1Department of Zoology, Government Mata Jijabai Postgraduate Girls College, Indore. M.P.
2Department of Zoology, Government Postgraduate College, Badnagar, Ujain, M.P.
Corresponding author Email: anshujain7284@gmail.com
Article Publishing History
Received: 22/06/2018
Accepted After Revision: 24/07/2018
An attempt has been made to analyse the physicochemical and bacteriological examination of ground water (Tap water) seasonally (rainy, winter and summer seasons) at selected regions of Mhow Tehsil area (Sangi Street, Raj Mohalla, Kali Mata Mandir area, Cantonment Board area and Main Street) for two years, during 2011-2013. During physicochemical examination, Water Colour, Temperature (°C), pH values, Total hardness (mg/lit), Specific conductivity (µmhos/cm), Total alkalinity (mg/lit), TDS (mg/lit), Chloride (mg/lit), Fluoride (mg/lit), Nitrate (mg/lit), Phosphate (mg/lit), Sulphate (mg/lit) and BOD (mg/lit), DO (mg/lit) and COD (mg/lit) values have been analyzed however, the total coliforms (MPN/100ml) and faecal coliforms (MPN/100ml) were also estimated during bacteriological examination. Continuous monitoring and environment management programs should be run properly to manage the elements in limit range which is necessary to control drinking water pollution.
Tap Water, Mhow Tehsil Area, Physicochemical And Bacteriological Examination
Jain A, Khatri A, Siddiqui A. Tap Water Quality Assessment of Some Selected Regions of Mhow, District Indore India. Biosc.Biotech.Res.Comm. 2018;11(3).
Jain A, Khatri A, Siddiqui A. Tap Water Quality Assessment of Some Selected Regions of Mhow, District Indore India. Biosc.Biotech.Res.Comm. 2018;11(3). Available from: https://bit.ly/2lYGqJh
Introduction
Ground water plays an important role in our environment and our economics. In the environment it supports rivers, lakes, wetlands, springs, ponds, marshland, swamps, streams and used as an important sources of freshwater around the world. Groundwater is available at purest form in nature which is colourless and tasteless. Safe potable water is not likely to be harm humans which keeps healthy lives throughout the world. The quality of ground water depends on various physiochemical constitutes and their geographical data of the particular region. Ground water is the major sources of drinking water. The clean drinking water is one of the essential compounds that profoundly influence life. The deficiency of the clean water increases day by day due to pollution of water. In recent years, an increasing threat to ground water quality is the results of human activity at ground water. Now water is getting contaminated, which can cause water borne diseases and gets health hazards, (Shahida and Ummatul, 2015, Singh 2016, Jena and Sinha, 2017, Nagaraju et al., 2018 and Patil 2018).
It has been reported that the more than 90% of population of various states in our country are dependent upon groundwater source for drinking and other purposes (Ramachandraiah, 2004; Tank, 2010). Now days, groundwater is used in agricultural and industrial sector (Ramesh and Soorya, 2012). However, due to increase in industrialization and urbanization, deterioration in the quality of groundwater has been noticed (Laluraj et al., 2005 Leelavathi et al., 2016; Prajapati and Rokde, 2016; Behailu et al., 2017; Soni and Singh, 2018).
The availability of pure water through surface and groundwater resources has become more critical day today. Only 1% of surface and groundwater resources are available on earth for drinking purpose, domestic purpose, power generation, industrial consumption, agricultural purpose, transportation and waste disposal (Mishra et al., 2002; Tahir, et al., 2008). The majority of the recent problems related to drinking water contamination, associated with pollution of surface and ground water resources and with the formation of reaction by-products resulting from the use of disinfectants and oxidants in drinking water treatment, are closely connected with the rapid advances in analytical techniques. It has been noticed that the most common and wide spread danger associated with drinking water is the direct or indirect contamination by sewage, human and agriculture, chemical and industrial influents etc. (Clark et.al., 1982 and WHO, 1985). It is therefore considering the importance of health problem in context of ground water (Tap water) contamination.
Mhow is that the cantonment of the Indore district in M.P. The water from the main source is the municipal corporation supply to fulfil the need of population. Thus quality of potable and non potable Tap water resource of Mhow is an urgent need to protect human health. Hence, it was thought to study physico-chemical and bacteriological parameters of different Tap water resources of Mhow. Therefore a continuous periodical monitoring of water quality is necessary. In order to protect human health from different water borne and water related diseases appropriate steps should be taken. The study will be helpful in the management of water resources and save human health of Mhow Tehsil from environmental pollution.
Although various workers have contributed in the field of water monitoring related to health problems in various parts of India, yet most of the parts of Madhya Pradesh are neglected even today. Mhow is a cantonment of Indore, but there is no work done in this area. Therefore, looking to the importance from health point of view, the present work has been aimed to analysis of potable or non-potable Tap water resources of Mhow Tehsil. The undertaken work has been aimed to study the physicochemical and bacteriological examination of ground water (Tap water) seasonally (rainy, winter and summer seasons) at selected regions of Mhow Tehsil area (Sangi Street, Raj Mohalla, Kali Mata Mandir area, Cantonment Board area and Main Street) during 2011-
2013.
Materials and Methods
The physicochemical and bacteriological examinations of ground water (Tap water) were analyzed seasonally (rainy, winter and summer seasons) as per standard methods of APHA (2005). The samples were collected seasonally (Winter–December/January, Rainy–July/August, Summer–April/May) from the various sampling station in the morning (between 8 a.m. to 11 a.m.) for the consecutive two year (2011- 2012 and 2012 -2013). For the analysis of physicochemical parameter of ground water (Tap water) APHA, 2005 was applied.
- Hydrogen ion concentration (pH): The hydrogen ion activity of the water sample was measured with the help of calibrated analyzer (pH meter).
- Colour: Colour of water was observed by visual comparison method.
- Temperature: The water samples were collected in suitable container, measured with the help of mercury thermometer.
- Specific conductivity: It was measured with the help of a Conductivity meter. The unit of conductivity measurement is µmhos/cm.
- Dissolved oxygen: Dissolved Oxygen was measured titrimetrically by Winkler’s method
- Biochemical oxygen demand: Biochemical oxygen demand was estimated by 5 days BOD method (APHA, 2005).
- Chemical oxygen demand: Chemical oxygen demand was estimated through titrimetric method
- Alkalinity: Total Alkalinity was estimated by titrimetric method
- Sulphate: Sulphate was estimated by gravimetric method.
- Phosphate: Phosphate was measured by spectrometric method
- Nitrate: Nitrate was measured by spectrometric method
- Total Hardness: Hardness was the combination of Ca or Mg ions. The total hardness was estimated by titrimetric method
- Chloride: Chloride was estimated by argentometric method
- Fluoride: Fluoride was estimated by SPADNS calorimetric method
- Methods for analysis of bacteriological parameter
Bacteriological parameters of ground water (Tap water) were also analysed as per standard methods of APHA (2005).Total coliform microbes (Bacteria) – Total coliform in ground water were estimated by multiple tube method. Results were obtained in MPN (Most Probable Number) per 100 ml by consulting the MPN table.Faecal coliform microbes (Bacteria) – Faecal coliform were also estimated by multiple tube method by using BGB broth. Results were also documented in MPN (Most Probable Number) per 100 ml by consulting the Most Probable Number table.
Results and Discussion
The results obtained in present investigation have been summarized by Figures (1-5). The true colour has been noticed for tap water around all the studied area during rainy, winter and summer seasons during 2011-2013. The highest and the lowest temperature (°C) were recorded as 40oC (Summer, 2012 -2013) for Main street and 13 oC (Winter, 2011-12) for Sangi Street, respectively. The pH values were noticed the lowest as 7.1 (Rainy, 2011 -2012) for Main street and the highest as 8.1 (Summer, 2012-13) for Main street also. Whereas, the total hardness (mg/lit) were estimated the lowest as 100 mg/lit (Rainy, 2011-12, for Sangi Street and Raj Mohalla both) and this value were analysed the highest as 139 mg/ lit (Summer, 2012-13, for Cantonment Board area). Specific conductivity (µmhos/cm) value were noticed the lowest and the highest as 176 µmhos/cm (Rainy, 2011 -2012) for Sangi Street and 412 µmhos/cm ((Rainy, 2012-13) for Kali Mata Mandir area, respectively. Total alkalinity (mg/ lit) value were found to be the lowest as 74 mg/ lit (Rainy, 2012-13, for Raj Mohalla both) and the highest as 110 mg/ lit (Summer, 2011 -2012 for Kali Mata Mandir area and Main street both).
 |
Figure 1: Physicochemical and bacteriological examination of Tap Water of Sangi Streetduring Rainy, Winter and Summer seasons (2011-2013). |
 |
Figure 2: Physicochemical and bacteriological examination of Tap Water of Raj Mohalladuring Rainy, Winter and Summer seasons (2011-2013) |
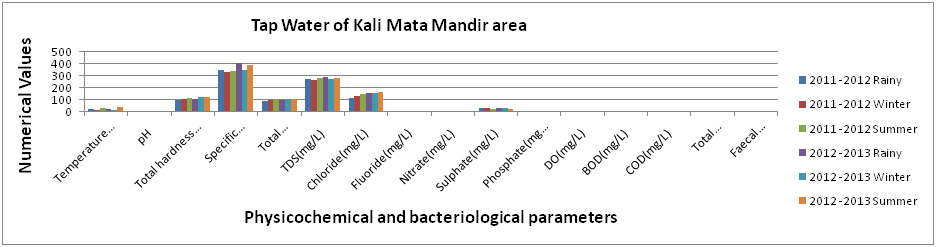 |
Figure 3: Physicochemical and bacteriological examination of Tap Water of Kali Mata Mandir areaduring Rainy, Winter and Summer seasons (2011-2013). |
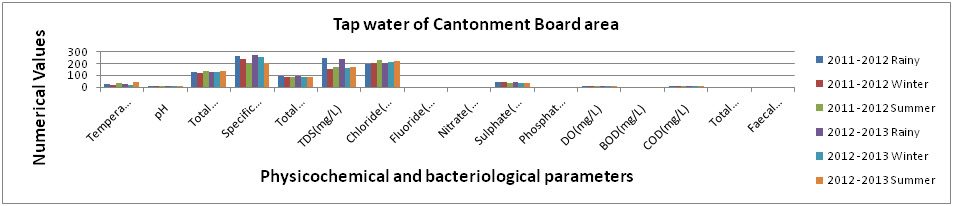 |
Figure 4: Physicochemical and bacteriological examination of Tap Water of Cantonment Board areaduring Rainy, Winter and Summer seasons (2011-2013). |
 |
Figure 5: Physicochemical and bacteriological examination of Tap Water of Main Streetduring Rainy, Winter and Summer seasons (2011-2013). |
The values of TDS(mg/ lit) were noticed highest as 289 mg/ lit (Rainy, 2012-13, for Kali Mata Mandir area) and lowest as 158 mg/ lit (Winter, 2011-12, for Cantonment Board area).Whereas, Chloride (mg/ lit) value has been calculated the lowest as 120 mg/ lit (Rainy, 2011 -2012 for Kali Mata Mandir area) and the highest as 230mg/ lit (Summer, 2011 -2012, for Cantonment Board area). Fluoride (mg/lit), Nitrate (mg/lit), Phosphate (mg/lit) and BOD (mg/lit) value were fluctuated in the range of 0.1 -0.6, 1.1-1.7, 0.01-0.02 and 1.0-2.0 respectively during studied season of experimental period. The value of Sulphate (mg/lit) were found the highest as 82mg/lit (Rainy, 2011-2012) for Sangi Street and the lowest as 19mg/lit (Summer, 2011-12) for Main street.
The DO(mg/lit) contents were found to be in the ranged between 6.1 mg/lit (Winter, 2011-12, for Cantonment Board area) to 7.9 mg/lit (Summer, 2012-13, for Main street). Whereas, COD (mg/lit) value were also noticed the highest as 10.4 mg/lit(Rainy, 2012-13, for Cantonment Board area) and the lowest as 7.2 mg/lit (Winter, 2011-12, for Raj Mohalla). However, the total coliform (MPN/100ml) were found 2 to 4 (Sangi Street and Raj Mohalla area) during rainy seasons only but faecal coliform (MPN/100ml) were found to be nil throughout the studied season of experimental period.
Analysis of groundwater quality is unavoidable because its poor quality may badly affect its users (Prasanna et al., 2010). Various agencies like industrial effluents, agricultural runoff, sewage contributes several kinds of pollutants and nutrients in to the water bodies that brings about a series of changes in the physicochemical characteristics of water, which becomes the need of several investigations (Mahananda et al., 2010).Whereas, water quality parameters has been assessed in Veeranna Cheruvu, Hasnapur, Mahabubnagar District, Telanagana State. It was found that the water quality parameters were within the permissible limits of standards and during the study period it has been noticed that many water quality parameters were minimum in monsoon and maximum in pre monsoon periods (Nagaraju et al., 2018).
It has been reported that the lowest and highest values of the borehole and spring water samples such as pH ranged between 6.34–6.37 and 6.34–7.92, EC between 627.33–621 µmho/cm and 566.33–569 µmho/cm, Total dissolve solids between 407.67–414.33 and 355.33–351.67 mg/lit and Total suspended solids between 14.37–14.83 and 13.00–13.08 mg/lit, respectively. Total hardness (TH), both calcium and magnesium hardness in terms of calcium carbonate concentration, ranged between 63.63–66.61 and 32.44–38.76 mg/lit respectively. Whereas, the highest and lowest concentration of NO2 were ranged between 0.11–0.12 and 0.05–0.06 mg/lit, NO3 between 1.10–1.89 and 2.83–8.40 mg/lit, SO4 2- between 26.33–33.00 and 17.00–18.33 mg/lit and PO4 3- between 0.21–0.30 and 0.16–0.22 mg/lit in the borehole and spring water respectively (Shigut et al., 2017).
However, assessment of Water Quality Index (WQI) of groundwater in Rajkot District, Gujarat were done and noticed that the pH values ranged between 7.38-8.27 indicates that samples was changes from neutral to slightly alkaline. The TDS varied from 309-4858 mg/lit, chloride concentration ranged from 57-2237 mg/lit, total hardness ranged from 127 to 1582.40 mg/lit, Sulphate concentration ranged from 3-120 mg/lit and nitrate concentration in groundwater samples ranged from 1-876 mg/lit (Krishan al., 2016). Results of present investigation also in conformities with the finding of previous authors in context of studied parameters. Besides this, results of present work are also supported by several workers, (Chapolikar, and Ubale 2010; Manjappa et al., 2011; Arya al., 2012; Nagarnaik and Patil, 2012; Kumar et al., 2013; Chandne, 2014; Leelavathi
et al., 2016; Prajapati and Rokde, 2016; Behailu et al., 2017; Soni and Singh, 2018).
According to WHO standard, potable water should be free of coliform bacteria (WHO, 2008) but the data from other study proves that the tube wells are commonly contaminated with faecal organisms (Luby et al., 2008; Omezuruike et al., 2008). It was found that out of the 454 samples, 49% (221/454) samples were contaminated with total coliform, ranges from 1.45±4.15 to 10780±33814 MPN/100ml and 14% (65/454) samples were contaminated with E.coli with concentrations ranges from 0.09±0.43 to 24.95±104.37 MPN/100ml and; 3% (13/454) of the samples were contaminated with Salmonella species, noticed as 0.06±0.41 MPN/100ml during analysing the status of groundwater contamination in Rural Area, Kelantan (Idrus et al., 2014). The total coliform contents of the samples ranged from zero to 16 MPN of coliform/ 100ml analysed during bacteriological assessment of selected borehole water samples in Ilorin metropolis (Agbabiaka and Sule, 2010). Whereas, the total coliform and faecal coliform were noticed in the range of 03-97 MPN/100 ml and 00-78 MPN/100 ml during microbiological analysis of groundwater of Khulais Province, Kingdom of Saudi Arabia (Saleem and Algamal, 2016).
Significant high coliform bacteria were noticed in borehole water, qualitatively correlate with levels of possible pollution in the immediate surroundings environment in some communities (Anima et al., 2010). However, more or less similar patterns of total coliform (MPN/100ml) and faecal coliform (MPN/100ml) reported in present study are also in conformities with the findings of previous authors.
The present investigation can be concluded that there is a need of continuous monitoring of water quality and also proper environment management programs should be run to control drinking water pollution.
References
Agbabiaka, T.O. and Sule, I.O. (2010). Bacteriological assessment of selected borehole water samples in Ilorin metropolis. International Journal of Applied Biological Research, 2(2): 31–37.
Anima, F., Nyame, F.K. and Armah, T. K. (2010). Coliform status of water bodies from two districts in Ghana, West Africa: implications for rural water resources management. Department of Geology, University of Ghana.
APHA (2005). Standard methods for the examination of water and waste water.17thed. American Public Health Association.Washington.D.C, U.S.A.
Arya, S., Kumar, V. and Sharma, S. (2012). Analysis of water quality parameters of groundwater in and around diamond cements industry, Jhansi, Central India. International Journal of Current Research, 4 (03): 075-077.
Behailu, T.W., Badessa, T.S., Tewodros, B.A. (2017). Analysis of Physical and Chemical Parameters in Ground Water Used for Drinking around Konso Area, Southwestern Ethiopia. Journal of Anaytical and Bioanalytical Techniques, 8 (5): 3-7.
Chandne, S.G. (2014). Study of Groundwater Quality in Concern with Fluoride of Village Rampur from Ghatanji Tehsil, Yavatmal, Maharashtra (India). IOSR Journal Of Environmental Science, Toxicology And Food Technology, 8(1): 96-99.
Chapolikar, A.D. and Ubale, M.B. (2010). A correlation study on physico- chemical characteristics of ground water in Thane-Belapur Industrial area, Mumbai. Current World Environment, 5(1): 67-71.
Clark, J.A., Bung, G.A. and Sabatiros, L.E. (1982). Characteristics of indicator bacteria in municipal, dam water, drinking water and new main water sample and land. Journal. of Microbiology, 28:1002-1013.
Idrus, A.S., Fauziah, M.N., Hani, M.H., Wan Rohaila, W.A. and Wan Mansor, H. (2014). Status of Groundwater Contamination in Rural Area, Kelantan. IOSR Journal of Environmental Science, Toxicology and Food Technology, 8(1): 72-80.
Jena, V and Sinha, D. (2017). Physicochemical analysis of ground water of selected areas of Raipur city, Chhattisgarh, India. Indian Journal of Science and Research, 13 (1):61 -65.
Krishan, G., Singh, S., Kumar, C.P., Gurjar, S. and Ghosh, N.C. (2016). Assessment of Water Quality Index (WQI) of Groundwater in Rajkot District, Gujarat, India. Journal of Earth Science & Climatic Change, 7(3): 1-4.
Kumar, P.P., Yadav, P. R. and Kodaparthi, A. (2013). Bacteriological and Physico-Chemical Quality of Main Drinking Water Sources. Pollution. Journal of Environmental. Studies. 22(3): 825-830.
Laluraj, C. M., Gopinath, G. and Dinesh Kumar, P. K. (2005).Groundwater chemistry of shallow aquifers in the costal zones of Cochin. India Applied Ecology and Environmental Research, 3(1): 133-139.
Leelavathi, Ch.., Sainath, U.K. and Rabbani, A.K. (2016). Physicochemical Characterization of ground water of Autonagar, Vijayawada, Krishna district. International Journal of Engineering Development and Research, 4(2): 1324-1328.
Luby, S.P., Gupta, S.K., Sheikh, M.A., Johnston, R.B., Ram, P.K. and Islam, M.S. (2008). Tubewell water quality and predictors of contamination in three flood-prone areas in Bangladesh. Journal. of Applied. Microbiology, 105: 1002-1008.
Mahananda, M. R., Mohanty, B. P. and Behera Mahananda, N. R. (2010). Physicochemical analysis of surface and ground water of Bargarh district, Orissa, India. IJRRAS, 2: 26 -30.
Manjappa, R.B., Puttaiah, T.and Nagarajappa, P. (2011). Physico-chemical analysis of underground water of Harihara Taluk of Davanagere District, Karnataka, India. Advance in Applied Science Research, 2(5):143-150.
Mishra, K.R. Pradip Tripathi, S.P. ( 2002). Groundwater quality of open wells and tube wells, Acta Ciencia Indica, 2:179.
Nagaraju, C., Rajashekhar, A. V. and Kumar, A.S. (2018). Preliminary Studies on Water Quality Assessment of Veeranna Cheruvu, Hasnapur, Mahabubnagar District, Telanagana State, India. IJSRST, Themed Section: Science and Technology, (4) 2: 275-279.
Nagarnaik, P.B. and Patil, N.P. (2012). Analysis of Ground Water of Rural Areas of Wardha-City Using Physico – Chemical and Biological parameters. International Journal of Engineering Research and Applications. 2(3): 803-807.
Omezuruike, O.I., Damilolan, A.O., Adeola, O.T., Enobong, A. and Olufunke, B. (2008). Microbiological and physicochemical analysis of different water samples used for domestic purposes in Abeokuta and Ojota, Lagos State, Nigeria. African Journal of Biotechnology, 7: 617-621.
Patil, S.R., Patwari, J.M and Mushtaq, A.D.( 2018). Assessment of ground water suitability for drinking purpose from Narangal, (MS), India. Journal of Bioscience Discovery. 9(3): 396-402.
Prajapati, R. and Rokde, R. (2016 ). Bacteriological Study of Drinking Water in South Indore City (M. P.). International Journal of Science and Research, 5 (8): 1193 -1194.
Prasanna, M.V., Chidambaram, S., Shahul Hameed, A. and Srinivasamoorthy, K. (2010). Study of evaluation of groundwater in Gadilam basin using hydrogeochemical and isotope data. Environmental Monitoring and Assessment, 168: 63-90.
Ramachandraiah, C. (2004). Right to drinking water in India. Centre for Economic and Social Studies, 56.
Ramesh, K. and Soorya, V. (2012). Hydrochemical analysis and evaluation of groundwater quality in and around Hosur, Krishnagiri District, Tamil Nadu, India. International Journal of Research in Chemistry and Environment, 2(3): 113-122.
Saleem, Q.M. and Algamal, Y. (2016). Assessment of Physico-chemical and Biological Properties of Ground Water of Khulais, Province, Kingdom of Saudi Arabia. International Journal of science and Research Methodology, 5 (1): 504-521.
Shahida, P and Ummatul, F. (2015). Physico –Chemical analysis of ground water quality in Aligarh city. Uttar Pradesh. International journal of science and nature. 6(3): 397 -405.
Shigut, D.A., Liknew, G., Irge, D.D. and Ahmad, T. (2017). Assessment of physico-chemical quality of borehole and spring water sources supplied to Robe Town, Oromia region, Ethiopia. Applied Water Science, 7: 155–164.
Singh, S. (2016). Study of ground water quality in Ujjain. Ph.D. thesis. Barkatullah University, Bhopal. (M.P.): 2-4.
Soni, S. and Singh, R.K. (2018). Study of ground water quality in tap and hand pump water of Tonk city, Rajasthan, India. International Journal of Scientific Research and Management, 6(1): 1-7.
Tahir, M.A., Rasheed, H. and Malana, A. (2008). A method of development for arsenic analysis by modification in spectrophotometric technique. Drink- Water-Eng. Sci. Discuss., 1135- 154.
Tank, D.K. and Singh, C.C.P. (2010). Analysis of major ion constituents of ground water of Jaipur city. Nature Science, 8: 1-7.
WHO. (1985). Guidelines for drinking water quality (Drinking water quality control in small community supplier), World Health Organization, Geneva 3: 121.
WHO. (2008). Water Guidelines for drinking-water quality. Recommendation of the World Health Organization. WHO, 3rd ed. 29.


