1Dr. Indu Dayal Meshri College of Science and Technology, Patan – 384265, Gujarat (India)
2Department of Chemistry, Hemchandracharya north Gujarat University, Patan – 384265, Gujarat India
Article Publishing History
Received: 14/01/2020
Accepted After Revision: 20/03/2020
In the present paper we discuss about the biogenic synthesis of gold nanoparticles using Aegle marmelos extract. The gold nanoparticles were synthesized via eco-friendly and low cost effective method. Preparation of the aqueous leaf extracts of Aegle marmelos was carried out deionized water and the extracts acted as reducing agent as well as capping agent. The synthesized gold nanoparticles were characterized by UV-Visible spectroscopy, Fourier transform infrared spectroscopy (FTIR), X-ray Diffraction (XRD), Field emission gun Scanning electron microscopy (FEG-SEM) with EDS and High Resolution Transmission electron microscopy (HR-TEM). The elemental composition and purity of gold nanoparticles was analysed by EDS. XRD patterns showed average particle size of 22 nm and also UV absorption peak showed around 534 nm. The antibacterial activity of gold nanoparticles was studied against micro organisms like against Bacillus subtilis (MTCC 121) and Escherichia coli (MTCC 119).
Gold nanoparticles, Aegle marmelos, Biogenic Synthesis, Antimicrobial Activity
Shah R, Vaghela H, Pathan A. Synthesis and Characterization of Biogenic Gold Nanoparticles Using Aegle marmelos Extracts: Antibacterial Assay. Biosc.Biotech.Res.Comm. 2020;13(1).
Shah R, Vaghela H, Pathan A. Synthesis and Characterization of Biogenic Gold Nanoparticles Using Aegle marmelos Extracts: Antibacterial Assay. Biosc.Biotech.Res.Comm. 2020;13(1). Available from: https://bit.ly/2IGgWrW
Copyright © Shah et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Nanostructures with various properties like physical, chemical and electrical empower broad applications such as an antibacterial, catalysis, optical, electrical, as an energy transformation as well as reservoir devices and production of biomedicals. In the past, research has shown that antioxidant compounds present in Aegle marmelos inactivate the free radicals or make them less reactive and thus protect against reactive oxygen species. Health-promoting compounds present in Bael include Terpenoids, Flavonoids, Steroids, Phenolic Compounds, Alkaloids, Tannins, and Saponins. Out of all these, tannins have a very strong free radical scavenging property and act as a primary antioxidant, (Iqbal et al.,2015 Ashar et al.,2016 Cao et al.,2016 Iqbal et al.,2017).
The Bael plant or Aegle marmelos grows in the soil with a pH range between 5 – 10. The Aegle marmelos tree tolerates all kinds of soil situation and stands water logging situation. This tree has a wide tolerance for temperature and grows well in the temperature range 7º C to 48ºC. It grows best on rich, well-drained soil. it grows very well in summer and its growth declines during winter. The preliminary step in the synthesis of AuNPs is the reduction of gold ions (Au1+) to neutral gold atoms (Au0). It occurs by reduction of chloroauric acid (H [AuCl4]) in a solution in the presence of suitable reducing agent. The gold nanoparticles synthesized using plant extracts do not require reducing agent as the compounds which readily exists in plants acts as reducing agents as well as stabilizing agents. The synthesis of gold nanoparticles using plant extracts has been tried and it has become successful. Biosynthesis of gold nanoparticles from plants like Hibiscus rosa-sinensis (Daizy et al., 2010), Cinnamomum camphora, Azadirachta indica (Shankar et al., 2004), Geranium, (Shankar et al., 2003), lemon grass (Grunwald et al., 2004), Aloe vera (Chandran et al., 2006), Moringa pterygosperma and Boerhavia diffusa (Vaghela et al., 2018), Crateva Religiosa (Parmar et al., 2016), and Bauhinia variegate (Vaghela et al. (2017,2018).
Recently, synthesized AgNPs using fresh fruit extract of Phyllanthus emblica have been and evaluate for their antibacterial efficacy against pathogen Acidovorax oryzae strain RS-2 of rice bacterial brown stripe (Masum et al., 2019). Balasubramanian et al., (2020) have reported biogenic synthesis of gold nanoparticles using Jasminum auriculatum leaf extract and have reported their catalytic, antimicrobial and anticancer activities. Vo et al. (2020) have reported biosynthesized silver and gold nanoparticles from Lactuca indica leaf extract and their applications in catalytic degradation of toxic compounds. Uzma et al. (2020) have reported biogenic synthesis of gold nanoparticles using Commiphora wightii and their cytotoxic effects on breast cancer cell line (MCF-7). Similarly, Aisida et al. (2020) have reported biogenic synthesis of iron oxide nanorods using Moringa oleifera leaf extract which showed good antibacterial activity. Siddiquee et al. (2020) have reported green synthesis of silver nanoparticles from Delonix regia leaf extract and its application in cytotoxicity interaction studies with bovine serum albumin.
In this study, we report the plant-mediated synthesis of gold nanoparticles using the aqueous leaf extracts of Aegle marmelos an evergreen shrub which is found in many parts of India. We prepared metallic gold nanoparticles via green biogenic synthesis. The reduction of aqueous Au+ ions with the thallus broth of marine algae, P. pavonica its characterization and tested its anti-microbial activity tested against microorganisms namely Escherichia coli and Bacillus subtilis. The gold nanoparticles (AuNPs) synthesized were characterized by UV-Vis spectroscopy, SEM, TEM, XRD, EDAX, and FTIR. The Antibacterial/Antimicrobial Activity effects of gold nanoparticles.
MATERIALS AND METHODS
All the chemical reagents used in this experiment were of analytical grade purchased from s.d. fine chemicals. The fresh and healthy leaves were collected around patan (N.G.) and washed with distilled water to remove dust particles.All the chemical reagents used in this experiment were of analytical grade purchased from s.d. fine chemicals. The fresh and healthy leaves were collected around patan (N.G.) and washed with distilled water to remove dust particles.After washing the leaves were spread evenly in a clean paper and the leaves were allowed to dry in shade for about 3-4 days. When the leaves were dried completely they were finely powdered using mixer and was used for extract preparation. First we cut all the leaf and then Initially 10 gm of leaf boiled with 100 ml double distilled water for 60 min. in heating on Soxhlet at temperature 600C. The mixture was brought to the room temperature and the aqueous leaf extract was collected using Whatman filter paper. The resulting product was filtered and stored in refrigerator for further use.
All solutions were prepared using double distilled water. Initially 10 gm of leaf Aegle Marmelos were boiled with double distilled water and heated on Soxhlet for 60 min. than it was filtered using Whatman filter paper No.1 after filtration, 20 ml extract was added in 30 ml gold (AuNO3) which was put on magnetic stirrer with hot plate for around 8 hours. The resultant solution was dark black color which turned to dark brownish color within one hour. The solid products were obtained from it. Subsequently the solution was then centrifuged at 12000 rpm for 20 min. The supernated centrifugation was used as plant extract. Gold nanoparticles were synthesized by adding 20 ml of plant extract to 1×10-3M AuNo3 (ACS extra pure).
RESULTS AND DISCUSSION
After completion of reaction, reaction mass was observed for the colour changes from dark black to dark brownish in comparison to the control solution. The colour change is the visual method of detection of synthesis of gold nanoparticles. The gold nanoparticles were characterized by using UV-Vis spectroscopy (Shimadzu UV 1800 UV-Visible spectrophotometer) reduction of gold ions was monitored by measuring the UV-Vis range of the reaction mixture at 8 hour (Martínez et al., 2012). FTIR spectroscopy analysis was carried out to identify the biomolecules responsible for the reduction of Au+ ions (Elia ET AL., 2014). FTIR spectroscopy analysis was carried out to find the biomolecules that were bound specifically on the gold oxide nanoparticles surface. The morphology of the obtained nanoparticles was characterized by using a high-resolution transmission electron microscope (HR-TEM) (Krishnaswamy et al., 2014). Chemical composition of the obtained nanoparticles were analysed by EDAX technique using scanning electron microscope (FEG-SEM) (Hezard et al., 2012). The crystallographic structure of Gold nanoparticles and the phase properties was examined by XRD measurements using Rigaku D/max 40 kV diffractometer (Yan et al., 2005).
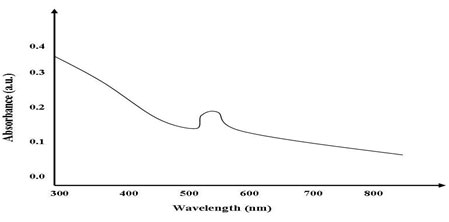
UV-Vis spectroscopy measurements (Shimadzu UV 1800) were carried out at room temperature in the region 800–200 nm as a function of time of the reaction. The UV–Visible absorption spectrum was used for the analysis of optical properties of Biogenic synthesized Gold oxide nanoparticles. The mono dispersed Gold oxide nanoparticles are shown in synthesis figure-1. The room temperature spectra exhibited strong excitonic absorption peaks at 534 nm for samples respectively, which is in good agreement with previous work (Islam et al., 2015).
Figure 1: Uv-visible spectra of Biogenic synthesized Gold nanoparticles.
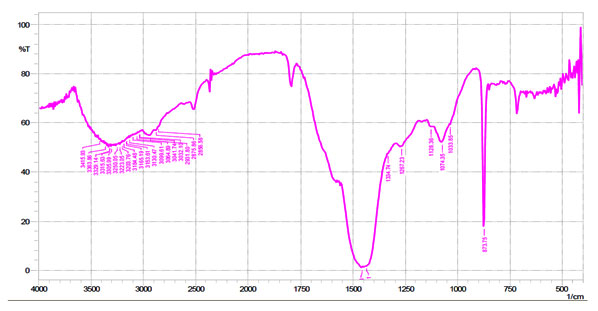
FTIR spectroscopy analysis was carried out to identify the biomolecules of extract responsible for the reduction of Au+ ions. FTIR spectroscopy analysis was carried out to find the biomolecules that were bound specifically on the Gold nanoparticles surface. Fig. 2 shows the FTIR spectra of Biogenic synthesized Gold nanoparticles. The spectrum showed sharp bands at 873 cm-1 corresponding to metal–oxygen (M–O). Strong bands were observed at 1033, 1074 and 1450 cm-1 and have been referred to as alcohols and phenolic groups, C–N stretching vibrations of aliphatic and aromatic amines, respectively. The bands obtained at 3000 cm-1 have been representing to stretching vibrations of primary alkanes, amines and water molecules.
Figure 2: FTIR spectra of Biogenic synthesized Gold nanoparticles.
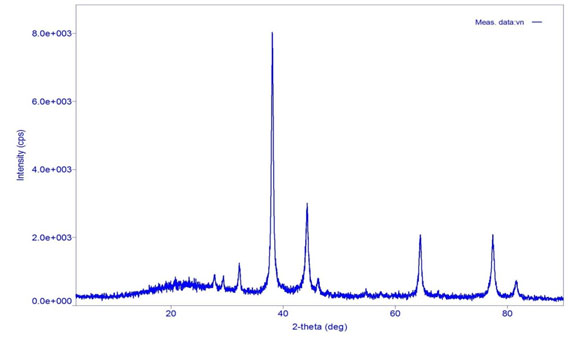
X-ray diffraction (XRD) measurement of the Biogenic synthesis of Gold nanoparticles carried out on a Rigaku D/max 40 kV diffractometer equipped with the graphite mono chromator and Au target. Fig. 3 shows the XRD analysis of Biogenic synthesized Gold oxide nanoparticles. This is used for further confirmation of Gold oxide phase of nanoparticles. The observed intense peaks are 380, 440, 640 and 770respectively representing the (111), (200), (222) and (311)reflections indicating the face centered cubic (fcc) structure of AuNPs XRD pattern reveals the face centered cubic structure indicating the crystalline nature of AuNPs and the particle size calculated using Debye-Scherrer equation,
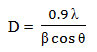
Where, D is average Particles size, λ is wavelength (1.5418 Å), θ is the Bragg’s angle and β is full width half maximum (FWHM) of corresponding peek. The Scherer’s formula was used to estimate the particles sizes and was found to around 22 nm (Dubey et al., 2010).
Table 1. XRD spectral data of gold nanoparticles
| 2Ɵ | Particle size (nm) | (h k l) |
| 38 | 19.94 | (111) |
| 44 | 16.34 | (200) |
| 64 | 20.68 | (222) |
| 77 | 34.24 | (311) |
| Average Particle size D = 22 nm | ||
Figure 3: XRD spectra of Biogenic synthesized Gold nanoparticles.
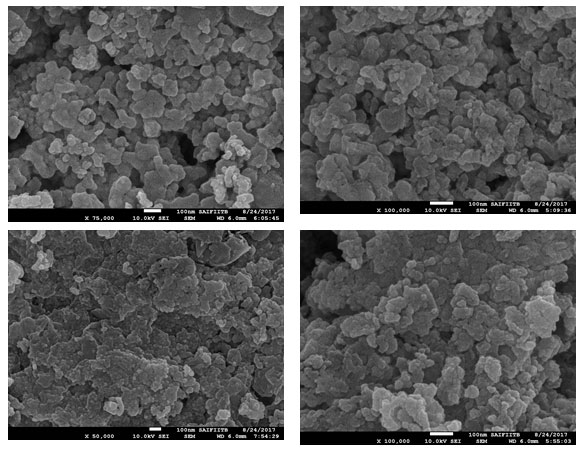
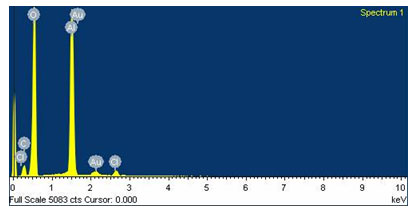
Field emission gun scanning electron microscope (FEG-SEM) and EDS images were recorded on a JSM-7600F series instrument. FEG-SEM spectra of Biogenic synthesis of Gold nanoparticles are shown in Fig.4. Gold nanoparticles by this method show nearly mono dispersed distribution of particle sizes. The average particle size of the Au nanoparticles is around 15-30 nm. The composition of Gold nanoparticles was further probed by energy-dispersive X-ray (EDS) analysis. Fig. 5 shows the EDS pattern of AuNPs prepared using Gold sulphate, which indicates the presence of Au and small amount of oxygen. EDS spectrum of Gold nanoparticles shows the peaks for Gold and respective elements indicating the formation of Gold nanoparticles. Peak indexing of the elements is oxygen 0.5 keV and Gold 1.5 & 2.2 keV. The compositions in the mass percentage of the elements are oxygen 45.12% and Gold 41.21 %. The experimental composition matches with the theoretically calculated composition.
Figure 4: FEG-SEM spectra of Biogenic synthesis of Gold nanoparticles
Figure 5: EDS spectra of Biogenic synthesis of Gold nanoparticles
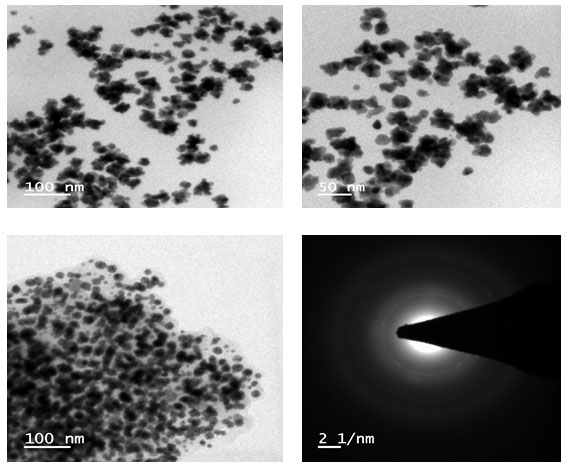
High-resolution Transmission electron microscope (HR-TEM) images were recorded on a Tecnai G2-F30 electron microscope. Fig. 6 shows the HR-TEM images of Au NPs prepared using Mixtures. The sample preparation was carried out via the coating on carbon coated grid Cu Mesh 300 prior to the measurement. High-resolution Transmission electron microscopy (HR-TEM) has been employed to characterize the size, shape and morphology of synthesized Gold nanoparticles.
Figure 6: HR-SEM spectra of Gold nanoparticles
The antibacterial screening test of the synthesized Gold nanoparticles was performed by both agar well diffusion method against Bacillus subtilis (MTCC 121) and Escherichia coli (MTCC 119).
Table 2. Antimicrobial activity data for synthesized gold nanoparticles.
|
Bacteria |
Zone of Inhibition of Biosynthesized Gold Nanoparticles with different concentrations | Average zone of
Inhibition |
||
| 20 μL | 40 μL | 60 μL | ||
| B. subtilis | 4mm | 8mm | 13mm | 8.3 mm |
| E. Coli | 7mm | 12mm | 17mm | 12mm |
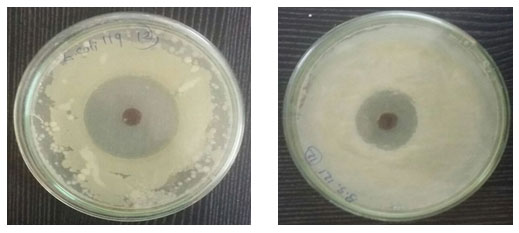
The antibacterial activity of Gold nanoparticles was carried out by agar cup plate method. The antibacterial activity of Gold nanoparticles was evaluated against Bacillus subtilis and Escherichia coli. Fresh overnight Culture of each strain swabbed uniformly by cotton on plates containing Mueller Hinton agar and 4 wells (diameter size- 6 mm) were prepared using cup borer. Different concentration (20, 40, 60 μL) samples of nanoparticles pour into each well and incubated it for 24 hr at 370C, after that around the well diameter of inhibition zone was observed in millimeter (Figure 7)(Table 2). Inhibition zone of bacterial growth is due to inhibitory compounds from the tested sample. We concluded that agar well diffusion method exhibited good antibacterial activity in which Escherichia coli showed excellent zone of inhibition and Bacillus subtilis showed weak results compare to Escherichia coli bacteria. Plant extract didn’t give any results (Bhawasar et al., 1965, Behl and Srivastava, 2002, Shah and Qadry, 1971, Zafar, 1994). Experiments with each strain performed three times for good results.
Figure 7: Antimicrobial activity of Au nanoparticles
In summary, the biogenic synthesis of gold nanoparticles was performed using Aegle marmelos extracts without involving any toxic chemicals. In this reduction reaction metal ions were reduced (Au+2 to Au+1) very rapidly and reaction was finally completed within 8 hours to produce Gold nanoparticles. Synthesized gold nanoparticles mediated from Aegle marmelos leaves exhibited excellent antibacterial activity against gram-positive (Bacillus subtilis) and gram negative (Escherichia coli) bacteria which exhibited considerable zone of inhibition. Increase in the dose of AuNPs resulted in the rise in antibacterial activity. So it can concluded that biologically synthesized gold nanoparticles using Aegle marmelos extracts can be used as environment-friendly low cost formulation with excellent antibacterial activity against Escherichia coli microorganisms.
REFERENCES
Ashar, A., Iqbal, M., Bhatti, I. A., Ahmad, M. Z., Qureshi, K., Nisar, J., & Bukhari, I. H. (2016). Synthesis, characterization and photocatalytic activity of ZnO flower and pseudo-sphere: Nonylphenol ethoxylate degradation under UV and solar irradiation. Journal of Alloys and Compounds, 678, 126-136.
Aisida, S. O., Madubuonu, N., Alnasir, M. H., Ahmad, I., Botha, S., Maaza, M., & Ezema, F. I. (2020). Biogenic synthesis of iron oxide nanorods using Moringa oleifera leaf extract for antibacterial applications. Applied Nanoscience, 10(1), 305-315.
Balasubramanian, S., Kala, S. M. J., & Pushparaj, T. L. (2020). Biogenic synthesis of gold nanoparticles using Jasminum auriculatum leaf extract and their catalytic, antimicrobial and anticancer activities. Journal of Drug Delivery Science and Technology, 101620.
Behl, P. N., & Srivastava, G. (2002). Herbs useful in dermatological therapy (Vol. 11). CBS Publishers & Distributors.
Bhawasar, G. C., Guru, L. V., & Chadda, A. K. (1965). Antibacterial activity of some indigenous medicinal plants. Medical-Surgical Nursing, 5, 11-14.
Cao, J., Sun, X., Zhang, X., & Lu, C. (2016). Homogeneous synthesis of Ag nanoparticles-doped water-soluble cellulose acetate for versatile applications. International journal of biological macromolecules, 92, 167-173.
Chandran, S. P., Chaudhary, M., Pasricha, R., Ahmad, A., & Sastry, M. (2006). Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnology progress, 22(2), 577-58
Dubey, S. P., Lahtinen, M., & Sillanpää, M. (2010). Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochemistry, 45(7), 1065-1071.
Elia, P., Zach, R., Hazan, S., Kolusheva, S., Porat, Z. E., & Zeiri, Y. (2014). Green synthesis of gold nanoparticles using plant extracts as reducing agents. International journal of nanomedicine, 9, 4007.
Grunwald, A. (2004). The case of nanobiotechnology. EMBO reports, 5(S1), S32-S36.
Hezard, T., Fajerwerg, K., Evrard, D., Collière, V., Behra, P., & Gros, P. (2012). Gold nanoparticles electrodeposited on glassy carbon using cyclic voltammetry: Application to Hg (II) trace analysis. Journal of Electroanalytical Chemistry, 664, 46-52.
Iqbal, M., & Bhatti, I. A. (2015). Gamma radiation/H2O2 treatment of a nonylphenol ethoxylates: degradation, cytotoxicity, and mutagenicity evaluation. Journal of hazardous materials, 299, 351-360.
Iqbal, M., Nisar, J., Adil, M., Abbas, M., Riaz, M., Tahir, M. A.& Shahid, M. (2017). Mutagenicity and cytotoxicity evaluation of photo-catalytically treated petroleum refinery wastewater using an array of bioassays. Chemosphere, 168, 590-598.
Islam, N. U., Jalil, K., Shahid, M., Rauf, A., Muhammad, N., Khan, A. & Khan, M. A. (2015). Green synthesis and biological activities of gold nanoparticles functionalized with Salix alba. Arabian Journal of Chemistry.
Krishnaswamy, K., Vali, H., & Orsat, V. (2014). Value-adding to grape waste: Green synthesis of gold nanoparticles. Journal of Food Engineering, 142, 210-220.
Martínez, J. C., Chequer, N. A., González, J. L., & Cordova, T. (2012). Alternative methodology for gold nanoparticles diameter characterization using PCA technique and UV-VIS spectrophotometry. Nanosci. Nanotechnol, 2(6), 184-189.
Masum, M. M. I., Siddiqa, M. M., Ali, K. A., Zhang, Y., Abdallah, Y., Ibrahim, E. & Li, B. (2019). Biogenic synthesis of silver nanoparticles using Phyllanthus emblica fruit extract and its inhibitory action against the pathogen Acidovorax oryzae Strain RS-2 of rice bacterial brown stripe. Frontiers in microbiology, 10.
Parmar, Kokila A., A. K. Pathan, Kavita R. Desai, J. R. Jadhav and Rahul Shah. (2016). Synthesis of Biogenic Silver Nanoparticles from Medicinal Plant and its Antibacterial Activity. IOSR Journal of Applied Chemistry, 9(8), 29-33.
Philip, D. (2010). Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Physica E: Low-Dimensional Systems and Nanostructures, 42(5), 1417-1424.
Shah, C. S., & Qadry, J. S. (1971). A textbook of pharmacognosy. Messrs BS Shah.
Shankar, S. S., Ahmad, A., & Sastry, M. (2003). Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnology progress, 19(6), 1627-1631.
Shankar, S. S., Rai, A., Ahmad, A., & Sastry, M. (2004). Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. Journal of colloid and interface science, 275(2), 496-502.
Siddiquee, M. A., ud din Parray, M., Mehdi, S. H., Alzahrani, K. A., Alshehri, A. A., Malik, M. A., & Patel, R. (2020). Green synthesis of silver nanoparticles from Delonix regia leaf extracts: In-vitro cytotoxicity and interaction studies with bovine serum albumin. Materials Chemistry and Physics, 242, 122493.
Vaghela, H., Shah, R., & Pathan, A. (2018). Palladium nanoparticles mediated through Bauhinia variegata: potent in vitro anticancer activity against mcf-7 cell lines and antimicrobial assay. Current Nanomaterials, 3(3), 168-177.
Vaghela, Hiral, Rahul Shah, and Kokila A. Parmar. (2018). Plant Mixture Mediated Biogenic Copper Nanoparticles: Antibacterial Assay. Current Nanomaterials 3(2), 86-94.
Vo, T. T., Dang, C. H., Doan, V. D., Dang, V. S., & Nguyen, T. D. (2020). Biogenic synthesis of silver and gold nanoparticles from Lactuca indica leaf extract and their application in catalytic degradation of toxic compounds. Journal of Inorganic and Organometallic Polymers and Materials, 30(2), 388-399.
Uzma, M., Sunayana, N., Raghavendra, V. B., Madhu, C. S., Shanmuganathan, R., & Brindhadevi, K. (2020). Biogenic synthesis of gold nanoparticles using Commiphora wightii and their cytotoxic effects on breast cancer cell line (MCF-7). Process Biochemistry.
Shah, C. S., & Qadry, J. S. (1971). A textbook of pharmacognosy. Messrs BS Shah.
Yan, W., Petkov, V., Mahurin, S. M., Overbury, S. H., & Dai, S. (2005). Powder XRD analysis and catalysis characterization of ultra-small gold nanoparticles deposited on titania-modified SBA-15. Catalysis Communications, 6(6), 404-408.
Zafar, R. (1994). Medicinal plants of India CBS Publications and Distributors. New Delhi, 105.