using Sabouraud dextrose broth (150 ml), prepared in 500ml Erlenmeyer flasks and autoclaved at 15 lbs for 15 min. The medium was inoculated with isolated fungus of 3 mycelial disc (7 mm diameter) punched out from the edges of its 8 days old colonies from Petri plates. The flasks were incubated at 30 ± 2 °C for 72 hrs. The fungus was inoculated into substrates flasks for enzyme production.
Substrate preparation for cellulase enzyme production: Four different types of agro wastes were used as a substrate for cellulase enzyme production such as wheat bran, sugarcane bagasse, cotton waste and discarded paper. Cotton Waste and discarded paper were pretreated with 3% H2SO4. This 2.0 gm of substrates were supplemented to the basal medium. The composition of Basel medium includes KH2PO4: 2gm, (NH4)2PO4: 1.4 gm, Urea: 0.3 gm, CaCl2.2H2O: 0.3 gm, MgSO4: 0.3 gm, Peptone: 1.0 gm, FeSO4.7H2O: 5.0 mg, MnSO4.7H2O: 1.6 mg, ZnSO4.7H2O: 1.4 mg, CoCl2.6H2O: 2 mg, Tween 80: 0.2% (v/v), D/W 1000 ml and pH: 5.5 (Mandel and Resse, 1957). Substrate flask was incubated at 300C at 6 pH.
Preparation of crude enzyme: Enzymes were extracted from substrate flask by addition of 5 ml of cold 0.05 M acetate buffer (pH 4.8). The material was filtered through muslin cloth and the filtrate was centrifuged at 5000 rpm at 4°C for 15min. The supernatant was used for determine the enzyme activity such as carboxyl methyl cellulase (CMCase) and filter paper activity (FPase).
Enzyme Assay:Filter paper assay (FPase): According to the method of Mendels and Weber (1969) filter paper activity of the culture filtrates were determined. Whatman filter paper strips containing 50 mg (1cm X 6cm) weight was inoculated in 1 ml of 0.05 M sodium acetate buffer (pH 4.8) and kept at 50 °C in a water bath. 0.5 ml aliquots of enzyme source were added to the above mixture and incubated for 60 minutes at 50°C. After incubation, the released reducing sugar was estimated by the 3, 5-dinitrosalicylic acid (DNSA) method. Control without enzyme was simultaneously run with sample. Activity of cellulase was expressed in filter paper units which were defined as the amount of enzyme releasing 1 mole of reducing sugar from filter paper /ml /min.
Endoglucanases enzyme assay (CMCase): Endoglucanase activity in the culture filtrates was quantified by carboxy-methyl cellulase (CMCase) method as describe by Ghosh (1987). In this method the reaction mixture of 0.5 ml enzyme, 0.5 ml of 1% carboxy methyl cellulose in 0.05 sodium acetate buffers with pH 5.0 and 1ml DNSA were incubated at 50 °C in a water bath for 20 minutes. Appropriate control without enzyme was simultaneously run. The release of glucose due to the enzyme activity was assayed by 3, 5 Dinitro salicylic acid measured at 540 nm using spectrophotometer (Miller, 1959).
Coctail of in house producing enzymes from wheat bran: Four individual cultures (A4, A8, A10, and A11) with their 6 combinations were tested for efficient combination of enzyme in wheat bran under SSF at 300C. Cellulase enzyme extracted from wheat bran using four cultures were mixed in equal amount and find out their FPase and CMCase activity. Crude enzyme was extracted as above 2.1 methods.
Effect of Incubation time, temperature and pH on cellulase enzyme production from wheat bran: Among four different cellulosic waste wheat bran was selected for enzyme production. The influence of temperature (30 to 50 °C), pH (4.0, 5.0, 6.0, 7.0 and 8.0) and incubation period (2-6 days) on enzyme production was studied using A-11 by keeping all other parameters constant. Samples were withdrawn after every 24hr of incubation up to 6 day. The material was filtered through muslin cloth and the filtrate was centrifuged at 5000 rpm at 4°C for 15 min and the supernatant was analysed for FPase and CMCase activity.
RESULTS AND DISCUSSION
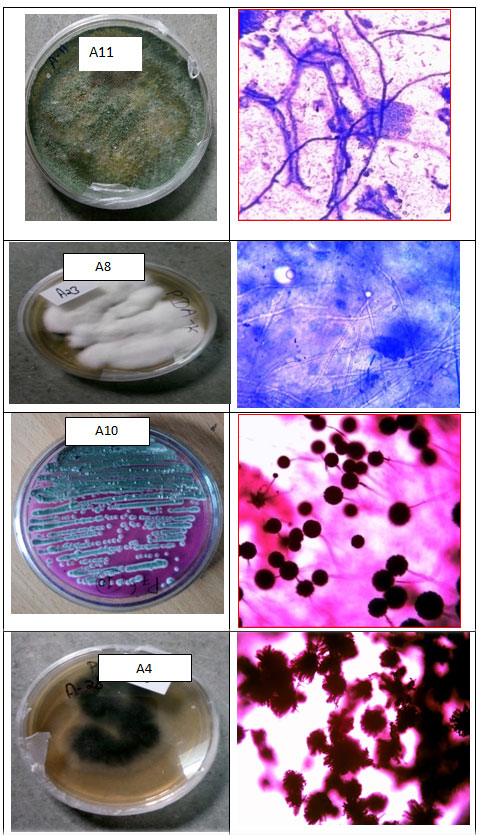
Isolation, Primary screening and identification of cellulolytic Fungi: Total 34 different isolates were selected by the primary screening technique from which 4 isolates were showing (Table 1) higher cellulase activity. Potential isolates were obtained from wood furnishing region, paper industry waste and compost wheat bran waste shown- in table 1. In the Primary screening, the isolates were subjected to screening procedure on Carboxy Methyl Cellulose (CMC) plates as described earlier by Narra et al. (2014). After 72 hrs of incubation cellulase activity was measured as a clear zone of hydrolysis. The fungal strains were selected based on cellulose utilization; the zone of hydrolysis was observed after flooding with iodine solution. A4, A8, A10 and A11 were selected for secondary screening of cellulase activity from four different agro wastes (Figure 1).
Table 1. Colony morphology and microscopic observation of selected culture
| Isolated Culture | Isolation from | Colony Morphology | Cellulase Zone (mm) |
| A1 | Soil | White to green, dark green spore | 7 |
| A2 | Cotton waste | White to green, dark green spore | 14 |
| A3 | Cotton waste | Green to black | 12 |
| A4 | Vegetable waste | Green to Black, Black mycelium | 21 |
| A5 | Wood | White to Green, Green spore | 18 |
| A6 | Cotton waste | White to green, dark green spore | 13 |
| A7 | Soil | Green to Black | 21 |
| A8 | Plant | White mycelium,
Greyish green |
12 |
| A9 | Soil | White to green, dark green spore | 12 |
| A10 | Paper waste | White to Green | 18 |
| A11 | Wheat bran | White to light Green, light and dark green mycelium | 25 |
| A12 | Plant | White to light Green | 22 |
| A13 | Plant | White to green, dark green spore | 13 |
| A14 | Plant | White to light green, light green mycelium | 8 |
Figure 1: Morphological and Microscopic Observation of Fungi
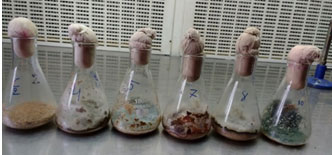
Solid state fermentation (SSF) for cellulase enzyme production: Inoculum preparation was carried out using Sabouraud dextrose broth. The medium was inoculated with selected fungi and fungi were grown in 72hrs at 30 ± 2 °C (Figure 2A). The fungus was inoculated into substrates flasks for enzyme production (Figure 2B).
Figure 2A: Inoculum preparation for SSF
Figure 2B: Solid State Fermentation for cellulase production
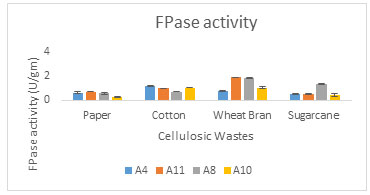
Various agro residues viz. Paper waste, cotton ginning waste, wheat bran and sugarcane bagasse were used for cellulase production by A8, A10, A11 and A4 fungal culture with Mandel’s medium as the moistening media under solid state fermentation (SSF). According to Srivastava et al. (2018), Aspergillus and Trichoderma are the main fungal sources of commercial cellulase enzyme production from wheat bran. In the present study optimum FPase activity 1.92U/gm and 1.8U/gm were obtained from wheat bran on 5th day of incubation time at 300C by A11 culture and A8 respectively (Figure 3A).
Figure 3A: FPase activity from cellulosic waste
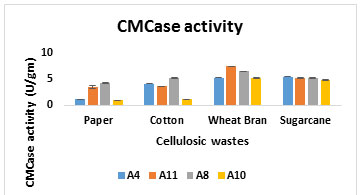
In case of CMCase activity 7.51 U/gm by A11 and 6.54U/gm A8 were obtained high in wheat bran on 5th day of incubation time at 300C (Figure 3B).
Figure 3B: CMCase activity from cellulosic waste
Vyas (2005) have also found that T. viride produced 2 U/ml CMCase and 0.25 U/ml FPase activity on 7th day of incubation. Raghuwanshi et al. (2014) found that CMCase activity: 13.2 IU/g and FPase activity: 2.2 IU/g by Trichoderma from wheat bran on 7th day of incubation. While yield of FPase and CMCase were low with rest of the substrates (Figure 1 and 2). In present study, all selected fungal strains give maximum cellulase activity in wheat bran, which was selected for further cellulase enzyme production.
Earlier it was reported that, Wheat bran is the universal substrate among various substrates because it acts as a complete nutritious feed for microorganisms having all the ingredients and remains loose even under moist conditions providing a large surface area. It is the need of the time to search for cheaper substrates for cellulase enzyme production so that can be reduced in the cost of biofuels production while as wheat bran is cheap and easily available substrates in India. Furthermore, the biochemical composition of wheat bran indicates that it contains various fermentable sugar such as glucose, xylose, arabinose, galactose, etc. which are helpful for the initiation of growth of microorganisms (Archana and Sathyanarayana, 1997).
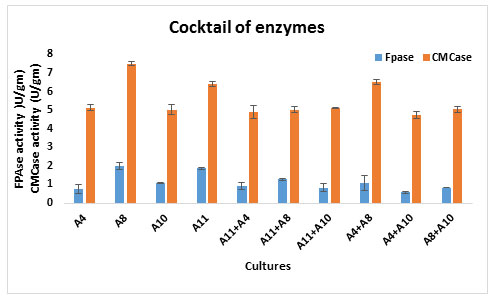
Coctail of in house producing enzymes from wheat bran: Four individual cultures with their 6 combinations were tested for efficient combination of enzyme production using wheat bran. The results of FPase and CMCase activity were shown in Figure 3A and 3B respectively. From the result of FPase activity, all the isolates analysed for their monoculture efficiency in enzyme production using wheat bran (Figure 1), it was only A 8 and A11 that had a higher FPase activity (1.98 U/gm and 1.86U/gm respectively) than other mono culture. Co-culture enzyme production was not much better comparing to individual and this shows antagonism effects between cultures (Lequart et al., 1999). Combination of A11 and A8 showed better FPase activity (1.27 U/gm) and combination of A4 and A8 was 6.5U/gm showed higher CMCase activity compare to another enzyme bland. According the Vyas A. (2005) the combination of Aspergillus and Trichoderma showed higher cellulase as well as FPase activity in wheat bran.
Figure 4: Coctail of in house producing enzymes
Most of the fungi obtained significantly better enzyme production in monocultures which is observed in Figure 4. Maximum CMCase production was found using A8 culture (7.51 U/gm), while A11 gave 6.53 U/gm activity. The cocktail of enzyme also shows better CMCase activity compare to FPase.
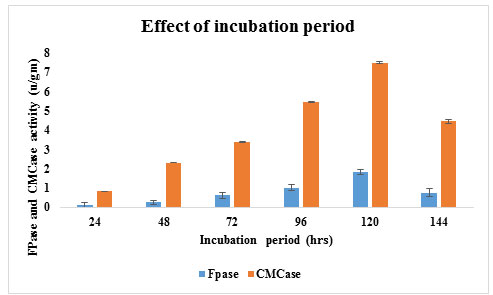
Effect of incubation time on cellulase production: The incubation period directly effects enzyme production. Using SSF A11 (Trichoderma sp.) isolate gave highest enzyme activity of CMCase and FPase 7.51 U/gm and 1.92 U/gm (Figure 5) at 120 hrs of incubation at 300C temperature which was suitable the commercial point of view. Earlier Gomes I et al. (2006) reported that the cellulase activities were reached to maximal levels within 5 – 7 days of incubation. After 120 hr cellulase activity was decline it might be due to the depletion of nutrients which stressed the fungal physiology resulting in the inactivation of secretory machinery of the enzyme (Nochure et al., 1993).
Figure 5: Effect of incubation time on cellulase production
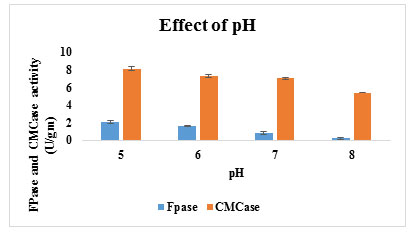
Effect of Initial pH on cellulase production: pH is an important physical parameter which is effect on the growth rate of fungal strain and significantly affect the enzyme production. Generally, the agro-industrial wastes when used act as a unique buffering action and have an advantage for enzyme production because it has many soluble sugars for growth of microbes. The influence of pH on cellulase production was studied by adjusting the initial medium (Mandal’s medium) pH from 4 to 8. The optimum pH for cellulase production was found to be pH 5.0 with an enzyme activity of 2.1U/g of FPase and 8.12 U/gm CMCase using wheat bran by A11 isolates. According to literature’s evidence, many researchers reported that acid pH values between 4-6 have favoured cellulase enzyme production (Kuhad et al., 1998). At pH 6.0, 7.0 and 8.0 enzyme activity were found to be 1.67U/g/m, 0.83U/g/m and 0.24U/gm of FPase and 7.34 U/gm, 7.24U/g and 5.4U/gm of CMCase produced respectively (Figure 6).
Figure 6: Effect of Initial pH on cellulase production
The enzyme activity was decreased with increasing pH after 5 pH. Highly acidic pH may limit the growth and production by reducing associability of the hemicellulosic substrates, besides that the nature of substrate also has a strong influence on pH kinetics, due to the buffering effect of lignocellulosic substrates.
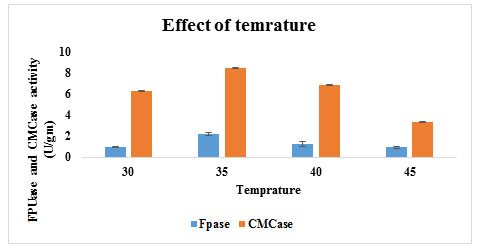
Effect of Temperature on cellulase production: Temperature has a significant role in the development of biological process as it influences the protein denaturation, enzyme inhibition and cell growth. In the present study the optimum temperature for maximum enzyme production by fungal isolate A11 using Wheat bran as substrate showed was 8.49 U/g and 2.23 U/g activities of CMCase and FPase, respectively recorded at 35ºC on 5th day of incubation period (Figure 7).
Figure 7: Effect of temperature on cellulase production
CONCLUSION
In the present study a potential cellulolytic fungal strain Trichoderma sp. (A11) was isolated from decomposed wheat bran. Production of cellulases was carried out under SSF at 35ºC. The major factors influencing the production of cellulases were found to be temperature, pH, and incubation period. The results revealed that there was an overall increase in FP, β-glucosidase, endoglucanse and exoglucanase activities after the optimization of physical conditions. The newly isolated fungus Trichoderma sp. (A11) has the ability to produce cellulase under solid state fermentation in Wheat bran having optimum activity of CMCase 8.49 U/gm and FPase 2.23 U/gm at 120 hrs of incubation, pH 5.0 and at 350C. This extracted endoglucanase and exoglucanases from Trichoderma sp. (A11) has a potential to be utilized for biofuel applications owing to its ability to enhance saccharification of cellulosic waste. Hence the cellulase enzyme obtained in this study hold promise in bioethanol production in bio- processing industrial applications.
ACKNOWLEDGEMENTS: We would like to thank KSV University, Department of Biotechnology for giving an opportunity to work and supporting throughout the work.
Conflicts of Interest: No potential conflict of interest was reported by the authors.
REFERENCES
Archana S and Sathyanarayana T. (1997) Xylanase production by thermophilic Bacillus lichenformis A99 in solid-state fermentation. Enzyme and Microbial Technology, 21, pp 12–17.
Ashish Vyas and Deepak Vyas. (2005) Production of fungal cellulase by solid state bioprocessing of ground nut shell wastes. Journal of scientific and industrial research, 64, pp 767-770.
Cai C, Bao Y, Zhan X, Lin X, Lou H and Pang Y. (2019) Recovering cellulase and increasing glucose yield during lignocellulosic hydrolysis using lignin- MPEG with a sensitive pH response. Green Chemistry, 21, pp 1141–1151.
Lequart, JM Nuzillard, B. Kurek and P. Debeire. (1999) Hydrolysis of wheat bran and straw by an endoxylanase: production and structural characterization of cinnamoyl-oligosaccharides. Carbohydrate Research, 319, pp 102–111.
Darwesh, Osama M., Salma H. El-Maraghy, Hany M. Abdel-Rahman, and Rashed A. (2019) Improvement of Paper Wastes Conversion to Bioethanol Using Novel Cellulose Degrading Fungal Isolate. Fuel, 262, pp 116518.
Devanathan A, Shanmugan T, Balasubramanian and Manivannan S. (2007) Cellulase production by Aspergillus niger isolated from coastal mangrove debris. Trends in Applied Science Research, 2, pp 23-27.
Ezeilo, Uchenna R., Roswanira Abdul Wahab, and Naji Arafat Mahat (2020) Optimization Studies on Cellulase and Xylanase Production by Rhizopus Oryzae UC2 Using Raw Oil Palm Frond Leaves as Substrate under Solid State Fermentation. Renewable Energy, 156, pp 1301–12.
Ghosh TK. (1987) Measurement of cellulase Activities. Pure and Applied Chemistry, 59(2), pp 257-268.
Gomes I, Mohammad S, Sabita RR and Donald JG. (2006) Comparative studies on production of cell wall degrading hydrolases by Trichoderma reesei and T. viride in submerged and solid- state cultivations. Bangladesh Journal of Microbiology, 23(2), pp 149 -155.
Hart TD, Leij DF, Kinsey G, Kelley J and Lynch JM. (2002) Strategies for the isolation of cellulolytic fungi for composting of wheat straw. World Journal of Microbiology and Biotechnology, 18, pp 471-480.
Hui L, Wan C, Hai-tao D, Xue-jiao C, Qi-fa Z and Yu-hua Z. (2010) Direct microbial conversion of wheat straw into lipid by a cellulolytic fungus of Aspergillus oryzae A-4 in solid-state fermentation. Bioresource Technology, 101, pp 7556-7562.
Jahangeer S, Khan N, Jahangeer S, Sohail M, Shahzad S, Ahmad A and Khan SA. (2005) Screening and characterization of fungal cellulases isolated from the native environmental source. Pakistan Journal of Botany, 37(3), pp 739-748.
Kuhad RC, Manchanda M and Singh A. (1998) Optimization of xylanase production by hyper xylanolytic mutant and strain of Fusarium oxysporum. Process Biochemistry, 33, pp 641-647.
Mandel M. and Resse ET. (1957) Induction of cellulase in fungi in Trichoderma viride as influencing carbon source. Journal of Bacteriology, 37, pp 268-298.
Mandels M and Weber J. (1969) The production of cellulases, In; Cellulases and its application. Advances in chemistry Series. American Chemical Society, Washington, DC, pp 391-414.
Miller GL. (1959) Use of dinitro salicylic acid reagent for the determination of reducing sugars. Analytical Chemistry, 131, pp 426-428.
Narra M, Dixit G, Divecha J, Kumar Kiran, Madamwar D and Shah AR. (2014) Production, purification and characterization of a novel GH 12 family endoglucanase from Aspergillus terreus and its application in enzymatic degradation of delignified rice straw. International J Biodeterioration Biodegradation, 88, pp 150–161.
Nochure SV, Roberts MF and Demain AI. (1993) True cellulase production by Clostridium thermocellum grown on different carbon source. Biotechnology Letters, 15, pp 641-646.
Raghuwanshi S, Deswal D, Karp M and Kuhad RC. (2014) Bioprocessing of enhanced cellulase production from a mutant of Trichoderma asperellum RCK2011 and its application in hydrolysis of cellulose. Fuel, 124, pp 183–189.
Singh A, Kuhad RC and Ward OP. (2007) Industrial applications of microbial cellulase, in lignocelluloses Biotechnology Future prospects. I.K International publishing house Private Limited, pp 345-358.
Souza PM. and Magalhaes PO. (2010) Application of microbial – amylase in industry-A review. Brazilian Journal of Microbiology, 41, pp 850-861.
Srivastava N, Srivastava M, Gupta VK, Ramteke PW and Mishra PK. (2018) A novel strategy to enhance biohydrogen production using graphene oxide treated thermostable crude cellulase and sugarcane bagasse hydrolyzate under co-culture system. Bioresource technology, 270, pp 337–345.
Srivastava N, Srivastava M, Manikanta A, Ramteke PW, Singh RL, Mishra PK and Upadhyay SN. (2018) Fungal Cellulases Production for Biodegradation of Agriculture Waste. Microorganisms for Green Revolution, pp 75–89.
U.R. Ezeilo, II. Zakaria, F. Huyop and R.A. Wahab (2017) Enzymatic breakdown of lignocellulosic biomass: the role of glycosyl hydrolases and lytic polysaccharide monooxygenases, Biotechnology and Biotechnology Equipment. pp 1-16.
Waill A, Elkhateeb, Daba Ghoson M, Thomas Paul W and Wen Ting-Chi. (2019) Influence of Two Extraction Methods on Essential Oils of Some Apiaceae Family Plants. Egyptian Pharmaceutical Journal, 18, pp 160–64.
Xue Y, Wang X, Chen X, Hu J, Gao MT and Li J. (2017) Effects of different cellulases on the release of phenolic acids from rice straw during saccharification. Bioresource technology, 234, pp 208–216.
Zeng W and Chen HZ. (2009) Air pressure pulsation solid state fermentation of feruloyl esterase by Aspergillus niger. Bio-resource Technology, 100, pp 1371-1375.