Department of Biotechnology, IMS Engineering College, Ghaziabad (UP)
Corresponding author Email: priyaranjan.biet07@gmail.com
Article Publishing History
Received: 22/03/2019
Accepted After Revision: 15/05/2019
Tuberculosis, one of the most appalling disorders of the ancient times and still the major fatality affecting bacterial infection, is the condition principally concerned with the pulmonary complexities as well as sometimes damaging extra-pulmonary regions in the body also, demands proper medication and monitoring to be rectified effectively. In countries like India, the incremental prevalence of the resistant TB makes it obligatory to act rigorously in order to control the TB cases and adhere to the TB management programmes. Resistant tuberculosis represents the irrepressible state where the available TB drugs become insufficient for the treatment, worsening the lives of the sufferers. The situation is emerging in such a way that there would the time when the encountered totally-drug resistant cases become effectively high, emphasizing the necessity to develop drugs with improvised antagonistic capabilities to eradicate the existence of Mycobacterium. This review represents a thorough study of the dreadful existence of the pathogen providing all the required information for a better understanding of the disease and its resistance.
Tuberculosis, Mycobacterium tuberculosis, Resistant Tuberculosis, Active tuberculosis, Latent tuberculosis
Yadav V, Bisht D, Kumar P. R. Tuberculosis: A Comprehensive Study on its Evolution, Variants, Causes and Treatment Related Challenges. Biosc.Biotech.Res.Comm. 2019;12(2).
Yadav V, Bisht D, Kumar P. R. Tuberculosis: A Comprehensive Study on its Evolution, Variants, Causes and Treatment Related Challenges. Biosc.Biotech.Res.Comm. 2019;12(2). Available from: https://bit.ly/2IrOlWO
Copyright © Yadav et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
The paleopathological evidence suggests the ubiquity of a scourging lung disease about thousand years back, invoked the terror of death amongst the people since then. The dynamicity of the disease, Tuberculosis, prevails in the names which were used in the history to indicate the presence of disease, namely, phthisis, consumption and the great white plague among many others (Frith, 2014). Tuberculosis is a contagious disease in which the lungs get severely damaged by the invasion of Mycobacterium tuberculosis, the causative agent of pulmonary disease, results in the lethality to the host (Adigun and Bhimji, 2018). TB is a prominent cause of mortality enlisted in the top ten fatal diseases worldwide, reported in 2018 WHO Global Tuberculosis report. The emergence of the disease has caused 1.3 million deaths and 10 million new TB cases in 2017, required 10.4 US Dollar funding in the year 2018 for TB interventions. The severity of the incidence of the disease is prone to the South-East Asia Region (44%), followed by Africa (25%), whereas the Region of the Americas and Europe contributed least with 2.8% and 2.7% incidence respectively(WHO Key facts, 2018;WHO Global Tuberculosis Report, 2018).
The subjected risk factors include bacillary load, proximity to an infectious case, immunosuppressive conditions, malnutrition, alcohol and many others, create a favourable environment to increase the chances of contagion (Narasimhan, et al., 2013). The symptoms encountered at the onset of active TB disease are prolonged cough, fever and weight loss, eventually worsens with time (Heemskerk, et al., 2015;Shanmuganathan and Shanmuganathan, 2015). Mycobacterium, a member of the actinomycete group, classified into fast and slow-growers, collectively estimates for 150 identified species, possess the pathological ability to cause critical pulmonary, disseminated and cutaneous diseases to the host (Todar,2008-2012; Talbot and Raffa, 2015;King, et al., 2017). The mycobacterial invasion of the host organism not necessarily culminates in the active infection due to the robust host immune system, thus results in latent tuberculosis. The latent tuberculosis infection (LTBI) persists in one-third of the global population, but consequently, 5-10% develops active tuberculosis within the foremost five years of initial infection, a process termed as TB reactivation(Flynn and Chan, 2001;WHO Latent tuberculosis infection (LTBI) – FAQS, 2019).
The first milestone research happened to combat TB was BacilleCalmette-Gue´rin (BCG) vaccine which took 13 years(1908-1921), discovered by Calmette and Gue´rin, followed by the development of antimycobacterial drugs starting from the discovery of streptomycin in 1943(Luca and Mihaescu,2013; Podany and Swindells, 2016). Besides an efficient treatment regimen implemented all over the world, the unexpected multidrug-resistant tuberculosis (MDR-TB) outrage in 1980 due to lacking docility towards the continued regimens along with the repeated use of already existed drugs. The concern has become tremendous due to the rise of extensively drug-resistant tuberculosis (XDR-TB), and currently totally drug-resistant tuberculosis (TDR-TB) (Smith, et al., 2013).
In response to the prevailing failed treatment and persisting resistant TB, in the early years of the 1990s, WHO has suggested a short course, Directly Observed Treatment (DOTS), to ensure the improvement in the adherence towards the treatment (Rabahi, et al., 2017). Globally every year the rate of TB is deteriorating with 2%, saving approximately 54 million lives, between 2000-2017, with the help of appropriate diagnosis and treatment provided (WHO Key facts, 2018). The extensive duration and complex treatment, its side effects, HIV coinfection, are amongst the many profound challenges in the TB treatment which are discussed later in the article, subjecting an individual prone to develop TB (Shehzad, et al., 2013; Boogaard, et al., 2008).
History Of Tuberculosis
Tuberculosis discovery cannot be attributed to a single individual as it took the involvement of a huge number of people to recognise the etiology and pathogeny of such a dreadful illness. Pioneers for this were Aristotle (384-322 BCE), Hippocrates (410- 400 BCE), Cassius Felix (447 CE) and Aretaeus of Cappadocia who autonomously identified the disease, specifying it with different names such as Scrofula, Phthisis and Pott’s disease (Frith, 2014). Phthisis, as described by Hippocrates, was the weakness of the lungs, a lethal and contagious disorder affecting youthful inhabitants, causing cough, fever and characteristic lung injuries. Scrofula signified the malady of throat’s lymph nodes, also termed as King’s evil and assumed to be healed by royal touching in England and France (Barberis,et al., 2017) while Pott’s disease was the one where spines were damaged. Consumption, Robber of the youth, The great white plaque and Graveyard cough are some of the other names used, expressing the discouragement and miseries people were undergoing, Frith, 2014; Frith, 2014). The primary archaeological shreds of evidence for the prevalence of consumption have been observed by the investigations conducted on the Egyptian mummies, implying disease incidence during 3000–2400 BC (Al-Humadi, et al., 2017), due to the presence of spinal and rib tubercular wounds, as well as the distorted bones found at several Neolithic localities in the Middle East, Italy and Denmark (4000 years ago) ( Daniel, 2006; Smith, 2003). The initially available written documents on TB were reported in India and China, dating back to 3300 and 2300 years ago, respectively (Daniel, 2006 Barberis, et al., 2017).
The 19th centenary witnessed magnificent contribution in the field of tuberculosis studies commencing with the invention of the stethoscope by Laennec in 1816, facilitating a proper analysis of subjects by hearing their chests recognising any sort of abnormalities present. He persuaded that the factor accountable for the numerous forms of the disease is the same Tubercle, possessing the capabilities to produce pulmonary as well as the extra-pulmonary infections and rendered detailed information on the miliary and caseous(cheese-like) forms directing the pus or cavity formation within the lungs or in the different organs. He himself died grieving from the same disease. In 1834, a German physician Johann Lukas Schönlein described the disease with tubercle as Tuberculosis but did not correlate it with phthisis or scrofula. Jean Antoine Villemin, a French military surgeon in 1865 identified that the soldiers from the countryside or who worked more on the fields were healthier and had fewer possibilities of phthisis contrasting to those at closed spaces for long times. He illustrated that this ailment is contagious by confirming its transfer to the rabbits from human or cattle (Sakula, 1983 Frith, 2014).
Pasteur’s germ theory of infectious disease (1862) appeared as the basis to conclude the presence of a microbiological entity liable for disease outbreaks, consequently search for cause of TB began, preceding to which Robert Koch in 1882 identified the Tubercle bacillus, the causative agent for the consumption and proposed the etiological perspectives of the tuberculosis infection (Frith, 2014;Murray, 2004). This led to the classification of the disease as Koch’s disease or Koch’s bacillus (Al-Humadi, et al., 2017; Sakula, 1983). Afterwards, the importance of immunisation for the prevention of TB was highlighted with the experiments conducted by Clemens Freiherr von Pirquet who scouted an outline of Latent Tuberculosis and commenced the development of the BCG vaccine, (Daniel,2006). Search for curative medications for the treatment started with the development of Streptomycin and Para-aminosalicylic acids proving advantageous for subduing the adverse consequences of the disorder (Zhang, 2005 Frith,2014 ).
Table 1: Chronological events of tuberculosis discovery (Frith, 2014; Frith, 2014; Al-Humadi, et al., 2017; Sakula, 1983; Murray, 2004; Youmans,et al., 1947; Chakraborty Table 1: Chronological events of tuberculosis discovery (Frith, 2014; Frith, 2014; Al-Humadi, et al., 2017; Sakula, 1983; Murray, 2004; Youmans,et al., 1947; Chakraborty and Rhee, 2015and Rhee, 2015).
| YEAR | EVENT |
| 410 BCE | Hippocrates discovered Phthisis |
| 2400 BC | Evidence of TB in Egyptian Mummies |
| 174 CE | Claudius Galen of Pergamum suspected TB as contagious |
| 1363 | French surgeon Guy de Chauliac proposed the removal of Scroufulos organ as the cure |
| 1650 | Sylvius described Tubercle |
| 1689 | Richard Morton explained the pathogeny of consumptive and additional forms of the infection |
| 1702 | Jean-Jacques Manget gave the term “miliary tuberculosis” |
| 1720 | Benjamin Marten, in his publication “A new theory of Consumption” speculated contagious nature of TB |
| 1779 | Sir Percivall Pott identified as Pott’s disease |
| 1793 | Matthew Bailledescribed the caseous (“cheese-like”) characteristics of phthitic ulcers |
| 1803 | Gaspard-Laurent Bayle of Vernet defined the pulmonary and other kinds of tubercle infection |
| 1816 | Stethoscope invented by Laennec |
| 1834 | Tuberculosis term coined by Johann Lukas Schönlein |
| 1843 | Philipp Friedrich Hermann Klenckeinoculated rabbits with TB |
| 1844 | Friedrich Gustav Jakob Henle postulated phthisis as infectious |
| 1854 | Sanatorium cure started |
| 1862 | Pasteur’s germ theory of infectious disease |
| 1865 | Jean Antoine Villemin inoculated guinea pigs and rabbits with tubercle from cows and humans |
| 1882 | Koch discovered Tubercle Bacillus |
| 1907 | Clemens Freiherr von Pirquet introduced Latent TB |
| 1908 | Albert Calmette and Camille Gue developed BCG vaccine |
| 1921 | BCG vaccine first used on humans |
| 1943 | Streptomycin discovered |
| 1946 | PAS(para-aminosalicylic acid) discovered by Gerhard Domagk |
| 1950 | Golden era of Anti-TB Drug |
Mycobacterium
Mycobacterium tuberculosis (MTB), a dreaded agent of TB infection, belongs to the family Mycobacteriaceae, in phylum Actinobacteria.
 |
Figure 1: Taxonomical classification of Mycobacterium tuberculosis (Todar,2008-2012) |
MTB is a rod-shaped, non-motile obligate aerobic bacterium, which is neither Gram-positive nor Gram-negative and thus classified as acid-fast bacteria (Todar,2008-2012). There are nearly 150 known species under Mycobacterium genus which are further classified as fast-growing( visible growth within 7 days) and slow-growing species(more than 7 days for visible growth)(King, 2017; Stahl and Urbance, 1990). The slow growers are comparatively more host pathogenic which comprises human and animal infectious pathogens namely, Mycobacterium tuberculosis, Mycobacterium bovis, Mycobacterium paratuberculosis and members of the Mycobacterium avium complex. Nevertheless, both groups involve pathogens that can cause pulmonary, disseminated and cutaneous diseases (Wards and Collins, 2000; King, 2017).
The Mycobacterium tuberculosis complex (MTBC) covers all the closely related humans and animal infecting species with an interrelated genome. They vary on the basis of epidemiology, pathogenicity, host specificity and ability to resist drug. MTBC involves pathogens M. tuberculosis, M. bovis, M. bovis BCG, M. africanum, M. microti, and M. canetti amongst which M. tuberculosis and M. africanum targets human as host whereas M. bovis infects wild and domestic animals and may infect humans too (Richter,et al., 2003; Bayraktar,et al., 2011). Mycobacterium leprae, another species of Mycobacterium genus, has the preference for the skin and nerves and causes Leprosy (Hansen’s disease) (Bhat and Prakash, 2012). Mycobacterium avium complex (MAC) is different from MTBC, which comprises multiple nontuberculosis mycobacterial species (NTM) and is ubiquitous in nature. MAC infects HIV-immunocompromised human, children with cystic fibrosis, people with existing pulmonary disease and as well as lung disease and bronchiectasis in patients without underlying lung disease. Apart from pulmonary infections, MAC also causes skin and soft tissue infections, musculoskeletal infections, and lymphadenitis as well (Field, et al., 2004; Shin,et al., 2010; Akram and Attia, 2018).
Table 2: LTBI vs ATBI vs NTMI (Akram and Attia, 2018; Schluger, 2008; Kendall,et al., 2011;Ryu,et al., 2016; Centers for Disease Control and Prevention,2013)
| FEATURES | LATENT TUBERCULOSIS INFECTION (LTBI) | ACTIVE TUBERCULOSIS INFECTION | NON-TUBERCULOUS INFECTION (NTMI) |
| Causative organism | Mycobacterium tuberculosis complex including: Mycobacterium tuberculosis | Mycobacterium tuberculosis complex including: Mycobacterium tuberculosis | Mycobacterium avium complex including: M. avium, Mycobacterium intracellular, Mycobacterium paraintracellulare |
| Type of infection | Inactive or dormant MTB sequester inside granuloma | Activated MTB causes chronic pulmonary and extra pulmonary infection | NTM causes pulmonary infections, Hypersensitivity pneumonitis (HP), musculoskeletal infections, and lymphadenitis |
| Transmission | Not contagious | Contagious to other individual | Not contagious |
| Test for indication | TB skin test or TB blood test reaction | TB skin test or TB blood test reaction | Positive sputum culture, Bronchoscopy, transbronchial or lung biopsy |
| Sputum or Smear test | Negative | positive | Positive |
| Radiography reports | Normal | May be abnormal | Radiological patterns visible |
| Symptoms | asymptomatic | cough, fever, and/or weight loss | common clinical symptoms with pulmonary TB like chronic cough, dyspnea, low-grade fever, malaise and weight loss |
| Risk factors | Direct contact with infected person | Immunosuppressive drug consumption, malnutrition, HIV co-infection | HIV immunocompromised individual, Children with cystic fibrosis |
Pathogenesis
The primary exposure of MTB follows dormancy or latency in 90% of the human host, determined as latent tuberculosis infection (LTBI) while the remaining 10% human develops the clinical disease (Ilievska-Poposka, et al., 2018). The Latent TB is an asymptomatic clinical condition in which the person lacks the active disease by remaining in non-replicating or dormant bacilli state in response to the host immune system (Schraufnagel, 2016). The dormant tubercle bacilli persist in the human host for years, develops LTBI and consequently emerge as an active TB infection and thus becomes contagious to non-infected individuals (Centers for Disease Control and Prevention, 2013). The pathogeny of TB infection initiates when an infected person releases aerosol in the environment in the form of tiny droplets, carries loads of MTB in it to spread, enters the pulmonary region of the non-infected person when inhaled. The immune response is generated by the existing resident alveolar macrophages, by developing bacterial-macrophage interaction to phagocytose MTB. Pathogenic MTB can also enter and thrives in Alveolar epithelial type II pneumocytes, which is present in plenty of number than macrophage (Smith, 2003; Kaplan, et al., 2003). When the MTB ingested by immune cells including macrophages and dendritic cells, they get killed often but other times succeed in escaping the bactericidal effects of the alveolar macrophages. It is due to the characteristic property of the MTB to prevent phagolysosomal fusion and thus hinder phagosome acidification which leads to the formation of inactivated macrophages, where the MTB multiplies and continuously damages it. The infected macrophage triggers the activation of other immune response including oxygen and nitrogen reactive species, blood monocytes and other inflammatory cells, which slows down the replication rate but cannot eradicate the bacteria efficiently. The granular structure develops finally, in which the activated T-cells and macrophages accumulate to restrict the infection within the structure, which is the consequence of host’s efficient immune response, to limit the growth and spread of MTB to surrounding cells, denoted as latency (Warner, 2015; Sasindran and Torrelles, 2011). It requires both innate and adaptive immunity of a host organism to form a granuloma, keep the MTB growth intact inside the granuloma and its rupture (Ehlers and Schaible, 2012).
Intracellularly, the granular structure becomes necrotic and hypoxic due to which MTB undergoes dormancy and stays asymptomatic. Although the granuloma can effectively restrict the infection with the host’s strong immunity but if the host’s immunity fails due to various factors including consumption of immunosuppressant drugs, HIV coinfection, malnutrition, may eventually lead to the reactivation and uncontrolled proliferation of the bacteria, followed by bursting of granuloma. At this stage, the escaped bacteria from granuloma start infecting the surrounding pulmonary cavity and consequently emerges as an active TB infection from LTBI. The active TB infection may also infect other tissues and emerges as extra pulmonary TB or military TB (Smith, 2003;Sasindran and Torrelles, 2011 and Warner 2015).
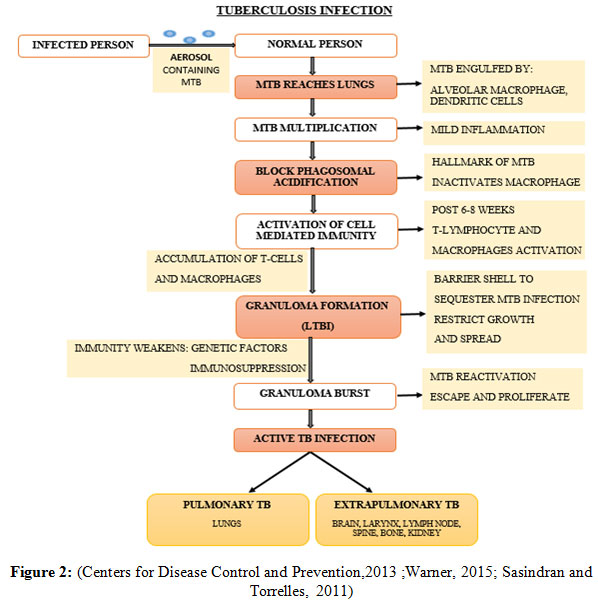 |
Figure 2: (Centers for Disease Control and Prevention,2013 ;Warner, 2015; Sasindran and Torrelles, 2011) |
Tb Comorbidities And Associated Risk Factors
Tuberculosis, being a chronic contagious disease, gets clinically active due to the breakdown in immunity surveillance of host organism, reflecting a positive association with both communicable diseases (CDs) or non-communicable diseases (NCDs) (Bates,et al.,2015). Studies revealed NCD’s can be easily developed by the TB patients (including Diabetes mellitus, cancer, chronic lung disease, alcohol-abuse disorder) (Peltzer, 2018), demonstrating a strong association with high TB mortality rates (Zerbini, et al., 2017). These medical conditions which attribute towards the risk factors for TB and the resulting poor outcomes in the treatment of TB disease are the TB associated comorbidities (Peltzer,2018; TB comorbidities and risk factors, WHO, 2019). The emerging cases of comorbidities in the general population contribute to the TB burden and result in the enormous challenge faced by health care services (Peltzer, 2018).
Consequently, preventing the failure of recognizing the comorbidities can help to encourage joint management approaches and to control the TB epidemic (Bates, et al., 2015). Risk factor induces the occurrence of clinically active TB disease due to the fact, not every individual with existing bacterial infection develops TB disease (Shanmuganathan and Shanmuganathan, 2015). The risk factors associated with TB constitutes distal factors and proximate factors. The distal factors include socioeconomic status while proximate determinants are the host and environmental factors (Taha,et al., 2011). HIV coinfection, most critical immunity compromising factor, amongst other considerable risk factors including alcohol abuse, poverty, overcrowding, malnutrition and many others needed to be addressed to control the TB disease progression (Narasimhan, et al., 2013).
Resistant Tb
Drug resistance Tuberculosis, a condition when the causative microbiological agent for TB develops endurance due to the spontaneous modifications happening within the genetic constitution of the bacteria itself, resulting in the failure of the patient’s immune system to respond to the drugs prescribed for treatment. Resistant TB is a man-made phenomenon; non-adherence to the drug therapy regimen, single drug or improper combination of prescribed drugs, delayed diagnosis, inadequate quality of drugs, first-hand contact with a drug-resistant TB sufferer, incompetent drug formulations, are some of the reasons for its development making it worse to treat (Davies,2001). It can develop in two distinct manners, Primary and Secondary Resistance. Primary resistance, when the patient is originally affected with the resistant bacterial strain, capable of forwarding this drug resistance to others as well, having no known prior treatment history while Secondary resistance is acquired during the course of treatment due to negligence in proper treatment (Centers for Disease Control and Prevention, 2013). Mutations in the target genes accountable for drug activation are the significant basis for a reduction in drug efficacy, leading to interference with numerous crucial biochemical processes (Seifert et al.,2015).
Mechanism of drug resistance involves Acquired antibiotic resistance and Intrinsic antibiotic resistance; Acquired antibiotic resistance, when the once susceptive strains of Mycobacterium underwent chromosomal alterations, enhancing the likelihoods of survival against the potent drugs by causing cellular mutagenesis, chiefly attributed to the higher release of reactive oxygen species (Smith et al.,2013) Intrinsic antibiotic resistance involves numerous intracellular mechanisms which help the mycobacterium to strive against the antibiotics, neutralising their effect and enhancing its survival potentialities. This boosts the competence of the Mycobacteria to tackle the drug activity as well as affecting the generation of the new drugs against this bacteriological agent. Intrinsic resistance, primarily concerned with diminishing receptiveness of the targets, can be categorised into passive resistance and specialized resistance mechanisms. Passive mechanisms include cell wall impermeability, the envelope comprising of an outer cell membrane, mycolic acid, arabinogalactan, peptidoglycan, arabinomannan and plasma membrane layers around the mycobacterial cell rendering it further protection from the antibiotic action. The non-availability of porin channels lessens the permeability of cell wall for hydrophilic antibiotics, MTB’s capability of existing in Latent or dormant states also favours the survival possibilities against drugs as the metabolic activity of the bacteria is remarkably low during this phase, resulting in lower production of ordinarily targeted molecules (Nasiri et al.,2017).
Specialized mechanism involves Modification of drug targets, either via conformational variations in the target to depreciate the binding affinity of antibiotics or by directly inactivating the drug with some chemical modification, Enzymatic degeneration of drugs, usually done by degrading the antibiotics with hydrolases, Molecular mimicry of drug targets, Enzymatic modification of antibiotics, where the mycobacterial modifying enzymes restrict the drug from binding to its target site by opportunistically altering the antibiotic (Smith et al.,2013) and the presence of efflux pumps supports the expulsion of the drug molecule outside the cell, contributing survival advantage and a low-level of drug tolerance (Szumowski et al.,2013).
Resistant Tuberculosis can be further differentiated into MDR(Multidrug-resistant tuberculosis), XDR(Extensively drug-resistant tuberculosis) and TDR(Totally drug-resistant tuberculosis) depending upon the perceptivity of the drugs towards treatment. The emergence of MDR and XDR has been observed to expand globally amongst the new TB subjects as well as the already treated ones. MDR-TB, resistance to the principal first-line drugs for tuberculosis, isoniazid(INH) and rifampicin(RIF), diminishing the potency of these anti-tuberculous drugs (Dash,2013; Eker et al.,2008). According to WHO 2018 statistics, among the entire encountered cases of TB, 3.5% new cases and 18% earlier treated TB patients had MDR-TB, the major shareholding countries include India, China and Russia (WHO Global Tuberculosis Report, 2018).
Rifampicin resistance, termed as RR-TB is observed to be more prevalent, it functions by repressing the transcription of the MTB to obstruct its functioning but mutation in the rpoB (β-subunit of RNA polymerase) gene limits rifampicin interaction and makes the cells resistant (Seung et al.,2015; Hameed et al.,2018) while isoniazid functions by inhibiting mycolic acid biosynthesis, which is hindered by the alterations in the katG (catalase-peroxidase), inhA (enoyl-acyl carrier protein reductase) or ahpC (alkyl hydroperoxide reductase) genes (Chhabra et al.,2012; Gillespie,2002; Telenti,1998). INH is a pro-drug activated by the katG gene whereas the inhA gene facilitates fatty acid elongation in mycolic acid biosynthesis, any modification in these can lead to resistance. The probability of resistance development is more in case of INH as compared to other anti-TB drugs (Shehzad, et al., 2013). This resistance towards existing drugs elevates the complexities in the treatment, prolonging the remedial period and the risks of contamination. XDR-TB is the resistance to either isoniazid or rifampicin accompanied with fluoroquinolones (ofloxacin, levofloxacin or moxifloxacin) and second line injectables such as kanamycin, capreomycin or amikacin. Social factors such as smoking, alcohol abuse, unemployment are few additional risk determinants for XDR-TB, Studies reveal that possibilities of MDR transforming to XDR-TB are comparatively higher in females than males (Kurz et al.,2016).
Its treatment relies on more noxious, expensive drugs with reduced potency (Calligaro et al.,2014). TDR-TB, the extreme situation when no response for any of the first line or second-line drugs is there, all the accessible drugs fail to cure the disease. Bedaquiline and delamanid, the recently developed drugs are also incapable to produce some sort of effect on TDR patients, hence declared untreatable by the Centre of disease control and prevention (Matteelli et al.,2014; Prasad et al.,2007). Numerous morphological variations are also identified in the TDR strains such as MTB with thicker walls and a highly branched structure (Velayati et al.,2013). Henceforth, minimising the probabilities for its occurrence is the only permissible alternative to tackle this condition, by getting the MDR and XDR-TB properly treated with the correct dose of drugs for appropriate time duration.
Table 3: Comparison between Drug susceptible and Drug resistant TB (WHO Global Tuberculosis Report, 2018; Seung et al.,2015; Louw et al.,2009; Chang and Yew,2013; Collantes et al.,2016; Yang et al.,2017).
| PARAMETER | DRUG SUSCEPTIBLE TB | MDR-TB | XDR-TB |
| Definition | Mycobacterial infection sensitive to available drugs | TB resistant to the two main first-line drugs | Resistance to few first-line drugs along with some second-line injectables |
| Resistant drugs | Sensitive to the available drugs; no resistance | Isoniazid, Rifampicin | Isoniazid, Rifampicin, Fluoroquinolones, kanamycin, capreomycin, amikacin |
| Mutated Genes | – | katG, InhA, ahpC, kasA, ndh,
rpoB |
katG, InhA, ahpC, kasA, ndh,rpoB,
gyrA, gyrB, rrs, tlyA |
| Symptoms | Chronic cough, Blood in sputum, weight loss | Same as drug susceptible TB | Same as drug susceptible TB |
| Cases notified (WHO 2018) | 6.7 million | 1,60,684 | 10,800 |
| Burdened countries | Angola,Brazil, Cambodia, China, Central African Republic, Ethiopia, India, Indonesia, Kenya, Liberia, Mexico, Mozambique, Myanmar, Namibia, Nigeria, Papua New Guinea, Philippines, Russian, Federation, South Africa, Thailand, VietNam, Zambia, Zimbabwe | Angola, Bangladesh, China, Democratic People’s Republic of Korea, DR Congo, Ethiopia, Indonesia, Kazakhstan, Kenya, Mozambique, Myanmar, Nigeria, Pakistan, Philippines, Russian Federation, Somalia,
South Africa, Thailand Ukraine, Uzbekistan |
The Russian Federation, India, Ukraine South Africa and Belarus |
| Preventive measures | Vaccination, strong immunity, early diagnosis, ventilated surroundings | Adherence to the provided treatment regimen | No negligence in MDR-TB treatment |
| Diagnostic technique | Chest X-ray,
Microscopy, Tuberculin skin testing |
Line Probe Assay,
Drug Sensitivity Testing, Microscopic Observation Drug Susceptibility |
Drug Sensitivity Testing, Microscopic Observation Drug Susceptibility |
| Side effects of Drugs | Nausea, vomiting, weight loss, hepatotoxicity, fatigue, abdominal pain, ataxia, anorexia, immunological reactions | High toxicity, hepatitis, joint pain, raised uric acid levels, renal insuffiency, allergic reactions, blurred vision, hypothyroidism, arthritis | Compartively more toxic than MDR drugs, longer treatment duration |
Diagnosis
Diagnosing Tuberculosis comprises numerous screening and affirmative analyses to detect the presence of the contagious microbial factor within the subject’s body. The choice of the diagnostic procedure is dependent on the site of infection either Pulmonary, Latent or Extra-pulmonary. Fundamental screening methods for analysis involves Chest X-Ray (CXR), the most followed microbiological identification test, intimating the presence of unusual deformities within the lungs or other body organs due to the growing tuberculin. It has been used for primary evaluation of TB for a long time, usually accompanied by the culturing of the sputum smear for confirmation. CXR, the diagnostic radiological test for severity prediction, provides the stage of infection depending upon the cavitation in the lungs (Ryu,2015). In case of Primary TB, X-ray displays abnormalities in the mid and lower lung portions, for Reactivated TB, nodules are found in the upper lung region and for Extra-pulmonary TB, X-ray shows nodules in the affected body organs. If immunosuppression is there, CXR may appear normal despite the presence of active bacteria (Cudahy and Shenoi,2016; Murthy et al.,2018).
Mantoux skin test, also named Tuberculin skin testing, standard for Latent TB infection identification (Brodie and Schluger,2005), is primarily a screening approach in the speculated individuals indicating the infection where a purified protein derivative (PPD) is introduced intradermally and skin induration at the site of reaction within subsequent hours indicates exposure to the bacteria. PPD is an antigenic amalgamation of mycobacteria leading to a hypersensitive skin response (Pai, 2005). It is cheap and comparatively easier to perform, although it might yield misleading results in the person previously immunised with the BCG vaccine, also in cases of inappropriate dosing of the PPD (The National Institute for Health and Care Excellence,2016).
Microscopy, the culturing of the mycobacteria collected from the sputum of the sufferer, evaluating the comprehensive TB burden, including viable as well the non-viable lifeless cells (Cudahy and Shenoi,2016). The cells grown in the laboratory conditions are stained using the Ziel- Neelson stain, staining the acid-fast species into bright red coloured cells. Since the mycobacteria is an acid-fast organism, it can be distinguished easily using this method (Parsons et al.,2011). It produces results with high specificity but minimum sensitivity. LED Microscopy is the latest advancement substituting the already existing microscopic technique (Cudahy and Shenoi,2016; Nema,2012).
Confirmatory tests for Tuberculosis covers several molecular approaches such as, Interferon Release Assays for quantification of the fraction of inflammatory cytokines particularly interferon-gamma (IFN-gamma) which are produced when mycobacterial cells are incubated into the patient’s blood only if the bacteria is already present in that system (Pai,2005; The National Institute for Health and Care Excellence, 2016). Currently, available tests involve T-SPOT.TB and the Quantiferon-TB assay, where isolated blood mononuclear cells and the complete blood are utilised respectively, helps in the detection of Latent as well as Active TB infection(Cudahy and Shenoi,2016; Brodie and Schluger,2005). Adenosine deaminase assays (ADAs) detect adenosine deaminase activity in serum and plasma samples, highly used in the detection of Extra-pulmonary TB (The National Institute for Health and Care Excellence, 2016).
Nuclear Amplification and Gene-Based Tests are the most advanced testing methods used for the confirmation of tuberculosis, DNA based molecular methods are employed to investigate the presence of Mycobacterium by identifying its genetic material which is amplified with the application of PCR (Ryu,2015; Parsons et al.,2011). Polymerase Chain Reaction finds its use in yet another molecular detection method termed as Line Probe Assay, facilitating accelerated identification of Mycobacterium Tuberculosis along with characterisation of rifampicin and isoniazid drug resistance in patients by the analysis of mutated gene sequences which make the bacteria resistant, with the aid of hybridization techniques (Lawn,2015; Desikan et al.,2017).
Drug Sensitivity Testing involves use of solid media cultures along with antibiotics to confirm the MTB growth and sensitivity towards the drugs (Cudahy and Shenoi,2016). Colorimetric Redox Indicator(CRI), Nitrate Reductase Assay(NRA) and Microscopic Observation Drug Susceptibility(MODS) are some of the non- commercial drug susceptibility testing approaches providing phenotypic characterization of the mycobacterium by testing mainly the rifampicin and isoniazid resistance in the cells. MODS analyses the early growth of bacteria in the culture with use of inverted microscope, aiding for faster detection of MDR-TB especially in high burden countries (Migliori et al.,2008). CRI and NRA are based on the colorimetric identification of the infectious agent present in the sputum directly or in the developed culture while NRA specifically focuses on the utilisation of the nitrate reducing capability of mycobacterium, further detected colorimetrically (Ahmad,2009). These both methods are used in the low- resource countries for faster and economical outcomes, still not endorsed by WHO due to insufficient evidences available (Heemskerk, et al., 2015; Desikan et al.,2017).
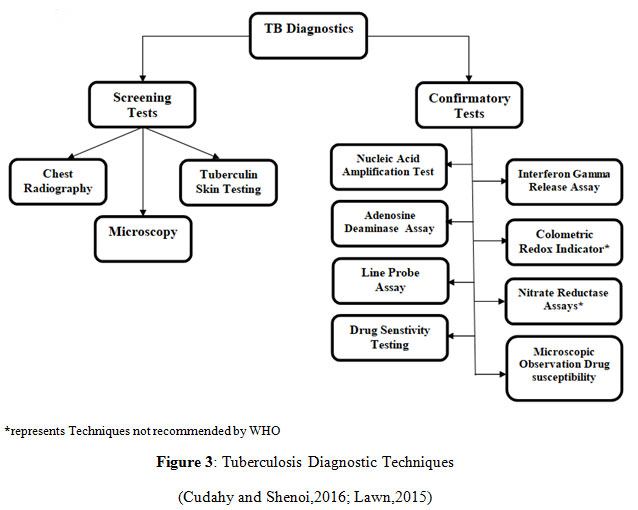 |
Figure 3: Tuberculosis Diagnostic Techniques (Cudahy and Shenoi,2016; Lawn,2015) |
Treatment and Its Challenges
The discovery of drugs and vaccine in history have been the evidence in the struggle against tuberculosis prevalence and the complexities still persist. Before the onset of TB drugs, remedies in the past relied on herbal solutions, dietary support and climatic prescripts along with some measures implemented during the 17th-19th century which included ameliorate lifestyle and suggesting sanatorium to the infected person, resulted in reduced incidences (Al-Humadi, et al., 2017; Iseman,2002; Zhang, 2005).
Starting from 1908, the work for developing BCG with the help of a virulent bovine strain implemented and finally reached to the human for trial in 1921 (Luca and Mihaescu,2013). It was only after World War II, the first-ever vaccine for tuberculosis was especially encouraged by UNICEF, World Health Organization (WHO), and by Scandinavian Red Cross Societies, which then extensively adopted in the US, Great Britain, and Germany (Al-Humadi, et al., 2017; Luca and Mihaescu,2013). The current 2017 data indicates high coverage by the BCG vaccine, provided to 158 countries out of which 120 reported 90% coverage (WHO Global Report, 2018).
The development of anti-TB drugs around the 1930s embarked the age of modern TB chemotherapy, derived from two anti-microbial sources of chemical and antibiotic origin (Zhang, 2005). The magnificent discovery of penicillin in 1928 and sulphonamide-prontosil rubrum in 1935 was the drastic move in the antimicrobial therapy (Iseman,2002; Tan and Tatsumura,2015; Jeśman,2011; Lesch,2008). Later in 1938, sulfa drug was used to treat TB infection in an experimental guinea pig which showed a significant result but didn’t perform well in humans. The drug was then further formulated to produce thiosemicarbazones, which was comparatively effective than sulfa drugs but not more than streptomycin (Zhang, 2005).
Modern chemotherapy took a steep turn in the range of anti-TB drugs with the discovery of streptomycin in 1943 by Waksman(Gonzales,1994). Being an active anti-mycobacterial agent, it possessed the hindrance property towards the tubercle and its infection in both the in vitro and in vivo conditions respectively(Hinshaw,1947). Soon after the discovery of streptomycin, Lehmann reported bacteriostatic property of para-aminosalicylic acid (PAS) on tubercle bacillus in 1943 with the first clinical trial on 1944 (Lehmann,1964; Kanabus,2018). Streptomycin and PAS, when provided individually, were responsible for drug resistance due to which combined dose prescribed for effective TB therapy but had certain side-effects. The year of coincidence 1951, released a potential anti-TB drug, isoniazid, which was coincidentally discovered by two different pharmaceutical specialists around the same time in 1912. Isoniazid demonstrated enormous supremacy among existing anti-TB drugs, with immense potency, mycobacterial specificity, tolerance and reasonability. Despite its superiority, the resistant TB infection still prevailed due to its individual practice, which subsequently induced the necessity of multiple drug treatment. The “triple therapy” recommended as the standard regimen for about 15 years, which included the combination of oral isoniazid altogether PAS for nearly two-years with intramuscular streptomycin for six months, was a great success (Chakraborty and Rhee,2015; Iseman, 2002;Murray et al.,2015).
The decade after the 1950s remarkably experienced augmented number of TB drugs and symbolized the Golden era in the history of Tb drug discoveries. The nicotinamide derived analogue, Pyrazinamide (PZA) (Zhang et al.,2013), synthesized in 1936 but recognized in 1952, followed by the development of Rifamycins (1957), ethambutol (1961) and clinical trial of rifampin in 1966 (Zhang, 2005; Murray et al.,2015).
Current Tb Regimen
DOTS, Directly Observed Treatment Short course, an efficient and economic internationally approved supervision method strongly recommended by the WHO to combat global tuberculosis (Tuberculosis, What is DOTS, WHO). The WHO’s Global Tuberculosis programme (GTB) promoted DOTS in 1993, which represented an amalgamation of a technical and managerial constituent, employed to prevent TB transmission by effectively restoring infectious cases(What is DOTS? : A guide to understanding the WHO-recommended TB control strategy known as DOTS. Geneva: WHO, 1999). With the launch of Stop TB strategy in 2006, the performance of DOTS accelerated (Treatment of Tuberculosis: Guidelines,WHO, 2010). Currently, the WHO has executed End TB Strategy in 2014, targeted to curb the global TB pandemic by 2035 (Implementing the end TB strategy: the essentials,WHO,2015). The ongoing WHO approved regimens categorized on the basis of types of TB cases which is depicted in the table:
Table 4: TB cases and its regimens (Treatment of Tuberculosis: Guidelines,WHO, 2010; Guidelines for treatment of drug-susceptible tuberculosis and patient care, 2017 update, WHO,2017)
| TYPES OF TB CASES | TB TREATMENT REGIMEN | |||
| Intensive phase | Continuation phase | |||
| Months | Drugs | Months | Drugs | |
| 1) New case
§ New patients § New patients with high level of Isoniazid resistance |
2
2 |
HRZE
HRZE |
4
4 |
HR
HRE |
| 2) Previously Treated case
(no documented resistance) |
2 | HRZE | 4 | HR |
| 3) HIV-positive TB patients | 6 months standard regimen (2HRZE+4HR) followed by ART within 8 weeks of treatment or 2 weeks for profound immune-compromised patients. | |||
Challenges
Mtb Clinical Manifestation
It demonstrates atypical properties possessed by the pathogen which involves high lipid content, unique physicochemical cell wall composition, intrinsic and acquired drug resistant properties, virulence, dormancy at a complex pulmonary cavity to avoid drug interaction and also the ability to cause extra pulmonary infection in the brain, kidney and bones (Al-Humadi, et al., 2017; Nasiri et al.,2017; Rabahi et al.,2017).
Treatment Drawbacks
Despite being a globally adopted regimen, the lag in the efficacy of DOTS prevails due to the non-adherence by the patients. The possible reasons for the non-adherence involve the non-economic, long-term chemotherapy (18-24 months) as seen in the case of MDR-TB. The standard treatment regimen involves multiple drugs to eradicate the pathogen effectively and their lengthy courses cause the pile-up of side-effects and related toxicity in the patient’s body. The first line drugs cause Hepatotoxicity, rashes, peripheral neuritis, sideroblastic anaemia whereas second-line drugs cause more toxicity to the patients including ototoxicity, vertigo, ataxia, and nystagmus, hearing loss, gastrointestinal side effects(nausea, vomiting), psychiatric disorder, depression psychosis, arthralgia, arthritis and gout. Due to the following reasons, the patient gets more detached from the therapy leading to either mortality or more drug resistant cases such as XDR-TB and even worse in the condition of Totally Drug Resistant (TDR), also there are increased chances of TB re-infection among the TB patients (Yang et al.,2017; Rabahi et al.,2017; Padgilwar et al.,2016; Tousif et al.,2015).
Hiv Co-Infection And Role Of Diabetes
HIV is an immunosuppressive virus, which drastically hampers the immunity of the person, causes the co-infection with TB more scourging and fosters profoundly resistant MTB strains which makes the TB management exigent and tough. The HIV coinfection with active TB, resistant TB or latent TB cases develops more fatal conditions due to which the provided treatment needs to be more effective, less time-consuming and achieving minimum probable drug-drug interaction between antitubercular and antiretroviral drugs (D’Ambrosio ,2015;Padgilwar,2016;Wells,2007). Diabetes also raises the probability of active TB development due to depleted immunity of an individual, which may account for emerging active TB cases (Shehzad, et al., 2013).There are certain factors which overpower the TB prevalence including poverty, exposure to the mass population, age, sex, immune response in an individual, nutritional level (Al-Humadi, et al., 2017).
Conclusion
Tuberculosis is an ever-growing disease, amongst ten lethal diseases, with the tendency to develop drug-resistant TB which intensifies the public health crisis. The 3 burden countries with resistant TB cases accounts for nearly half of world’s resistant cases, which involves India, China, and the Russian Federations. Mycobacterium Tuberculosis strain causes TB infection to the human beings, primarily inside the lungs for the majority of the cases whereas Extra pulmonary cases also accounts to the disease. MTB possesses inherent property and can acquire mechanisms through which they can thrive inside the host organism. There are various variables which accounts for the risk factors to the development of clinically active TB, by triggering immunosuppressive condition to the patient. Despite being the prominent cause of mortality, TB is preventable as well as curable, involving 6 months standard TB treatment regimen so as to control and manage TB. WHO introduced various strategies so as to eradicate the disease completely but due to various challenges it is still difficult to overcome the disease. The detailed updated information about the mycobacteria and its mode of action helps to understand the resistance mechanism as well as to develop drugs which could overcome them effectively. It is very important to account the various challenges confronted which hinders the effective TB treatment. The idea of creating this review is to provide the thorough information about the disease so that the maximum information can be available at a particular platform.
References
Adigun, R., Bhimji, S.S. 2018. Necrosis, Cell (Liquefactive, Coagulative, Caseous, Fat, Fibrinoid, and Gangrenous). In: Stat Pearls. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430935/ (Accessed on November 2018).
Ahmad, S. 2009. Recent advances in the diagnosis and treatment of multidrug-resistant tuberculosis. Respiratory Medicine,103, 1777-1790.
Akram, S.M., Attia, F.N. 2018. Mycobacterium avium In: StatPearls. Treasure Island (FL): StatPearls.
Available from: https://www.ncbi.nlm.nih.gov/books/NBK431110/
Al-Humadi, H.W., Al-Saigh, R.J., Al-Humadi, A.W. 2017. Addressing the Challenges of Tuberculosis: A Brief Historical Account. Frontiers in pharmacology, 8, 1-10.
Barberis, I., Bragazzi, N. L., Galluzzo, L., Martini, M. 2017. The history of tuberculosis: from the first historical records to the isolation of Koch’s bacillus. Journal of preventive medicine and hygiene, 58, E9-E12.
Bates, M., Marais, B. J., Zumla, A. 2015. Tuberculosis Comorbidity with Communicable and Noncommunicable Diseases. Cold Spring Harbor perspectives in medicine, 5, 1-15.
Bayraktar,B., Bulut,E.,Barış,A.B.,Toksoy,B., Dalgıc,N.,Celikkan,C., Sevgi, Dilek. 2011. Species Distribution of the Mycobacterium tuberculosis Complex in Clinical Isolates from 2007 to 2010 in Turkey: a Prospective Study. Journal of Clinical Microbiology, 49, 3837-3841.
Bhat, M., Prakash, C. 2012. Leprosy: An Overview of Pathophysiology. Interdisciplinary Perspectives on Infectious Diseases, 2012, 1-6.
Boogaard, J.V.D., Kibiki, G.S., Kisanga, E.R., Boeree, M.J., Aarnoutse, R.E. 2008. New Drugs against Tuberculosis: Problems, Progress, and Evaluation of Agents in Clinical Development. Antimicrobial Agents and Chemotherapy, 53, 849-862.
Brodie, D., Schluger, N W. The diagnosis of tuberculosis. Clinics in Chest Medicine, 26, 247-271.
Calligaro, G. L., Moodley, L., Symons, G., Dheda, K. 2014. The medical and surgical treatment of drug-resistant tuberculosis. Journal of thoracic disease, 6, 186-195.
Chakraborty, S., Rhee, K. Y. 2015. Tuberculosis Drug Development: History and Evolution of the Mechanism-Based Paradigm. Cold Spring Harbor perspectives in medicine, 5, 1-11.
Chang, K.C., Yew, W.W. 2013. Management of difficult multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis: Update 2012. Respirology,8, 8-21.
Chapter- 2 Centers for Disease Control and Prevention National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Tuberculosis Elimination. 2013. Core Curriculum on Tuberculosis: What the Clinician Should Know, 1-320. Available online: https://www.cdc.gov/tb/education/corecurr/index.htm
Chhabra, N., Aseri, M. L., Dixit, R., Gaur, S. 2012. Pharmacotherapy for multidrug resistant tuberculosis.Journal of pharmacology & pharmacotherapeutics, 3, 98-104.
Collantes, J., Solari, F. B., Rigouts, L. 2016. Rapid Detection of Mycobacterium tuberculosis Strains Resistant to Isoniazid and/or Rifampicin: Standardization of Multiplex Polymerase Chain Reaction Analysis. The American journal of tropical medicine and hygiene, 95, 1257-1264.
Communicable Diseases Cluster. 1999. What is DOTS? : A guide to understanding the WHO-recommended TB control strategy known as DOTS. Geneva: World Health Organization. Available online: http://www.who.int/iris/handle/10665/65979
Cudahy, P., Shenoi, S. V. 2016. Diagnostics for pulmonary tuberculosis. Postgraduate medical journal, 92, 187-193.
D’Ambrosio, L., Centis, R., Sotgiu, G., Pontali, E., Spanevello, A., Migliori, G.B. 2015. New anti-tuberculosis drugs and regimens: 2015 update. ERJ Open Research, 1, 1-15.
Daniel, T.M. 2006. The history of tuberculosis. Respiratory medicine, 100, 1862-1870.
Dash, M. 2013. Drug resistant tuberculosis: A diagnostic challenge. Journal of Postgraduate Medicine,59, 196-202.
Davies, P. D. 2001. Drug-resistant tuberculosis. Journal of the Royal Society of Medicine, 94, 261-3.
Desikan, P., Panwalkar, N., Mirza, S. B., Chaturvedi, A., Ansari, K., Varathe, R., Chourey, M., Kumar, P., Pandey, M. 2017. Line probe assay for detection of Mycobacterium tuberculosiscomplex: An experience from Central India. The Indian journal of medical research, 145, 70-73.
Ehlers, S., Schaible U.E. 2012. The granuloma in tuberculosis: dynamics of a host–pathogen collusion. Frontiers in Immunology, 3, 1-9.
Eker, B., Ortmann, J., Migliori, G. B., Sotgiu, G., Muetterlein, R., Centis, R., Hoffmann, H., Kirsten, D., Schaberg, T., Ruesch-Gerdes, S., Lange, C., German TBNET Group 2008. Multidrug- and extensively drug-resistant tuberculosis, Germany. Emerging infectious diseases, 14, 1700-1706.
Field, S.K., Fisher, D., Cowie, R.L. 2004. Mycobacterium avium complex pulmonary disease in patients without HIV infection. 126, 566–581.
Flynn, J.L., Chan, J. 2001. Tuberculosis: Latency and Reactivation. Infection and Immunity, 69, 4195–4201.
Frith, J. 2014. History of tuberculosis Part 1 – Pthisis, consumption and the White Plague. Journal of Military and Veterans’ Health, 22, 29-35.
Frith,J. 2014. History of Tuberculosis. Part 2 – the Sanatoria and the Discoveries of the Tubercle Bacillus. Journal of Military and Veterans’ Health, 22, 36- 41.
Gillespie, S.H. 2002. Evolution of Drug Resistance in Mycobacterium tuberculosis: Clinical and Molecular Perspective. Antimicrobial Agents And Chemotherapy, 46, 267–274.
Gonzales, J. Streptomycin was born fifty years ago. Histoire des sciences médicales, 28, 239-48.
Hameed, H., Islam, M. M., Chhotaray, C., Wang, C., Liu, Y., Tan, Y., Li, X., Tan, S., Delorme, V., Yew, W. W., Liu, J., Zhang, T. 2018. Molecular Targets Related Drug Resistance Mechanisms in MDR-, XDR-, and TDR-Mycobacterium tuberculosis Frontiers in cellular and infection microbiology, 8, 1-21.
Heemskerk, D., Caws, M., Marais. B., et al. 2015. Tuberculosis in Adults and Children. London: Springer. Chapter 2, Pathogenesis.
Available from: https://www.ncbi.nlm.nih.gov/books/NBK344406/
Hinshaw, H.C., Pyle, M.M., Feldman, W.H. 1947. Streptomycin in tuberculosis. The American Journal of Medicine, 2, 429-435.
Ilievska-Poposka, B., Metodieva1, M., Zakoska, M., Vragoterova, C., Trajkov, D. 2018. Latent Tuberculosis Infection – Diagnosis and Treatment.Open Access Macedonian Journal of Medical Sciences, 6, 651-655.
Iseman, M.D. 2002. Tuberculosis therapy: past, present and future. European Respiratory Journal, 20, 87s–94s.
Jeśman, C., Młudzik, A., Cybulska, M. 2011. History of antibiotics and sulphonamides discoveries. Pol MerkurLekarski, 30, 320-2.
Kanabus, A. 2018. History of TB drugs – PAS, Streptomycin, Waksman, Information about Tuberculosis, GHE.
Available online: https://www.tbfacts.org/history-of-tb-drugs/
Kaplan, G., Post, F.A., Moreira, A.L., Wainwright, H., Kreiswirth, B.N., Tanverdi, M., Mathema, B., Ramaswamy, S.V., Walther, G., Steyn, L.M., Barry C.E.III, Bekker L.G. 2003. Mycobacterium tuberculosis Growth at the Cavity Surface: a Microenvironment with Failed Immunity. Infection and immunity, 71,7099–7108
Kendall, B.A., Varley, C.D., Choi, D., Cassidy, P.M.,Hedberg, K., Ware, M.A, Winthropv, K.L. 2011. Distinguishing Tuberculosis from Nontuberculous Mycobacteria Lung Disease, Oregon, USA Brian. Emerging Infectious Diseases, 17, 506-509.
King, H.C., Butler, T.K., James, P., Oakley, B.B., Erenso, G., Aseffa A., Knight, R., Wellington, E.M., Courtenay, O. 2017. Environmental reservoirs of pathogenic mycobacteria across the Ethiopian biogeographical landscape. PlosOne, 12, 1-15.
King,H.C.,Khera-Butler, T.,James, P.,Oakley, B.B.,Erenso, G.,Aseffa, A.,Knight, R.,Wellington, E.M., Courtenay, O. 2017. Environmental reservoirs of pathogenic mycobacteria across the Ethiopian biogeographical landscape. Plos One, 12, 1-15.
Kurz, S. G., Furin, J. J., Bark, C. M. 2016. Drug-Resistant Tuberculosis: Challenges and Progress. Infectious disease clinics of North America, 30, 509-522.
Lawn, S. D. 2015. Advances in Diagnostic Assays for Tuberculosis. Cold Spring Harbor perspectives in medicine, 5, 1-18
Lehmann, 1964. Twenty years afterward historical notes on the discovery of the antituberculosis effect of Paraaminosalicylic acid (PAS) and the first clinical trials. The American review of respiratory disease, 90, 953-956.
Lesch, J.E. 2008. The first miracle drugs: how the sulfa drugs transformed medicine. Medical History. Medical History, 52, 416-417.
Louw, G.E.,Warren, R.M., Gey van Pittius, N.C., McEvoy, C.R., Van Helden, P.D., Victor, T.C. A Balancing Act: Efflux/Influx in Mycobacterial Drug Resistanc.Antimicrobial Agents and Chemotherapy, 53, 3181-3189.
Luca, S., Mihaescu, T. 2013. History of BCG Vaccine. Maedica, 8, 53-58.
Luca, S., Mihaescu, T. 2013. History of BCG Vaccine. Maedica, 8, 53-58.
Matteelli, A., Roggi, A., Carvalho, A. C. 2014. Extensively drug-resistant tuberculosis: epidemiology and management. Clinical epidemiology, 6, 111-118.
Migliori, G. B., Matteelli, A., Cirillo, D., Pai, M. 2008. Diagnosis of multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis: Current standards and challenges. The Canadian journal of infectious diseases & medical microbiology, 19, 169-172.
Murray, J.F. 2004. A Century of Tuberculosis. American Journal of Respiratory and Critical Care Medicine, 169, 1181–1186.
Murray, J.F., Schraufnagel, E., Hopewell, P.C. 2015. Treatment of Tuberculosis. A Historical Perspective. Annals of the American Thoracic Society, 12, 1749-1759.
Murthy, S.E., Chatterjee, F., Crook, A., Dawson, R., Mendel, C., Murphy, M.E., Murray, S.R., Nunn,A.J., Phillips, P.P.J., Singh, K.P., McHugh, T.D., Gillespie, S.H., REMoxTB Consortium. 2018. Pretreatment chest x-ray severity and its relation to bacterial burden in smear positive pulmonary tuberculosis. BMC Medicine, 16, 1-11.
Narasimhan, P., Wood, J., MacIntyre, C.R., Mathai, D. 2013. Risk Factors for Tuberculosis. Plumonary Medicine, 2013, 1-11.
Nasiri, M. J., Haeili, M., Ghazi, M., Goudarzi, H., Pormohammad, A., Imani Fooladi, A. A., & Feizabadi, M. M. 2017. New Insights in to the Intrinsic and Acquired Drug Resistance Mechanisms in Mycobacteria. Frontiers in microbiology, 8, 1-19.
Nema, V. 2012. Tuberculosis diagnostics: Challenges and opportunities. Lung India: official organ of Indian Chest Society, 29, 259-266.
Padgilwar, S.S, Manmode, R.S., Sahare, A.Y., Kadam, M., Manwar, J.V., Warade, P.P., Kumbhar, D.D. 2016. Recent Advances in Treatment for Tuberculosis: A Review. International Journal of Pharmaceutical Sciences Review and Research, 40, 162-172.
Pai, M. 2005. Alternatives to the Tuberculin skin test: Interferon-γ assays in the diagnosis of Mycobacterium tuberculosis Indian Journal of Medical Microbiology, 23, 151-158.
Parsons, L. M., Somoskövi, A., Gutierrez, C., Lee, E., Paramasivan, C. N., Abimiku, A., Spector, S., Roscigno, G., Nkengasong, J. 2011. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clinical microbiology reviews, 24(2), 314-350.
Peltzer, K. 2018.Tuberculosis non-communicable disease comorbidity and multimorbidity in public primary care patients in South Africa. African Journal of Primary Health Care & Family Medicine, 10, 1-6.
Podany, A.T., Swindells, S. 2016. Current strategies to treat tuberculosis. F1000Research, 5, 1-8.
Prasad, R., Singh, A., Balasubramanian, V., Gupta, N. 2017. Extensively drug-resistant tuberculosis in India: Current evidence on diagnosis & management. The Indian journal of medical research, 145, 271-293.
Rabahi, M. F., Silva Júnior, J., Ferreira, A., Tannus-Silva, D., Conde, M. B. 2017. Tuberculosis treatment. Jornalbrasileiro de pneumologia :publicacaooficial da SociedadeBrasileira de Pneumologia e Tisilogia, 43, 472-486.
Rabahi, M.F., Júnior, J.L.R.D.S., Ferreira, A.C.G., Silva, D.G.S.T., Conde, M.B. 2017. Tuberculosis treatment, JornalBrasileiro de Pneumologia, 43, 472-486.
Richter, E.,Weizenegger, M.,Rüsch-Gerdes, S.,Niemann, S. 2003. Evaluation of Genotype MTBC Assay for Differentiation of Clinical Mycobacterium tuberculosis Complex Isolates. Journal of Clinical Microbiology, 41, 2672-2675.
Ryu, Y. J. 2015. Diagnosis of pulmonary tuberculosis: recent advances and diagnostic algorithms. Tuberculosis and respiratory diseases, 78, 64-71.
Ryu, Y.J.,Koh, W.J., Daley, C.L. 2016. Diagnosis and Treatment of Nontuberculous Mycobacterial Lung Disease: Clinicians’ Perspectives. Tuberculosis and Respiratory Diseases, 79, 74-84.
Sakula, A. 1983. Robert koch: centenary of the discovery of the tubercle bacillus, 1882. The Canadian veterinary, 24, 127-131.
Sasindran, S.J., Torrelles, J.B., 2011. Mycobacterium tuberculosis infection and inflammation: what is beneficial for the host and for the bacterium?.Frontiers in Microbiology. 2, 1-16.
Schluger, N.W. 2008. Tuberculosis and Non-tuberculous Mycobacterial Infections in Older Adults. Clinics in chest medicine, 28, 1-11.
Schraufnagel, D.E. 2016. “Latent Tuberculosis Infection” Is a Term That Should Go Dormant, and the Significance of Latent Tuberculosis Should Be Rethought. Annals of the American Thoracic Society. 13, 593-594.
Seifert,M., Catanzaro,D., Catanzaro,A., Rodwell,T.C. 2015. Genetic Mutations Associated with Isoniazid Resistance in Mycobacterium tuberculosis: A Systematic Review. Plos One,10, 1-13.
Seung, K. J., Keshavjee, S., Rich, M. L. 2015. Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold Spring Harbor perspectives in medicine, 5, 1-20.
Shanmuganathan, R., Shanmuganathan, I.D. 2015. Clinical Manifestation and Risk Factors of Tuberculosis Infection in Malaysia: Case Study of a Community Clinic. Global Journal of Health Science, 7, 110-120.
Shehzad, A., Rehman, G., Islam, M.U., Khattak, W.A., Lee Y.S. 2013. Challenges in the development of drugs for the treatment of tuberculosis. The Brazilian Journal of Infectious Diseases, 17, 74-81.
Shin, S.J., Lee, B.S., Koh, W.J.,Manning, E.J.B., Anklam, K.,Sreevatsan, S.,Lambrecht,R.S., Collins, M.T. 2010. Efficient Differentiation of Mycobacterium avium Complex Species and Subspecies by Use of Five-Target Multiplex PCR. Journal Of Clinical Microbiology, 48, 4057-4062.
Smith, I. 2003. Mycobacterium tuberculosis Pathogenesis and Molecular Determinants of Virulence. Clinical microbiology reviews, 16, 463–496.
Smith, T., Wolff, K. A., Nguyen, L. 2013. Molecular biology of drug resistance in Mycobacterium tuberculosis.Current topics in microbiology and immunology, 374, 53-80.
Smith, T., Wolff, K.A., Nguyen, L. 2013. Molecular Biology of Drug Resistance in Mycobacterium tuberculosis. HHS Public Access, 374, 53-80.
Stahl, D.A., Urbance’, J.W. 1990. The Division between Fast- and Slow-Growing Species Corresponds to Natural Relationships among the Mycobacteria. Journal of bacteriology, 172, 116-124.
Szumowski, J. D., Adams, K. N., Edelstein, P. H., Ramakrishnan, L. 2013. Antimicrobial efflux pumps and Mycobacterium tuberculosis drug tolerance: evolutionary considerations. Current topics in microbiology and immunology, 374, 81-108.
Taha, M., Deribew, A., Tessema, F.,Assegid, S., Duchateau, L., Colebunders, R. 2011. Risk Factors of Active Tuberculosis in People Living with HIV/AIDS in Southwest Ethiopia: A Case Control Study. Ethiopian journal of health sciences, 21, 131-139.
Talbot, E.A., Raffa, B.J. 2015. Mycobacterium tuberculosis. Mycobacterium tuberculosis in Molecular Medical Microbiology (Second Edition). Academic Press, 3, 1637-1653.
Tan, S.Y., Tatsumura, Y. 2015. Alexander Fleming (1881–1955): Discoverer of penicillin. Singapore Medical Journal, 56, 366-367.
Telenti A. 1998. Genetics and pulmonary medicine. 5. Genetics of drug resistant tuberculosis. Thorax, 53, 793-797.
The National Institute for Health and Care Excellence.2016. Tuberculosis Prevention, diagnosis, management and service organisation. 1-551.
Todar, K. 2008-2012. Mycobacterium tuberculosis and Tuberculosis, The Good, the Bad, and the Deadly, Science Magazine, 304, 1-4.Available online: http://textbookofbacteriology.net/tuberculosis.html
Tousif, S., Ahmad, S., Bhalla, K., Moodley, P., Das, G. 2015. Challenges of Tuberculosis Treatment with DOTS: An Immune Impairment Perspective. Journal of Cell Science & Therapy, 6, 1-6.
Velayati, A. A., Farnia, P., Masjedi, M. R. 2013. The totally drug resistant tuberculosis (TDR-TB). International journal of clinical and experimental medicine, 6, 307-309.
Wards, B.J., Collins, D.M. 2000. Slow-Growing Mycobacteria. In: Eynard N., Teissié J. (eds) Electrotransformation of Bacteria. Springer Lab Manuals. Springer, Berlin, Heidelberg. Available online: https://link.springer.com/chapter/10.1007978-3-662-04305-9_20#citeas
Warner, D.F. 2015.Mycobacterium tuberculosis Metabolism. Cold Spring Harbor Perspectives in Medicine, 5, 1-23
Wells, C.D.,Cegielski, J.P.,Nelson, L.J.,Laserson, K.F.,Holtz, T.H.,Finlay, A.,Castro, K.G.,Weyer, K. 2007. HIV Infection and Multidrug-Resistant Tuberculosis—The Perfect Storm. The Journal of Infectious Diseases, 196, S86–S107.
World Health Organization. 2010. Treatment of Tuberculosis: Guidelines. 4th edition. World Health Organization.
Available online: https://www.who.int/tb/publications/2010/9789241547833/en/
World Health Organization. 2015. Implementing the end TB strategy: the essentials. World Health Organization. Available online: http://www.who.int/iris/handle/10665/206499
World Health Organization. 2017. Guidelines for treatment of drug-susceptible tuberculosis and patient care, 2017 update. World Health Organization. Available online: http://www.who.int/iris/handle/10665/255052.
World Health Organization. 2018. Global Tuberculosis Report. World Health Organization. Available online:
https://www.who.int/tb/publications/global_report/en/
World Health Organization. 2018. Tuberculosis, Key facts. World Health Organization. Accessed on September 2018.
Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis
World Health Organization. 2019. Tuberculosis(TB), Latent tuberculosis infection (LTBI) – FAQS. World Health Organization.
Available online: https://www.who.int/tb/areas-of-work/preventive-care/ltbi/faqs/en/
World Health Organization. Tuberculosis, What is DOTS (Directly Observed Treatment, Short Course), WHO
Available online: http://www.searo.who.int/tb/topics/what_dots/en/ Accessed on: December 2018.
World Health Organization.TB comorbidities and risk factors.
Available online: https://www.who.int/tb/areas-of-work/treatment/risk-factors/en/
Yang, T. W., Park, H. O., Jang, H. N., Yang, J. H., Kim, S. H., Moon, S. H., Byun, J.H., Lee, C. E., Kim, J. W., Kang, D. H. 2017. Side effects associated with the treatment of multidrug-resistant tuberculosis at a tuberculosis referral hospital in South Korea: A retrospective study. Medicine, 96, 1-5.
Youmans, G. P., Raleigh, G. W., Youmans, A. S. 1947. The Tuberculostatic Action of para-Aminosalicylic Acid.Journal of bacteriology, 54, 409-416.
Zerbini, E., Greco, A., Estrada, S., Cisneros, M., Colombo, C., Beltrame, S., Boncompain, C., Genero, S. 2017. Risk factors associated with tuberculosis mortality in adults in six provinces of Argentina. Medicina (Buenos Aires), 77, 267-273.
Zhang, Y. 2005. The Magic Bullets and Tuberculosis Drug Targets. Annual Review of Pharmacology and Toxicology, 45, 529–564.
Zhang, Y., Shi W.,Zhang, W.,Mitchison D. 2013. Mechanisms of Pyrazinamide Action and Resistance.Microbiology Spectrum, 2, 1-12.


