1Department of Laboratory Sciences, College of Sciences and Arts, Qassim University, Al-Rass, Saudi Arabia
2Department of Eco- physiology, Desert Research Center, 15753, Cairo, Egypt
3Chemical Department, Faculty of Science, Northern Borders University, Saudi Arabia
4Laboratory of Chemistry-Biology Applied to the Environment, Department of Biology, Moulay Ismail University, Meknes, Morocco
Corresponding author email: emad100sdl@yahoo.com
Article Publishing History
Received: 27/11/2016
Accepted After Revision: 26/12/2016
Ziziphus mauritiana is recognized for traditional use in different areas as its fruits are consumed locally in some parts of Saudi Arabia. However, little is known about the biological activities of the leaves. The current study has been aimed to evaluate some bioactive properties of the methanol leaf extract of Ziziphus mauritiana. Phytochemical analysis was performed using colorimetric methods:Disc diffusion, MIC and MBC method was used to determine the antimicrobial activity; DPPH scavenging activity and reduction capacity were determined spectrophotometrically for the antioxidant activity, and carrageenan-induced paw edema method using rat models was employed to evaluate the anti-inflammatory activity. The study revealed the presence of some bioactive phytochemical constituents such as saponins, tannins, alkaloids, phenolic compounds, terpenoids and flavonoids. The methanol leaf extract of Ziziphus mauritiana has significant antibacterial activity against Bacillus cereus ATCC 10876 and Proteus vulgaris (multi-drug resistant isolate); and varied degrees against other bacterial strains but it was not significant. The plant extract also has potent antioxidant (IC50 value of 0.024 g/L competitor to the ascorbic acid and the quercetin, with observed reducing power of Iron III to Iron II) and anti-inflammatory properties (71.83% reduction in inflammation at a concentration of 400 mg/kg body weight of rats). The results obtained in the present study suggest that leaves of Ziziphus mauritiana can be used as a source for functional ingredients for pharmaceutical drug industries.
Antibacterial, Antioxidant, Anti-Inflammatory, Ziziphus Mauritiana
Abdallah E. M, Elsharkawy E. R, Ed-dra A. Biological Activities of Methanolic Leaf Extract of Ziziphus Mauritiana. Biosc.Biotech.Res.Comm. 2016;9(4).
Abdallah E. M, Elsharkawy E. R, Ed-dra A. Biological Activities of Methanolic Leaf Extract of Ziziphus Mauritiana. Biosc.Biotech.Res.Comm. 2016;9(4). Available from: https://bit.ly/2WCTkuL
INTRODUCTION
No doubt, plants were the main source of therapeutics for human since ancient times and until the current era. Although, pharmaceuticals (mostly synthetic drugs) are the dominant drugs in modern medicine, but phyto-medicinal drugs (mostly plant derivatives) are more popular. The WHO reported that, up to 80% of the world population is depending on drugs derived from plants particularly in the developing countries (WHO, 1996). However, the approach for drug development from plants is very complicated and expensive, each new drug costs about 100-360 million US dollars and at least 10 years of intensive work in a form of multi-disciplinary and integrated activities including many fields like botany, chemistry, pharmacology, biomedical sciences, biotechnology and even anthropology (Rates, 2001).
If we put in consideration that, there are about 250,000 to 500,000 species of plants on earth, human and animals are consuming not more than 10% of these plant species (Abdallah, 2011), that means screening for bioactivity of medicinal plants is important and worthwhile in order provide the basic knowledge for discovery of new drugs. Two main constituents are produced from plants; primary compounds such as sugars, proteins and chlorophyll; secondary compounds (phytochemicals) such as flavonoids, terpenoids and phenolic compounds; the bioactive properties of some plants are attributed to the secondary or phytochemical compounds (Wadood et al., 2013 and Akhtar et al., 2016).
These bioactive phytochemical compounds have a great potential in the treatment of many human diseases such as diabetes, coronary heart diseases, infectious diseases and cancer (Chew et al., 2011). Inflammation is a complex series of events that occurs when any tissue or organ is injured or damaged by chemicals, micro-organisms, trauma, foreign bodies, surgery and ionizing radiation, involving the releases of different bioactive substances such as histamine, serotonin and the prostaglandins (Jackson-Robert and Morrow, 2011).
The classical analgesic drugs, opiates and non-steroidal anti-inflammatory drugs (NSAIDs) all have their origin in natural products that are used for centuries. Salicin, a bitter glycoside from the willow bark extract are known since the 18th century for its beneficial effect in fever and pain. The synthetic form, acetylsalicylic acid (ASA) was introduced into medicine as far back as the 19th century. Quite a number of derivatives of ASA and other newer drugs were discovered but these are associated with side effects limiting their use. These factors include allergy, gastric mucosal irritation and/or gastric ulceration due to the acidic nature of most NSAIDs and inhibition of a mucosal protective prostaglandin E (PGE). Others include prolonged vascular bleeding, NSAID-induced nephropathy, salt and water retention by the kidney as well as the displacement of other drugs from their protein binding sites due to the greater affinity of NSAIDs for plasma albumin are some of the other challenges facing this group of drugs (Foster, 1999). The roots and bark of Ziziphus Leaves and root bark of the plant are used as the remedy of inflammation by the local communities of eastern India. While, much work exists on extracts of leaves in animal model proving the anti-inflammatory action (Kumar and Sharma, 2010; Soliman 2011; Goyal et al., 2012 and 2013 Ashraf et al. (2015).
Ziziphus mauritiana from family Rhamnaceae commonly known as Ber or Indian jujube. The genus Ziziphus is very common plant found in many places in the world. Ziziphus mauritiana is a tropical shrub native to Indian Subcontinent, The Southeast Asia, Iran and some regions of Africa. Various parts of Ziziphus mauritiana are used for nutritional and medical purposes. However, leaves are employed traditionally as astringent and anti-typhoid (Akhtar et al., 2016, Najafi, 2013). In literature, many studies reported that Ziziphus mauritiana have some medical benefits such as antioxidant, anti-microbial, anti-diarrheal, anti-diabetic, hepato-protective and anti-cancer (Lim, 2013). The current study aimed to evaluate in vitro antibacterial, antioxidant and in vivo anti-inflammatory activity of the methanol leaf extract of Ziziphus mauritiana growing in Saudi Arabia.
MATERIAL AND METHODS
Plant Material
Leaves of Ziziphus mauritiana (Fig 1.) was collected in March 2016 from the garden of College of Sciences and Arts at Al-Rass, after verification and authentication by Dr. Wail Elsadig Abdalla (Plant taxonomist), voucher specimen was deposited in the herbarium of the department of laboratory sciences, College of Sciences and Arts at Al-Rass. Qassim University. Collected leaves were washed thoroughly with tap water, rinsed again in distilled water, and then dried in shade for up to one week. The dried leaves were crushed to fine powder using a blender (GEEPAS®, GCG 292) and kept until used.
Extraction
About 300 g of the dried powder of the leaves of Ziziphus mauritiana L. was macerated in 1.5 liter of 80% methanol (v/v) (HPLC grade, Fisher Scientific,UK ) to serve as a hydroalcoholic solvent and left for up to 3 days in a dark tighten container at room temperature (32-37 o C) with frequent soaking. In the 4th day, the macerate was filtered using Whatman filter papers No.1 (Whatman International Ltd, UK), the filtrate was put in the incubator (BINDER GmbH, Germany) at 45oC and allowed to evaporate for up to 10 days, till getting a semi-solid extract, which was used for the experiments.
Preliminary Phytochemical Analysis
Leaves of Ziziphus mauritiana were qualitatively screened for some bioactive phytochemical constituents by means of Colorimetric qualitative tests. The crude of methanolic extract was used to detect the presence of saponins and anthraquinones (Stahl, 1973), alkaloids (Yusuf et al., 2014), tannins (Ashfaq et al., 2012), Terpenoids and flavonoids (Mujeeb et al., 2014) and Phenolic compounds (Clarke, 1975).
| Table 1: The phytochemical analysis of 80% methanol extract (v/v) of Ziziphus mauritiana leaves. | |
| Phytochemicals | Methanol extract |
| Saponins | +++ |
| Tannins | ++ |
| Alkaloids | ++ |
| Flavonoids | + |
| Terpenoids | + |
| Phenolic compounds | ++ |
| Anthraquinones | – |
| +++ = Present in high amount, ++ = moderately present, + = Trace amounts, – = Absent. | |
Microorganisms
Four pathogenic bacterial strains (Staphylococcus aureus, Proteus vulgaris, Pseudomonas aeruginosa, and Klebsiella pneumoniae) and one referenced bacterial strain (Bacillus cereus ATCC 10876) were used in the antibacterial activity tests. The pathogenic strains were obtained from the Department of Pathology and Laboratory medicine, Al-Rass General Hospital, Saudi Arabia, they were identified by Dr. Mohamed Algadi. The sources of these pathogens and the antibiotics sensitivity profile are shown in (Table 2). While, the referenced type culture strain was brought from the department of laboratory sciences, College of Sciences and Arts at Al-Rass, Qassim University, Saudi Arabia.
Antibacterial Assay
The antibacterial activity of the leaves of Ziziphus mauritiana (Methanol extract) was evaluated by disc diffusion method as cited in (Abdallah, 2016) with minor modification. The tested bacterial strains were sub-cultured for 18 h, and then working bacterial samples were prepared and adjusted to be equivalent 0.5 McFarland (Approximately 1-2 × 108 CFU/ml). 20 ml of hot autoclaved Mueller-Hinton agar (Watin-Biolife, KSA), was poured to a sterile disposable Petri-dish (size 100x15mm) and left until solidified. 100 µl from the bacterial culture was loaded on the agar and swapped with a sterile cotton swap. Filter paper discs (cut off from Whatman No.1 filter paper) were immersed in the reconstituted extracts at 400 and 200 mg/ml. these discs were put on the agar plate, another antibiotic disc was loaded to the plate (gentamincin 10 µg/disc) to serve as a positive control, a sterile disc saturated with 80% methanol was also put on the plate to serve as negative control. The seeded plate was incubated at 37oC for 24 hours, the test was repeated twice, the mean inhibition zone and standard error of means were calculated.
Minimum Inhibitory Concentration
Test (Mic)
Only the bacterial strains which showed mean inhibition zone above 10 mm were subjected to MIC test, using the broth dilution method as reported in (El-Mahmood and Ameh, 2007) with slight modification. A sufficient quantity of test tubes containing 1 ml of Nutrient broth (Watin Biolife, KSA) was prepared. 1 ml of the plant extract (200 mg/ml) was dropped in the first tube and mixed well. Serial two fold dilutions were made subsequently to get 100, 50, 25, 12.5, 6.25, 3.123 mg/ml. 100 µl of the adjusted bacterial solution (1-2 × 108 CFU/ml) was put in each of the seven tubes. Positive control tube (all contents without the extract and with 1 ml of 5mg/ml Chloramphenicol), and a negative control tube (nutrient broth and 1 ml methanol) were prepared. All tubes were incubated for 18 hours at 37oC. The lowest dilution with no obvious growth was considered as MIC.
Determination Of Minimum Bactericidal Concentration (Mbc)
The MBC test was performed as cited in Doughari (2006) with minor modification. 100 μl from the MIC test tubes that showed no visible growth was loaded in new previously prepared sterile plates containing nutrient agar (Watin-Biolife, KSA) and spread over the agar with a sterile swap. To another 2 agar plates, one was inoculated with Chloramphenicol as a positive control and the other one was inoculated with 10%DMSO as a negative control. All cultured plates were incubated overnight at 37 oC and investigated for bacterial growth. Plates with no visible growth were considered as the MBC.
Dpph Scavenging Activity
The hydrogen atoms or electrons donation ability of the plant extract and some pure compounds were measured from the bleaching of a purple-colored methanol solution of DPPH (Kubola et al., 2008). Briefly, 1 mL of a 0.08 g/L solution of DPPH radical in methanol was added to 2 mL of the extract at different concentrations. The absorbance of the resulting solution was measured after 30 min in dark at 517 nm with a spectrophotometer. The percentage inhibition of activity was calculated as:
(A blank-A sample)
% Inhibition = × 100
(A blank)
Quercetin and ascorbic acid was used as positive control and the concentration providing 50% inhibition (IC50) were calculated from the graph of inhibition percentage plotted against the extract concentration.
Reductive Capacity: Iron (Iii) To Iron (II)
The reductive capacity of the extract was determined using ferric to ferrous iron reduction assay as determined spectrophotometrically from the formation of Perl’s Prussian blue colored complex (Dorman et al., 2003). Briefly, 1 mL of each sample, in methanol, was mixed with 2.5 mL of phosphate buffer (0.2 mol/L, pH 7.0) and 2.5 mL of potassium hexacyanoferrate K3Fe(CN)6 solution. After 30 min incubation at 50°C, aliquots (2.5 mL) of trichloroacetic acid (10%) were added to the mixture. Then, 2.5 mL of this solution was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%), and the absorbance was measured at 700 nm. The ascorbic acid standard was used for comparison.
Anti-Inflammatory Study
Anti-inflammatory effect was evaluated in rats using a carrageenan-induced paw edema (Winter et al., 1962). Experiments on animals were performed in accordance with the ethical guidelines and regulations set forth by Faculty of Science, Northern Borders University, Saudi Arabia. Four groups of animals (n=6) were fasted overnight prior to the study with free access to water. The 1st and 2nd groups (normal control and reference, respectively) were treated orally with the vehicle (5 mL/kg) and Indomethacin (10 mg/kg), respectively. The extract of Ziziphus mauritiana at 200 and 400 mg/kg was administered orally to rats of the 3rd and 4th groups, respectively. After 30 min, inflammation was induced by subplantar injection of 1% carrageenan (0.1 mL) in the left hind paw of all animals. The paw volumes up to the tibio tarsal joint were measured in mL using a plethysmometer (Ugo basile, Italy), immediately before and at 1, 2 and 3 h after carrageenan administration. Their mean increases in the injected paw volume were calculated and the percentage inhibition of paw edema was calculated using the following equation.
Inhibition (%) = {1- (Vt/Vc)}100
Where, Vt and Vc are the mean change in paw volume of treated and control rats, respectively.
Drug Preparation
Carrageenan (1% w/v), Indomethacin and the test extracts were prepared by suspending in 1% Carboxy methyl cellulose solution.
Statistical Analysis
Values were expressed as means ± S.E. Comparisons between means were carried out using one-way ANOVA followed by LSD (least significant difference) and Tukey multiple comparison tests. The P < 0.05 was accepted as being significant in all types of statistical tests. SPSS software (version 17) was used to carry out all.
RESULTS
Phytochemical Constituents
The investigation has revealed that methanol leaf extract of Ziziphus mauritiana contains some bioactive compounds such as saponins, tannins, alkaloids, phenolic compounds, and terpenoids. Flavonoids and anthraquinones did not detect in the methanol extract (Table 1).
Antibacterial Properties
For the antibacterial testing, both of the multi-drug resistant (MDR) and non-MDR gram- positive and gram-negative bacteria were chosen. As shown in (Table 2), clinical pathogenic strains resistant to three or more of the tested antibiotics were considered as multi-drug resistant, which was Proteus vulgaris, Pseudomonas aeruginosa, Klebsiella pneumoniae and Staphylococcus aureus. The other bacterial strains considered as non-MDR, which were Escherichia coli and Bacillus cereus ATCC 10876.
| Table 2: Antibiotics sensitivity profile of the clinical pathogenic strains and the referenced bacterial strain. | ||||||||
| Bacterial strain | Origin | Source | AK | GM | CPM | TC | PRL | IMI |
| Proteus vulgaris | Clinical | Pus | R | R | R | R | R | S |
| Pseudomonas aeruginosa | Clinical | Sputum | S | S | R | R | R | W |
| Klebsiella pneumonia | Clinical | Sputum | S | R | R | W | R | S |
| Escherichia coli | Clinical | Urine | S | S | S | S | S | S |
| Staphylococcus aureus | Clinical | Pus | R | R | R | S | S | S |
| Bacillus cereus | ATCC 10876 | – | S | S | R | R | S | S |
| AK: Amikacin 30 µg, GM: Gentamicin 10 µg, CPM: Cefepime 30 µg, TC: Ticarcillin 75 µg, PRL: Piperacillin 100 µg, IMI: Imipenem 10 µg, R: Resistant, S: Sensitive, (Bacterial inhibition zone ≤ 10 mm considered sensitive). | ||||||||
As shown in (Table 3), the disc diffusion test revealed various antibacterial efficacies of the plant extract. At concentration 400mg/ml, Bacillus cereus ATCC 10876, which is a referenced non-MDR bacterial strain showed the highest inhibition zone (13.00±0.00 mm) and Proteus vulgaris (8.75± 0.75 mm) which were statistically significant (p<0.05), followed by Staphylococcus aureus (7.25± 0.75 mm), Klebsiella pneumonia (6.50± 0.50 mm) and Pseudomonas aeruginosa (6.25± 0.25mm), respectively. There were also various degrees of antibacterial activity at the concentration 200 mg/ml of lesser strength. Figure (2) illustrates the antibacterial activity of the methanol leaf extract in comparison with the gentamicin (10µg/ml), after excluding the diameter of the paper disc (6 mm).
| Table 3: The antibacterial activity of the methanol leaf extract of Ziziphus mauritiana. | |||||
| Test | Mean zone of growth inhibition (mm)* | ||||
| Gram-positive | Gram-negative | ||||
| Sa | Bc | Pv | Pa | Kp | |
| Methanol extract (400 mg/ml) | 7.25 ± 0.75 | 13.00 ± 0.00** | 8.75 ± 0.75** | 6.25 ± 0.25 | 6.50 ± 0.50 |
| Methanol extract (200 mg/ml) | 6.50 ± 0.50 | 12.00 ± 0.00** | 7.50 ± 0.50 | 6.00 ± 0.00 | 6.50 ± 0.50 |
| Gentamicin (10 μg/disc) | 7.25 ± 0.75 | 20.00 ± 0.00** | 9.50 ± 0.50** | 10.00 ± 0.00** | 6.75 ± 0.25 |
| 80% Methanol | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| *Disc diameter=6 mm, zone off inhibition is the mean of two replicates ±standard error of means; 6 mm =no inhibition; Sa= Staphylococcus aureus, Bc= Bacillus cereus ATCC 10876 , Pv= Proteus vulgaris, Pa= Pseudomonas aeruginosa, Kp= Klebsiella pneumoniae. **Significant (p<0.05) | |||||
 |
Figure 1: The leaves of Ziziphus mauritiana. |
Bacteria with the highest sensitivity to the methanol leaf extract of Ziziphus mauritiana (Figure 2) were examined for MIC and MBC. The MIC and MBC values of Bacillus cereus ATCC 10876 and Proteus vulgaris were 25, 100 and 50, 100 mg/ml, respectively (Table 4).
 |
Figure 2: Representative photo showing the zone of inhibition of the extract. |
| Table 4: The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of bacteria showed significant sensitivity to the extract. | |||
| Microorganism* | MIC (mg/ml) | MBC (mg/ml) | MBC/MIC |
| Bc | 25 | 100 | 4 |
| Pv | 50 | 100 | 2 |
| *Bc= Bacillus cereus ATCC 10876, Pv= Proteus vulgaris | |||
Antioxidant Activity
In DPPH assay, the hydrogen donating ability of the extract was determined by converting the DPPH radical to nonradical by the reduction process. In this study, the methanol extract of Ziziphus mauritiana from Saudi Arabia was investigated for the antioxidant activity with DPPH scavenging assay. The results are shown in (Table 5).
| Table 5: IC50 (g/L) values of methanol extract of Ziziphus mauritiana to DPPH assay. | |||
| Antioxidant | Extract | Ascorbic acid | Quercetin |
| IC50 (g/L) | 0.024 g/L | 0.017 g/L | L |
The IC50 value was defined as the concentration of sample that scavenged 50% of the DPPH. In this study, the results showed an important antioxidant power of Ziziphus mauritiana extract compared to the standard product such as quercetin and ascorbic acid. The result (table 1) showed that the antioxidant activity of methanol extract of Ziziphus mauritiana has an IC50 value of 0.024 g/L, which was near to the inhibition capacity of the ascorbic acid. Then the quercetin has an IC50 value of 0.008 g/L. For the evaluation of the Reductive capacity of the methanol leaves extract of Ziziphus mauritiana (Iron III to Iron II. In the ferric to ferrous iron reduction assay, the electron donation capacity of the extract was assessed and compared to that of ascorbic acid, which known as strong reducing agent. The reducing power of Ziziphus mauritiana extract increased with the increasing of their concentrations (Figure 4).
| Table 6: Anti-inflammatory effect of Indomethacin and Extract using carrageenan-induced paw edema. | |||||||||
| Groups | Dose (mg/kg) | Paw volume (mL) | Inhibition % | ||||||
| 0 h | 1 h | 2 h | 3 h | 0 h | 1 h | 2 h | 3 h | ||
| Control | 0.0 | 0.350 | 0.402 | 0.420 | 0.342 | – | – | – | – |
| Indomethacin | 10 | 0.132 | 0.100 | 0.068 | 0.100 | 62.38 | 75.10 | 83.73 | 70.73 |
| Extrant | 200 | 0.183 | 0.167 | 0.197 | 0.258 | 47.62 | 58.51 | 53.17 | 24.39 |
| 400 | 0.142 | 0.113 | 0.152 | 0.225 | 59.52 | 71.78 | 63.89 | 34.15 | |
 |
Figure 3: The antibacterial activity of the methanol leaf extract of Ziziphus mauritiana compared to the antibiotic gentamicin. |
Anti-Inflammatory Activity
The anti-inflammatory activities were examined in vivo via inhibition of carragenan induced rat paw edema method at 200 and 400 mg/kg dose orally. The results of the anti-inflammatory activity obtained were compared to those of Indomethacin and those of the control, which received saline. The evolutions of inflammation for different groups are shown in Table 6.
According to the results (Table 6), the inflammation caused by carrageenan increases with time and reaches a maximum of 0.401 ±0.05mL for three hours. From these results, it appears that the plant extract inhibits significantly the inflammatory response. This inhibition is gathering of time, or at 1h the difference in paw volume is measured 0.167 ±0.06 ml, and the time equal to 2 hours the inhibition was 0.197 ± 0.06 mL and showed no significant difference compared to control. At time equal to 1 hour, the extract represents a highly significant inhibition of 0. 11 ±0.04 mL and which is very close to the effect of Indomethacin 0.10 ± 0.05 mL.
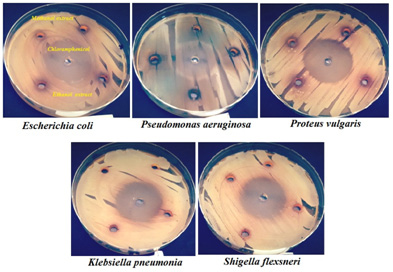 |
Figure 4: Reducing power of ascorbic acid and methanol extract of Ziziphus mauritiana. |
Then, this inhibition is continuous over time; one can deduce that the extract has an anti-inflammatory effect similar to the effect of Indomethacin. The anti-inflammatory activity (71.78% reduction in inflammation) was observed with extract at the concentration of 400 mg/kg body weight when administered orally to rats.
In the 1st hour, the MeOH extract at a dose of 200 mg/kg shows a percent inhibition of 47.62% lower than that obtained with Indomethacin, at a dose of 400 mg/kg at the third time the effect of the extract (71.78%) is almost similar to that of Indomethacin. (75.10%).) inhibition values.
The administration of Ziziphus mauritiana extract at a dose of 400 mg/kg prevents significantly (P <0.05) the plantar edema in rats from the second hour of treatment. This suggests the significant anti-inflammatory effect of the extract of the plant, it could be due to the richness of the methanol extract in bioactive compounds which are rich in Ziziphus, such as saponins, triterpenoic acids, fatty acids flavonoids and alkaloids.
DISCUSSION
The preliminary phytochemical screening showed various bioactive ingredients such as saponins, tannins, alkaloids, phenolic compounds, and terpenoids. The presence of these components is an indication that this plant has some medical properties. The findings of current study agreed with previous studies, Najafi (2013) reported that leaves of Ziziphus mauritiana revealed the presence of saponins, phenolic compounds, tannins and glycosides. Parmar et al. (2012) stated that leaves of Ziziphus mauritiana contain glycosides, saponins, phenols, lignins and tannins. Plants rich in saponins have anti-inflammatory activity and strengthen the immune system, Tannins are antibacterial compounds which damages the bacterial cell wall (Mainasara et al., 2012) Phenolic compounds, alkaloids, flavonoids, tannins, saponins and glycosides are good antioxidant compounds and controls the oxidative stress related disorders (Biapa et al., 2007).
The methanol leaf extract of Ziziphus mauritiana resulted in variable zone of inhibitions. However, Bacillus cereus ATCC 10876 (Gram-positive) and Proteus vulgaris (Gram-negative) exhibited the highest sensitivity. Similar studies published that leaves of Ziziphus mauritiana have antibacterial activity against different bacterial strains; Najafi (2013) cited that the methanol leaf extracts of Ziziphus mauritiana exhibited significant activity against Staphylococcus aureus and Escherichia coli. Ashraf et al. (2015) claimed that the methanol leaf extract of Ziziphus mauritiana has potent antibacterial effects against Escherichia coli, Bacillus subtilis and Staphylococcus aureus. However, in the current study the other clinical pathogens including MDR and non-MDR isolates did not show significant antibacterial activities. In addition, it was published that, leaves of Ziziphus mauritiana have no antibacterial effects against some bacterial strains.
Mainasara et al. (2012) mentioned that methanol and ethanol extracts did not show a significant effect against Pseudomonas aeruginosa, Salmonella typhi, Escherichia coli, Staphylococcus aureus and Streptococcus pyrogenes at 120 mg/ml. Accordingly, more studies regarding the mechanism and mode of action of this plant extract are required. The results also revealed that the ratios of MBC/MIC values are 4 and 2 for Bacillus cereus ATCC 10876 and Proteus vulgaris. It is considered that, to classify any plant extract as bactericidal the ratio of MBC/MIC should be ≤ 4, but if this ratio is > 4, so the plant extract classified as bacteriostatic (Djeussi et al., 2013). Accordingly, the methanol leaf extract of Ziziphus mauritiana have bactericidal effect on Bacillus cereus ATCC 10876 (Non-MDR referenced bacteria) and bacteriostatic effect on Proteus vulgaris (MDR-pathogen). Based on these interesting findings, more studies regarding the mechanism and mode of action of this plant extract are required in order to understand the nature of these antibacterial constituents. Free radicals, formed as result of oxidation, are one of the major causes of degenerative diseases (Phamhuy et al., 2008). Pharmacological evaluation of plant extracts is incomplete without assessment of their free radical scavenging activity. Therefore, in the present study, DPPH free radicals scavenging potential and the ferric reducing antioxidant power (FRAP) assay of methanol extract of Ziziphus mauritiana were appraised.
In this study, methanol extract of Ziziphus mauritiana has an IC50 of 0.024g/l, compared to ascorbic acid (IC50= 0.017 g/L) and Quercetin (IC50= 0.008 g/L). This result is in agreement with a previous study in Malaysia (Perumal et al., 2011), which reported an IC50 of Ziziphus jujuba leaf extract of 20.62 μg/mL. Another study in Pakistan shows an IC50 of 0.11 mg/mL (Ashraf et al., 2015).In Nepal, methanol extract of Ziziphus mauritiana has an IC50 of 47.50 μg/mL (Sharma et al., 2015). Methanol extract of Ziziphus mauritiana has an important antioxidant activity because it contains the substances having an antioxidant action such as ascorbic acids and flavonoids (Cheng et al., 2000; Pawlowska et al., 2000; Preeti and Tripathi, 2014). The flavonoids are considered as efficient radical scavengers and found in almost every plant. In addition, quercetin belongs to the flavonoids family, has a higher antioxidant activity than ascorbic acid, this results is reinforced by the study of Wybranowski et al. (2013).
In this study, methanol extract of Ziziphus mauritiana has good significant anti-inflammatory, the ratio of inhibition reached to 71.1% that close to positive control,The antioxidant and anti-inflammatory activity could be attributed to the presence of many bioactive compounds in Ziziphus mauritiana. Taking in to account that, it is plausible to suggest that the anti-inflammatory activity of extract of Ziziphus mauritiana involved, partly, synergistic action of alkaloids, flavonoids, condensed tannins, and saponins.
The cellular and molecular mechanism by which the ë-carrageenan induced inflammatory process is well known. It stimulates the release of histamine and serotonin from mast cells, starting it with a cascade of events that produce other mediators that contribute to the establishment of the acute inflammatory response (Cuzzocrea et al., 1998). Indeed, the carrageenan-induced during the early phase (1-2 h) of the inflammatory response, the production of pro-inflammatory factors such as histamine, serotonin, leukotrienes, PAF and prostanoids. These factors cause vascular changes leading to plasma exudation. In addition to their inhibition of the production of pro-inflammatory mediators, secondary metabolites (flavonoid, alkaloid and saponin) inhibit neutrophil recruitment to the pleural cavity through the inhibition of the expression of adhesion molecules on the endothelial cell wall veins (Middleton et al., 2000). Flavonoids block the migration of leukocytes to the inflammatory site by inhibiting adhesion molecules ICAM-1 and VCAM-1, and this regulation by TNF-á. (Tsuda et al., 2002) report that the administration of cyanidin 3-O-â-glucoside inhibits inflammation induced by zymosan. It has been reported indeed that quercetin blocks adhesion of leukocytes to the endothelial wall of the umbilical veins by inhibiting the expression of ICAM-1 (Anné et al., 1994). Gallic acid in turn inhibits leukocyte migration by inhibiting the molecules VCAM-1 adhesion, ICAM-1 and E-selectin in vascular endothelial cells, this inhibition is due to inhibition of IL-1, TNF-á and NF-kB (Murase et al., 1999). This agrees with our results at the high inhibition in the second hour of the experiment, which is related to the production of pro-anti-inflammatory compounds of the leaves of Ziziphus mauritiana.
CONCLUSION
Many pharmaceutical innovations are invented from natural products. Leaves of Ziziphus mauritiana are rich in phytochemical ingredients. These ingredients have antibacterial, antioxidant and anti-inflammatory properties. Isolation and purification of different bioactive phytochemicals may further yield significant antibacterial, antioxidant, anti-inflammatory or other curative properties against different ailments.
FUNDING
None
CONFLICT OF INTEREST
None-declared
REFERENCES
Abdallah, E. M. (2011): Plants: An alternative source for antimicrobials. J. Appl. Pharm. Sci., 1(6): 16-20.
Abdallah, E. M. (2016): Antibacterial efficiency of the Sudanese roselle (Hibiscus sabdariffa L.), a famous beverage from Sudanese folk medicine. J. Intercult. Ethnopharmacol., 5(2): 186-190.
Akhtar, N., Ijaz, S., Khan, H. M. S., Uzair, B., Reich, A. and Khan, B.A. (2016): Ziziphus mauritiana leaf extract emulsion for skin rejuvenation. Trop. J. Pharm. Res. 15(5): 929-936.
Anné, S., Agarwal, R., Nair, M. P., Schwartz, S. A., Ballow, M., Kandaswami, C. and Middleton, E. (1994): Inhibition of endotoxininduced expression of intercellular adhesion molecule-1 and of leukocyte adhesion to endothelial cells by the plant flavonoid quercetin. J. Allergy Clin. Immunol., 93: 276.
Ashfaq, M., Shah, K. W., Ahmad, S. and Singh, D. (2012): Preliminary phytochemical screening of alcoholic and aqueous extracts of Mentha longifolia Linn. leaves. Int. J. Biol. Pharm. Res., 3(3): 384-386.
Ashraf, A., Sarfrz, R. A., Anwar, F., Shahid, S.A. and Alkharfy, K. M. (2015): Chemical composition and biological activities of leaves of Ziziphus mauritiana L. native to Pakistan. Pak. J. Bot., 47(1): 367-376.
Biapa, P. C., Agbor, G. A., Oben, J. E. and Ngogang, J. Y. (2007): Phytochemical studies and antioxidant properties of four medicinal plants used in Cameroon. Afr. J. Trad. Complement. Altern. Med., 4(4):495-500.
Cheng, G., Bai, Y., Zhao, Y., Tao, J., Liu, Y., Tu, G., Ma, L., Liao, N..and Xu, X. (2000): Flavonoids from Ziziphus jujuba Mill var. spinasa. Tetrahedron., 56: 8915-8920.
Chew, Y. L., Chan, E. W. L., Tan, P. L., Lim, Y. Y., Stanslas, J. and Goh J. K. (2011): Assessment of phytochemical content, polyphenolic composition, antioxidant and antibacterial activities of Leguminosae medicinal plants in Peninsular Malaysia. BMC Compl. Altern. Med., 11:12. DOI: 10.1186/1472-6882-11-12
Clarke, E. G. C. (1975): Isolation and identification of Drugs. The pharmaceutical Press, London, UK.
Cuzzocrea, S., Zingarelli, B., Hake, P., Salzman, A. L. and Szabó, C. (1998): Antiinflammatory effects of mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, in carrageenan-induced models of inflammation. Free Radic. Biol. Med., 24(3):450–459.
Djeussi, D. E., Noumedem, J. A., Seukep, J. A., Fankam, A. G., Voukeng, I. K., Tankeo, S. B., Nkuete, A. H. and Kuete. V. (2013): Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complement. Altern. Med., 13: 164.
Dorman, H. J. D., Peltoketo, A., Hiltunen, R. and Tikkanen, M. J. (2003): Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem., 83: 255-262.
Doughari, J. H. (2006): Antimicrobial Activity of Tamarindus indica Linn. Trop. J. Pharm. Res., 5(2): 597-603.
Duh, P. D., Du, P. C. and Yen, G. C. (1999): Action of methanolic extract of mung hulls as inhibitors of lipid peroxidation and non-lipid oxidative damage. Food Chem. Toxicol., 37: 1055-1061.
El-Mahmood, A. M. and Ameh, J. M. (2007): In-vitro antibacterial activity of Parkia biglobosa (Jacq) root, bark extract against some microorganisms associated with Urinary tract infections. Afri. J. Biotech., 6(11): 195-200.
Gordon, M. H. (1990): The mechanism of antioxidant action in vitro. In: Foods antioxidants. Pp 1-18 (Ed) B. J. F. Hudson, London: Elsevier Applied Science, London UK.
Goyal, M., Sasmal, D. and Nagori, B.P. (2012): Analgesic and anti-inflammatory activity of ethanolic extract of Zizyphus nummularia. Res. J. Med. Plant, 6:521–528.
Goyal, M., Ghosh, M., Nagori, B. P. and Sasmal, D. (2013): Analgesic and anti-inflammatory studies of cyclopeptide alkaloid fraction of leaves of Ziziyphus nummularia. Saudi J. Biol. Sci., 20:365–371.
Jackson-Robert, L. and Morrow, J. D. (2011): Analgesic-Antipyretic and Anti-inflammatory agents and drugs employed in the treatment of gout. In: Goodman and Gilman’s the Pharmacological Basis of Therapeutics. Pp. 687-732 (Ed) Hardman, J.G., L.E. Limbird and A.G. Gilman, McGraw-Hill Medical Publishing Division 2011 New York USA.
Kubola, J. and Siriamornpun, S. (2008): Phenolic content and antioxidant activities of bitter gourd (Momordica charantia L.) leaf stem and fruit fraction extracts in vitro. Food Chem., 110: 881-890.
Kumar, S., Garg, V. K. and Sharma, P. K.. (2010): A review of Ziziphus nummularia. Pharmacologyonline, 2:565–574.
Lim, T. K. (2013): Edible Medicinal and Non-Medicinal Plants: Fruits, Springer Science+Business Media Dordrecht. 605-613. DOI 10.1007/978-94-007-5653-3_31
Mainasara, M. M., Aliero, B. L., Aliero, A. A. and Yakubu, M. (2012): Phytochemical and Antibacterial Properties of Root and Leaf Extracts of Calotropis procera. Nigerian J. Basic Appl. Sci., 20(1): 1-6.
Middleton, E. J. R., Kandaswami, C. and Heoradies T. C. (2000): The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev., 52: 673-751.
Mujeeb, F., Bajpai, P. and Pathak, N. (2014): Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. BioMed Res. Inter. Hindawi Publishing Corporation. Article ID 497606. http://dx.doi.org/10.1155/2014/497606
Murase, T., Kume, N., Hase, T., Shibuya, Y., Nishizawa, Y., Tokimitsu, I. and Kita, T. (1999): Gallates Inhibit Cytokine-Induced Nuclear Translocation of NF-kB and Expression of Leukocyte Adhesion Molecules in Vascular Endothelial Cells. Arterioscler Thromb. Vasc. Biol., 19:1412-1420.
Najafi, S. (2013): Phytochemical screening and antibacterial activity of leaf extract of Ziziphus mauritiana Lam. Int. Res. J. Appl. Basic Sci., 4 (11): 3274-3276.
Parmar, P., Bhatt, S., Dhyani, S. and Jain, A. (2012): Phytochemical studies of the secondary metabolites of Ziziphus mouritiana Lam. Leaves. Int. J. Curr. Pharm. Res., 4(3): 153-155.
Pawlowska, A. M., Camangi, F., Bader, A. and Braca, A. (2000): Flavonoids of Zizyphus jujuba and Zizyphus spina-christi (L) Wild (Rhamnaceae) fruits. Food Chem., 112: 858- 862.
Perumal, S., Mahmud, R., Piaru, S. P., Cai, L. W. and Ramanathan, S. (2012): Potential Antiradical Activity and Cytotoxicity Assessment of Ziziphus mouritiana and Syzygium polyanthum. Int. J. pharm., 8(6), 535-541.
Phamhuy, L. A., He, H. and Pham-Huy, C. (2008): Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci., 4(2): 89-96.
Preeti and Tripathi S. (2014): Ziziphus jujuba: a phytopharmacological review. Int. J. Res. Develop. Pharm. Life Sci., 3(3): 959-966.
Rates, S. M. K. (2001): Plants as source of drugs. Toxicon., 39: 603-0613.
Sharma, K. R., Kalauni, S., Awale, S. and Pokharel, Y. R. (2015): In vitro Free Radical Scavenging Activity of Methanol Extracts of Some Selected Medicinal Plants of Nepal. Austin J. Biotechnol. Bioeng., 2: 1-5.
Soliman, Y. H. (2011): Topical Anti-inflammatory and wound healing activities of herbal gel of Ziziphus nummularia L. (F. Rhamnaceae) leaf extract. Int. J. Pharm., 7:862–867.
Stahl, E. (1973): Drug analysis by Chromatography and Microscopy. Ann Arbor Scientific Publishers, Inc., Michigan, USA.
Tsuda, T., Horio, F. and Osawa, T. (2002): Cyanidin 3-O-β-D-Glucoside Attenuates the Hepatic Ischemia-Reperfusion Injury through a Decrease in the Neutrophil Chemoattractant Production in Rats. J. Nut. Sci. Vitam., 48 (2):134-141.
Wadood, A., Ghufran, M., Jamal, S. B., Naeem, M., Khan, A., Ghaffar R. and Asnad (2013): Phytochemical Analysis of Medicinal Plants Occurring in Local Area of Mardan. Biochem. Anal. Biochem. 2:144.
WHO (1996): WHO Guideline for the Assessment of herbal medicines. WHO Expert Committee on specification for pharmaceutical preparation. Technical Report series no. 863, Geneva.
Winter, C. A., Risley, E. A. and Nuss, G. W. (1962): Carrageenan-induced edema in hind paws of the rat as an assay for anti-inflammatory drugs. In: Proceedings of the Society for Experimental Biology and Medicine, New york, N.Y., 111: 544–547.
Wybranowski, T., Ziomkowska, B. and Kruszewski, S. (2013): Antioxidant properties of flavonoids and honeys studied by optical spectroscopy methods. Med. Biol. Sci., 27(4):53-58.
Yusuf, A. Z., Zakir, A., Shemau, Z., Abdullahi, M. and Halima, S. A. (2014): Phytochemical analysis of the methanol leaves extract of Paullinia pinnata lin. J. Pharm. Phyto., 6(2):


