1Department of Biological Sciences,Faculty of Science, King Abdulaziz University21589, Jeddah, Saudi Arabia
2Najla Bint Saud Al Saud Center for Distinguished Research in Biotechnology,Jeddah, Saudi Arabia
3Department of Biology, Faculty of Science, University of Jeddah,Jeddah, Saudi Arabia
Corresponding author email: salah_aboaba@yahoo.com
Article Publishing History
Received: 24/11/2021
Accepted After Revision: 25/03/2022
Cancer has become one of the most common clinical reasons of death all over the world, with more than nine million deaths each year. Throughout the history, many traditional plants have been used to treat various types of cancers. The scientifically researched anticancer plants, with over 3000 species are novel candidates for anticancer drugs, because more than 80% of society believes in traditional medicine as its cure. The aim of this study was to determine the effects of Costus speciosus root extract on human lung cancer (LC) cells (A549). Treatment of (A549) cells with ethyl acetate Costus root extract significantly lowered the cell viability.
IC50 was calculated, after 24, 48 and72 h, with concentration of 25, 40, 70 and 100 μg/100μl. The MTT assay was used to test the viability of the cells. The effect of (EACR) on cell adhesion and migration was demonstrated in a wound healing assay, mitochondrial membrane potential was detected in a JC-1 assay, and DNA damage and repair were demonstrated in a comet assay. There was a noticeable reduction in (LC) development. Experiments underlying the anti-cancer activity of (EACR) on (A549) cells may be regarded as one of the most promising targets for novel cancer treatment strategies (LC).
A549, Anti-cancer activity, Costus speciosus, Ethyl acetate, Cell viability
Omar A. S, Nasraldeen R. A, Albiheyri R, Kady R. H, Abo-Aba S. E. M. The Effects of Costus speciosus Root Extract on Cultured Human Lung Cancer Cells, A549. Biosc.Biotech.Res.Comm. 2022;15(1).
Omar A. S, Nasraldeen R. A, Albiheyri R, Kady R. H, Abo-Aba S. E. M. The Effects of Costus speciosus Root Extract on Cultured Human Lung Cancer Cells, A549. Biosc.Biotech.Res.Comm. 2022;15(1). Available from: <a href=”https://bit.ly/36zVOmp“>https://bit.ly/36zVOmp</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Dietary risks in addition to bad lifestyle and tobacco are factors that help cancer development and are the main risk factors which at end lead to lung cancer (LC) and accounting for 22% of cancer deaths According to the World Health Organization (WHO). Lung cancer (1.76 million deaths), colorectal cancer (862 000 deaths), and stomach cancer (783 000 deaths) are the most common causes of cancer death., Liver (782 000 deaths), Breast (627 000 deaths). In lung cancer, similar histo-type between patients is a biological prove for different treatment response for each patient, with different levels of molecular heterogeneity have been recognized. In the top 10 cancers affecting males and females in Saudi Arabia, the most affected group by LC is males (Marisa et al, 2019, Alqahtani, and Alghamdi 2020).
The main types and pattern of cancer treatments include surgery according to NCI, 2015, chemotherapy, radiation therapy according to American Society of Clinical Oncology 2016, immunotherapy and hormone therapy. Traditional medicinal herbs are used worldwide to treat cancer through the ages. With more than 3000 medicinal plants, the scientifically anticancer plants are novel for anticancer drugs, specially that up to 80% of society believe in traditional medicine as it was presented from (WHO), with more than $5 billion/year spent for herbal use in US alone (Andrew-Vickers et al., 2001; Wachtel-Galor, 2011; Lin et al., 2015; Thomas et al., 2015; Sharma et al., 2017; Chen et al., 2018; Hong-lian et al., 2019).
Furthermore, the pharmacologic screening for the treatments from herbs is under congesting advancing. The aerobic glycolysis manufacture of cancer cells is major amount of lactic acid whilst taking oxygen through mitochondrial oxidative phosphorylation. Due to a decrease in the number of the receptors in the cell surface according to Warburg observation. One of the functions of mitochondria is the regulation of apoptosis. By knowing the importance of the relation between cancer cells metabolism, mitochondria and apoptosis, it is vital to study relationship of the extracts of medicinal herbs and proliferation of cancer cell lines (Andrew-Vickers, et al., 2001; Rivlin et al., 2011; Guaman-Ortizet al., 2017; Mirza et al., 2018).
Costus speciosus grows on the moist, slopes environments, originally from South East Asia although it has been naturalized in some tropical areas such as Hawaii as well. But currently it is more found In India, Sri Lanka, Indonesia and Malaysia. Largely found in south India. This plant is cultivated for its roots which are used in perfume industry, incense and medicine. It has an acrid, sweet and bitter taste. Costus grandis and Costus speciosus is the most important used two plants in this family, Many scientific studies have been conducted or are now being conducted on these two plants in order to commercialize their positive properties and for pharmacological effects as well, although many of these studies are still in vitro phases, and not progressed onto human trials (Pandey et al., 2009; Vishalakshi et al., 2010; Pawar and Pawar, 2014; Waisundara, et al., 2015)
Some researchers discovered that this plant’s anticancer action is mediated by overexpression of proapoptotic and downregulation of antiapoptotic molecules, which together reduce cancer cell proliferation and progression. Anti-inflammatory Activity, Rheumatism, bronchitis, asthma, flatulence, constipation, leprosy, skin illness, and anemia are among the conditions for which it is prescribed. The oil extracted from the roots is known as Costus Oil. As a result of over-exploitation for various medical and commercial purposes, the supply of this vital plant in the wild is dwindling. As a result, the Indian government has banned the export of 29 medicinal and aromatic species. (Pandey et al, 2009; Pawar, and Pawar, 2014; El-Faret al., 2017; Selim and Jaouni 2017; Majiet al, 2020).
Therefore, the current study was carried out to investigate the effect of ethyl acetate extract of Costus cpeciosus roots on cultured human lung cancer cells (A549)
MATERIAL AND METHODS
Preparation of Costus roots’ extract: Fresh 500 g of C. speciosus roots was purchased from Mayajan abazeer in Makkah, KSA. The root material was extracted by macerating for 2 days in 75% methanol, filtered and dried. According to (Mohammed, et al., 2019), the single dose contains 0.05% DMSO to give the final desired concentration of methyl acetate of C. speciosus roots (ECR).
Using A549 Cell Line for culture: In a 15 ml tube, the collected cells were washed, centrifuged at 250 rpm for 5 min. and the obtained cell pellet was resuspended in one ml of cold cell medium (30 ml free medium with 1ml DMSO and 3ml FBS) and transferred to cryogenic vials, stored in the -80ºC.
Thawing of cells: The prepared cell suspension was transferred to a 15 ml tube, and mixed gently with 10 ml of complete culture medium. After centrifugation at 250 rpm for 5 min, the cells were collected in fresh culture medium, preserved in a CO2 incubator at 37oC and examined each 24 hours and sub cultured if necessary.
Cancer cell lines and Sphere forming culture: Human Lung cancer cells A549 were grown at 37 °C in RPMI medium supplemented with 10 % fetal bovine serum and 1 % penicillin and streptomycin under 5% CO2 and 95 % air. The cells were collected, washed with saline phosphate-buffered, incubated with 0.05 % trypsin/ EDTA and collected at the exponential phase to carry out all the experiments.
Cell Viability Assay (MTT Assay): The effects of C. speciosus roots on the cell viability and proliferation of A549 cells were detected by (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) (MTT) cell proliferation assay kit (Invitrogen), following manufacturer’s instructions.
Clonogenicity Assay: Clonogenic assay test was used to detect the efficacy of cytotoxic agents and other anti-cancer therapeutics on colony forming ability were done using different cell lines. In 6 wells plate, the suspended cells (3×10 3 cells/well) in 3 mL of culture medium, containing different concentration from the tested material (25, 40 and 70µg/100 µl) were incubated at 37°C and 5% CO2 for 24 hrs. After treatment for growth and colony formation, the medium was removed then replaced with fresh medium and incubated for another 7 days.
Colonies fixation and staining: Media was removed from each well by aspiration and by washing each well with 2ml PBS. colonies were fixed with 1.5 ml 10% formalin-based buffer solution for15-30 minutes. Washing the wells again with 2 ml PBS well done again, 600 μl of 0.25 % triton was added to the cells and washing with PBS to remove the tritone residues. Colonies were stained with 1.5 ml of Giemsa (1x) for 30-60 minutes, then removing the stains by washing with PBS then allowing cells dry. The observation was done using inverted microscope with a magnification lens x10.
Wound Healing Assays: The classic and commonly wound healing assays are a applied to study cell motility and biology according to (Jonkmanet al., 2014). In 6 wells plate, Cells (2×105cells/well) were incubated in 3 ml of the culture medium for 24 hrs at 37°C and 5% CO2. After 24 hrs, the formed cell ~70-80% confluence as a monolayer were scratched across the center of each well, washed by PBS incubated, treated with different concentrations the tested material (25, 40, 70 and 100 µg/500 µl), allowed to migrate and examined under inverted after 0, 24, 48 and 72 hrs post-induction of injury.
The Comet Assay, or Single Cell Gel Electrophoresis Assay (SCGE): DNA damage in individual cells can be detected by the common Comet assay (Product Manual, OxiSelect™ Comet Assay Kit (3- Well Slides). Cells (2×105cells/well) were incubated in 3 mL of culture medium in 6 wells plate for 24 hrs and treated by different concentrations of the tested material (25, 40, 70 and 100 µg/500 µl) for 24 hrs.
DNA fragmentation assay: Agarose gel electrophoresis was used to detect DNA fragmentation and distinguish apoptotic cell using to DNA purification kit (Promegacataloge#A1120).
Isolating Genomic DNA protocol: The DNA isolation methods were done according to Wizard® Genomic DNA Purification Kit, Promega, electrophorized using gel electrophoresis with EtBr staining method and visualized under UV illumination.
Measurement of the Mitochondrial Membrane Potential (ΔΨm) with JC-1: Apoptosis is a cellular process involving a genetically programmed series of events leading to the death of a cell. During this process, several key events occur in mitochondria, including loss of mitochondrial transmembrane potential (ΔΨm). For this reason, ΔΨm is an important parameter of mitochondrial function and has been used as an indicator of healthy cells.
The principle of all the following detection methods is done according to (Zhang et al., 2010). The cells were treated at different concentrations (25, 40, 70 and 100 µg/100µl) of plant extract for 24 hrs.100 µl of JC-1reagent was added to each well and incubated for 30 min. The fluorescence was measured by using a microplate reader with excitation/emission = 535/595 nm for red fluorescence (viable cells) and485/535 nm for green fluorescence (apoptotic cells).
Human Interleukin 32 ELISA Assay: The shortest and most abundant antibody is IL-32 alpha. Potential modifications include myristoylation and N-glycosylation. Transfected IL-32 alpha was more likely to be cell-associated as compared to IL-32 beta, suggesting an intracellular function. IL-32 is induced by mitogens in peripheral lymphocytes, by IFN-gamma in epithelial cells, and in turn induces cytokine expression.
Plate Preparation and assay: For coating a 96-well microplate, 100 μL per well of PBS (Capture Antibody working concentration) was used and the sealed plate overnight and incubated at room temperature. Each well was washed with 400 μL by the buffer. After removing the washing buffer, 300 μL of reagent diluent was added to each well and the plates were incubated at room temperature for an hour then 100 μL of reagent dilution was added to each well and incubation was applied to another two hours at room temperature.
100 μL of the detection antibody diluted in reagent diluent were added to each well, incubation 2 h at room temperature was done. 100 μL of the working dilution of streptavidin-HRP added to each well at room temperature in the dark for 20 min. 100 μL of substrate solution was added to each well with gentle tapping. 50 μL of Stop Solution was added to each well with gentle mixing. The microplate reader set used to measure the optical density at 450 nm.
Statistical analysis: Data was collected and mean value ± standard deviation (SD) from two independent experiments with three plicate was calculated. The statistical Package for Social Sciences (SPSS version 20) was used and variables between more than two groups was detected using one way ANOVA test. A probability at P< 0.05 was considered significant.
Results And Discussion
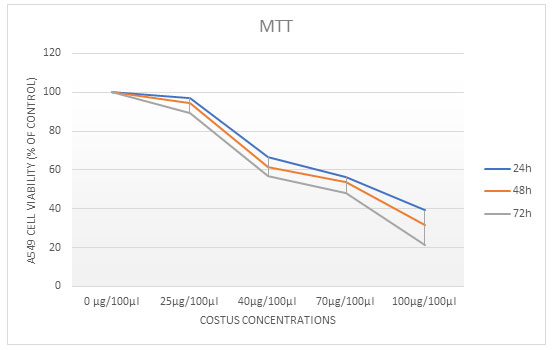
To investigate the effects of ECR on the proliferation of A549 cells were assayed by using MTT Assay test. Different increasing concentrations of treated cells (25, 40, 70 and 100µg/100µl) of ECR for 24, 48, or 72 h. ECR showed a dose- and time-dependent inhibitory effect on the growth of A549 cells. The extract caused 50% growth inhibition (IC50) at around (40 and 70µg/100µl) for 24, 48, and 72h, respectively (Table 1).
Table 1. The Effect of ECR on MTT levels in A549. Data were expressed as mean ± SD and
Statistical analyses were performed using One Way ANOVA
| Doses (µg/100µl) | 0
µg/100µl |
25
µg/100µl |
40
µg/100µl |
70
µg/100µl |
100
µg/100µl |
Sig.
(2-tailed) |
| Cost. 24h | 100
± 0.04879 |
97.075
± 0.087558 |
66.685
± 0.137552 |
56.407
± 0.131724 |
39.292
± 0.1101 |
.181 |
| Cost. 48h | 100
± 0.07103 |
94.704
± 0.128571 |
61.541
± 0.161742 |
53.593
± 0.139114 |
31.394
± 0.131354
|
.092 |
| Cost. 72h | 100
± 0.034648
|
89.136
± 0.033723 |
57.121
± 0.023381 |
48.161
± 0.027541 |
21.123
± 0.023049
|
.029 |
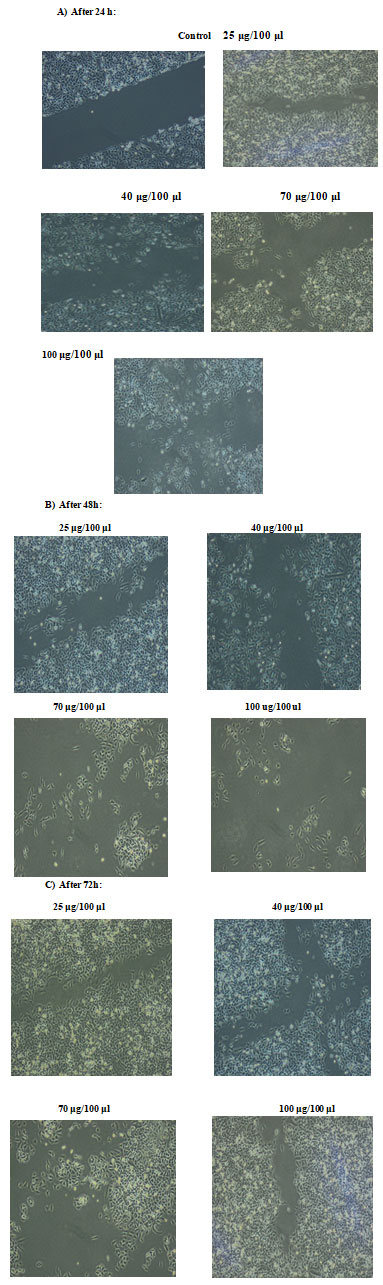
The Effect of ECR on A549 Cells Colony Formation: The anti-proliferative and cytotoxic effects of the ECR on A549 cells were further determined and verified using anchorage-dependent colony formation assay (also referred to as clonogenicity). The ECR suppressed colony formation in a dose-dependent manner demonstrates that (25, 40, 70 and 100μg/100/μl) of ECR reduced colony formation in A549 cells (Fig 1).
Figure 1: Cell viability was evaluated using MTT assay. The A549 cells were treated with different concentrations of extract for 24, 48 and 72 h. The values are represented as the mean ± SD, P ˂ 0.0microscope; magnification 4x.
To determine whether ECR inhibited cell growth by inducing apoptosis, A549 cells were treated with increasing concentrations for different time intervals and the cells were assessed using wound healing assay, comet assay and DNA degradation methods. The effect of ECR on migratory capacity of A549 cells was determined by wound healing assay. Treatment of A549 cells with ECR, dose-dependently, led to prevention of wound gap closure (Fig. 2a, b and c).
Figure 2: Effects of ECR on cell migration. A549 cell migration was determined by wound healing assay after treatment of A549 cells with increasing concentrations of the extract for A): 24, B): 48 and C0: 72 h; magnification ×10.
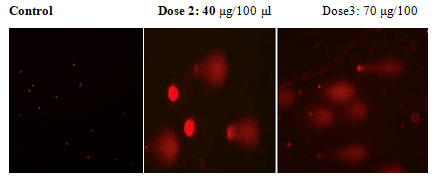
In an apoptotic assay, DNA degradation is an irreversible process. The ECR caused DNA fragmentation by DNA degradation. The comet assay is a reliable method for detecting single DNA strand breakage at the single-cell level, and it is widely used as an apoptosis biomarker. Treatment of A549 cells with increasing doses of ECR (40 and 70 μg//100μl) for 24 h resulted in significant DNA damage. The images show most DNA migrated out of nuclei indicating that ECR caused severe damage to the nuclear scaffold (Fig 3). These findings suggest that ECR induced DNA damage in A549 cells.
Figure 3: In commit assay test in A549 cells treated with ECR, shows the creation of a DNA tail and damaged DNA nuclei as shown a bright head and tail, whereas nuclei with undamaged DNA appear round with no tail.
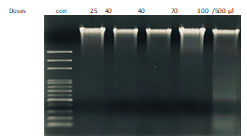
To obtain a further proof that ECR induces apoptotic death, cells were treated with increasing concentrations of the extract for 72 h and DNA fragmentation was visualized using agarose gel electrophoresis. The observations in (Figure 4) showed ECR induced inter-nucleosomal DNA cleavage forming discrete bands of 200 base pairs.
Figure 4 : ECR induced oligonucleosomal degradation of the genomic DNA. A549 cells were incubated with the
indicated concentrations of ECR for 72 h, then genomic DNA was extracted and electrophoresed.
One of the first intracellular events after the onset of apoptosis is the disruption of mitochondrial membrane potential. The vital mitochondrial dye JC-1 is a useful tool for investigating mitochondrial function.
To prove that ECR induces loss of mitochondrial membrane potential, the effect of ECR was monitored after labelling cells with the sensitive dye JC-1. Thus, A549 cells were treated with increasing concentrations of ECR for 24h, then the intensity of JC-1 fluorescence was assessed using microplate reader. Results in (Fig.5) indicated that ECR dose-dependently decreased the levels of JC-1 red/green ratio in A549 cells in a dose- and time-dependent manner (P<0.05) as compared to the untreated cells (Table 2).
Figure 5: Fluorescence microplate reader analyzed the dissipation of mitochondrial membrane potential in
A549 cells with increasing concentrations of ECR for 24 h and stained with JC-1 and the average
ratio of red/green fluorescence intensity, P<0.05 significantly different compared with control.
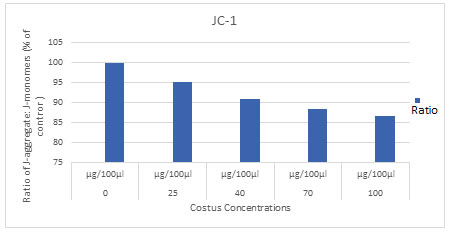
Table 2. The effect of ECR on JC-1 levels in A549. Data were expressed as mean ± SD and the
Statistical analysis was performed by using One Way ANOVA
| Doses
(µg/100μl) |
0
µg/100μl |
25
µg/100μl |
40
µg/100μl |
70
µg/100μl |
100
µg/100μl |
Sig.
(2-tailed) |
| Costus | 100
± 1.195203 |
95.16
± 0.177130 |
90.85
± 0.351164 |
88.20
± 0.372296 |
86.64
± 0.107190 |
.008 |
The metastatic and unrespectable conditions of LC patients have a poor prognosis. The disadvantages and low efficiency of available treatments of conventional chemotherapy, toxicity and drug resistance in metastatic patients (Qian,et al., 2017). Therefore, there is an urgent need to discover some more plant-based anti-cancerous agents with no or less cytotoxic effects. C. spesious is one of the important traditional medicinal herbs, having potent therapeutic capabilities (Vickers and Zollman, 2001; Hanahan and Weinberg 2013; Marinoet al., 2019).
Recently, the discovery of a natural antioxidants from different fruits and vegetables have been recently extensively studied for their antioxidant and/or free radical scavenging activity. A previous study indicated that the intake of fruits rich in antioxidants increases the antioxidant capacity of plasma and reduces the risk of chronic health ailments including cancer (Pandey et al., 2009; Lin et al., 2017; Abuelgasimet al., 2018).
The present study was carried out to investigate the potential anti-cancer activity of C. Spesious in the A549 cell line. Primarily, MTT assay was done to evaluate the antiproliferative effect of ECR on A549 cell line in a time- and dose-dependent manner for 24, 48- and 72-hours treatment (Jonkman et al., 2018).
Cancer is often characterized by too little apoptosis and too much proliferation of cells. So, the induction of apoptosis is an advantageous strategy for cancer therapy. Cancer therapies depends on the extent of their ability to induce the death of cancer cells while allowing the survival of healthy cells. The comet assay and gel electrophoresis in our data confirmed that the DNA damage by ECR, which is a characteristic feature of apoptosis. Cells with damaged DNA could undergo apoptosis as damaged DNA is hard to be repaired; therefore, the observation of these assays suggests that ECR treatments triggered events leading to DNA damage and initiation of apoptotic cascade, which leads to programmed cell death (Zhang et al., 2008: Ardekani and Jabbari 2009; Mazina et al., 2015; Pistritt et al., 2016; El-Far et al., 2018).
CONCLUSION
A key regulator of apoptosis is Mitochondria which have shown to be involved in the dissipation of mitochondrial membrane potential and integrating different pro-apoptotic pathways via the release of cytochrome c into the cytosol. The results of ECR depicted that mitochondrial membrane potential (ΔΨm) was gradually decreased with the increase of the dose, which is an important trigger to activate the intrinsic pathway.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
Conflict of Interest: Authors declare no conflicts of interests to disclose.
REFERENCES
Abuelgasim, A. K, Alsharhan, Y., Alenzi, T., Alhazzani, A., Ali A. Y. and Abuelgasim, J. A. (2018). The use of complementary and alternative medicine by patients with cancer: a crosssectional survey in Saudi Arabia. Complementary and Alternative Medicine 18 (1): 88.
Alqahtani, A. S. and Alghamdi, G. I. (2020). Epidemiology of Gallbladder Cancer in Saudi Arabia Cancer Manag Res. 2020; 12: 9527–9537.
Amararathna, M., Johnston, R. M. and H. P. Vasantha Rupasinghe, H. P. (2016). Plant Polyphenols as Chemo preventive Agents for Lung Cancer. International Journal of Molecular Sciences. 17: 1352.
Ardekani, M. A.and Jabbari, S. (2009). Nutrigenomics and cancer. Avicenna journal of medical biotechnology. 1 (1): 9-17.
Chen, Q., Kang, J. and Fu, C. (2018). The independence of and associations among apoptosis, autophagy, and necrosis. Signal Transduction and Targeted Therapy. Review Article 3 (18)
El-Far, A. E., Shaheen, M. H., Alsenosy, W A., El-Sayed, S. Y., Jaouni, K. S., Mousa. A. S. (2018). Costus speciosus: Traditional Uses. Phytochemistry, and Therapeutic Potentials Pharmacognosy Reviews. 12 (23): 120-127.
El-Far, H. A., Badria, A. F. and Shaheen, M. H. (2017). Possible Anticancer Mechanisms of Some Costus speciosus Active Ingredients Concerning Drug Discovery. Curr. Drug Discov. Technol. 13(3):123-143.
Guaman-Ortiz, G. L., Orellana, I. R. M., Ratovitsk, A. E. (2017). Natural Compounds as modulators of non-apoptotic cell death in cancer cells. Current Genomics. 18 (2): 132-155.
Hanahan, D and Weinberg, R. A. (2013). Hallmarks of Cancer: The Next Generation Cell. 144(5): 646–674.
Hong-lian Z, Zhang, A., Jian-hua M, Guang-li Yan, S. H., Wu, F. and Xi-jun W. (2019). Targeting regulation of typtophan metabolism for colorectal cancer therapy: a systematic review. Royal Society of Chemistry. 9 (6): 3072-3080.
Jonkman, E. N. J., Cathcart, A. J., Xu, F., Bartolini, E. M., Amon, E. J., Stevens, M. K. and Colarusso, P. (2014). An introduction to the wound healing assay using live-cell microscopy. Cell Adh. Migr. 2014;8(5):440-51
Jonkman, N.E; Cathcart, A. J; Xu, F.; Bartolini, E. M.; Amon, E.J.; Stevens, M. K. and Colaruss, P. (2014). An introduction to the wound healing assay using live-cell microscopy. Cell Adhesion and Migration 8:5, 440- 451
Lee, H. S. (2018). Chemotherapy for Lung Cancer in the Era of Personalized Medicine, review. Journal of Ethnopharmacology. 110: 397-390.
Lemjabbar-Alaoui, H., Hassan O. U., Yang, W.Y. and Buchanan, P. (2015). Lung cancer: biology and treatment options. Cell press. 5 (4): 189–210.
Lin, S.-R, Fu, Y.-S, Tsai, M.-J, Cheng, H, Weng, C.-F; Lin, S.-R & Weng, C.-F. (2017). Natural Compounds from Herbs that can Potentially Execute as Autophagy Inducers for Cancer Therapy. International Journal of Molecular Sciences, 18(7): 1412.
Lin, X., Zhangxiao Peng., Z. and Changqing, S. (2015). Potential Anti-Cancer Activities and Mechanisms of Costunolide and Dehydrocostuslactone. International Journal of Molecular Sciences. 16: 10888-10906.
Maji, P., Dhar, D. G., Misra, P. and Dhar, P. (2020). Costus speciosus (Koen ex. Retz.) Sm.: Current status and future industrial prospects. Industrial Crops & Products. 152: 112.
Marino, Z. F., Bianco, B. R., Accardo, M., Ronchi, A., Cozzolino, I., Morgillo, F., Rossi, G. and Franco, R. (2019). Molecular heterogeneity in lung cancer: from mechanisms of origin to clinical implications International Journal of Medical Scences. 16 (7): 981-989.
Mazina J, Vaher M, Kuhtinskaja M, Poryvkina L, Kaljurand M. Fluorescence, (2015). Electrophoretic and chromatographic fingerprints of herbal medicines and their comparative chemometric analysis. Talanta. 139.
Mirza, M. B, Elkady, A. I, Al-Attar, A. M, Syed, F. Q, Mohammed, F. A., & Hakeem, K. R. (2018). Induction of apoptosis and cell cycle arrest by ethyl acetate fraction of Phoenix dactylifera L. (Ajwa dates) in prostate cancer cells. J Ethnopharmacol, 218: 35-44.
Mohammed, A. F., Elkady, A. I., Syed, F. Q., Mirza, M. B. and Hakeem, K. H. (2018). Anethum graveolens (dill) – A medicinal herb induces apoptosis and cell cycle arrest in HepG2 cell line. Journal of Ethnopharmacology, 219. 15-22.
National Cancer Institute, Worldwide cancer data, World Cancer Research Fund (2018).
Pandey, K. B, Rizvi, S. I. (2009). Plant polyphenols as dietary antioxidants in human health and disease. Oxidative medicine and cellular longevity, 2(5):270-278.
Paul. M., Davey, B., Senf, B., Stoll, C., Munstedt. and Mucke, R. (2013). Patients with advanced cancer and their usage of complementary and alternative medicine. J Cancer Res Clin Oncol.139: 151.
Pawar, V. and Pawar, P. R. (2014). Costus speciosus: An Important Medicinal Plant. International Journal of Science and Research (IJSR). 3 (7): 28-33.
Pistritto, G., Trisciuoglio, D., Ceci, C., Garufi, A and D’Orazi, G. (2016). Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. AGING 8 (4): 604:619.
Qian,L. Y. Y. and Cui, J. (2017). Value of neutrophil-to-lymphocyte ratio for predicting lung cancer prognosis: A meta-analysis of 7,219 patients. Molecular and clinical oncology. 7 (3): 498-506.
Rivlin, N, Brosh, R, Oren, M, & Rotter, V. (2011). Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes & Cancer, 2(4), 466–74.
Selim, S. and Jaouni, A. S. (2017). Anti-inflammatory, antioxidant and antiangiogenic activities of diosgenin isolated from traditional medicinal plant, Costus speciosus (Koen ex.Retz.) Sm
Sharma, R., Hazra, H. and Prajapati, P. K. (2017). Nanophytomedicines: A Noval Approach to Improve Drug Delivery and Pharmacokinetica of Herbal Medicine. Bio Bull. 3 (1): 132-135.
Thomas, R., Elizabeth, F. M. and Butler, M. W. (2015). Phytochemicals in Cancer Prevention. Encyclopedia of Cancer, 8(2):2882–2885.
Vickers, A. and Zollman, R. C. (2001). Herbal medicine. The Western Journal of Medicine. 175 (2): 125-131.
Vishalakshi D. D and Urooj, A (2010). Nutrient profile and antioxidant components of Costus speciosus Sm. and Costus igneus Nak. Indian Journal of Natural Products and Resources. 1 (1): 116-118
Wachtel-Galor S., Benzie I. F. F., (2011). Herbal Medicine, An Introduction to its History, usage, Regulation, Current Trends and Research needs book. Chapter 1. Herbal medicine: A growing field with a long tradition.
Waisundara, V. W. Watawana, M. I. and Jayawardena, N. (2015). Costus speciosus and Coccinia grandis: Traditional medicinal remedies for diabetes. South African Journal of Botany. 98: 1–5
World Health Organization. (2018). Cancer key facts. The Lancet Global Health.
Zhang, W., Shi, Y., Chen, Y., Yu. S., Hao, J., Lou, J., Sha, X. and Fang. X. (2010). Enhanced antitumor efficacy by Paclitaxel-loaded Pluronic P123/F127 mixed micelles against non-small cell lung cancer based on passive tumor targeting and modulation of drug resistance, European journal of pharmaceutics and biopharmaceutics. 75(3):341-53
Zhang, Y, Seeram, N. P,Lee, R, Feng, L & Heber, D. (2008). Isolation and Identification of Strawberry Phenolics with Antioxidant and Human Cancer Cell Antiproliferative Properties. Journal of Agricultural and Food Chemistry, 56(3): 670–675.


