1Department of Microbiology and Biotechnology, Gujarat University, Navrangpura, Ahmedabad, India
2M G Science Institute, Dada Saheb Mavlankar Campus Opposite Gujarat
University Navrangpura, Ahmedabad 380009, Gujarat, India
3Shri P.H.G. Municipal Arts and Science College, Kalol, Gujarat, India
Article Publishing History
Received: 01/01/2025
Accepted After Revision: 25/03/2025
The increasing awareness about sustainable processes mandates the exploration of lignocellulosic agrowaste for the production of valuable industrial enzymes like xylanase and cellulase. This study investigates xylanase and cellulase production from an indigenously isolated Streptomyces lividans through substrate fermentation of agrowaste, highlighting the significance of optimizing various parameters for enhanced enzyme activity. The rationale behind this study stems from the urgent need of sustainable solutions to manage agricultural waste while producing commercially important xylanase and cellulase. A systematic approach involving response surface methodology was employed to evaluate the effects of key factors such as temperature (20-60 °C), pH (2-8), substrate concentration (5-25 mg %), ammonium sulphate, and dextrose on enzyme production.
The specific enzyme activities under optimal conditions were found to be 4.21 µmol/mg-minute for xylanase and 3.97 µmol/mg-minute for cellulase, with substrate concentration emerging as the primary contributor to enzyme yield, as indicated by the experimental design matrix and three-dimensional contour plots. These findings underscore the industrial relevance of Streptomyces lividans in enzyme production and suggest avenues for further research in optimizing fermentation conditions. Future studies should focus on large-scale applications and the potential enhancement of enzyme activity through genetic manipulation, thereby improving the viability of xylanase and cellulase in various industries, including biofuels, textiles, and bioremediation.
Activity; Purified Xylanase; Fermentation; Streptomyces lividans
Joshi K, Goyal N, Patwa R.Study of Enzyme Activity Profile of Crude and Purified Xylanase and Cellulase Produced Using Substrate Fermentation from Indigenously Isolated Streptomyces Lividans. Biosc.Biotech.Res.Comm. 2025;18(1).
Joshi K, Goyal N, Patwa R. Study of Enzyme Activity Profile of Crude and Purified Xylanase and Cellulase Produced Using Substrate Fermentation from Indigenously Isolated Streptomyces Lividans. Biosc.Biotech.Res.Comm. 2024;18(1). Available from: <a href=”https://shorturl.at/r5sSs“>https://shorturl.at/r5sSs</a>
INTRODUCTION
The need to save resources, combined with pollution issues from agro-industrial waste and limited disposal options, has encouraged the bioconversion of waste into industrially valuable products (Freitas et al. 2021; Phiri et al. 2024). Considerable amounts of agricultural biomass deemed ‘waste’ is now being explored for the production of biofuels, animal feed, chemicals, and enzymes, among other value-added items (Singh et al. 2021; Velvizhi et al. 2022; Alazaiza et al. 2025).
For example, abundantly and the cheaply available lignocellulosic agrowaste such as vegetable and fruit peels (Panda et al. 2016), rice and wheat husk (Devi et al. 2022; Abena and Simachew 2024), corn cobs (Devi et al. 2022; Joshi et al. 2023), sugarcane bagasse (Devi et al. 2022; Yadav et al. 2022), coconut coir (Khangwal and Shukla 2022), and wood pulp (Walia et al. 2013; Singh et al. 2023) etc. are increasingly being explored for production of important hydrolytic industrial enzymes such as xylanase and cellulase (Sadh et al. 2018; Waheeb et al. 2021; Yafetto 2022).
The polysaccharide components of lignocellulosic agrowaste are cellulose and hemicellulose. Cellulases consisting of endoglucanase (EC 3.2.1.4), exoglucanase (EC 3.2.1.91) and b-glucosidase (EC 3.2.1.21) act synergistically to convert the cellulose complex of biomass into glucose and oligosaccharide (Fatokun et al. 2016a; Tingthong et al. 2021). Xylanase is the class of enzymes which convert the polysaccharide beta-1,4-xylan into xylooligosaccharide, xylobiose and xylose subunits (Pandey et al. 2022; Chaudhary et al. 2023). Xylanase plays an important role in the conversion of the hemicellulose component of biomass into xylose, (Sarangi and Thatoi 2024).
Xylanases are the enzyme complexes responsible for hydrolysis of xylan, and they include endo-1,4-xylanases (EC 3.2.1.8) and -xylosidases (EC 3.2.1.37), in addition to side-chain-hydrolyzing enzymes (Kumar et al. 2014; Fatokun et al. 2016a). Xylanase and cellulase are important industrial enzyme that have series of applications in food, textile, agriculture, paper pulp and the bioremediation industry (Rodrigues and Odaneth 2021; Basit et al. 2021).The global market for industrially useful enzymes was estimated to be about $ 12.27 billion in the year 2022 and is expected to develop at a compound annual growth rate (CAGR) of approximately 6.5% over the period from 2023 to 2030 (Li et al. 2012; Grand View Research 2023).
Bacteria and fungi can secrete multiple lignocellulolytic enzymes, including xylanases and cellulases (Gudynaite-Savitch and White 2016; Talamantes et al. 2016). However, in recent times, bacterial enzymes for industrial applications are continually increasing due to their rapid growth rate and tenacity to be genetically engineered in contrast to fungi (Danso et al. 2022). Correspondingly, a variety of xylanase and cellulase-producing bacteria have been applied to lignocellulosic biomass for production of enzymes (Menendez et al. 2015; Chakdar et al. 2016; Kaur et al. 2024).
Streptomyces is one of the most important genera of Actinomycetes involved in fermentation process and it has been widely used for enzyme production (Javed et al. 2021; Khushboo et al. 2022). For example, recently Shrestha et al., reported production of multi-enzyme (pectinase, cellulase, and xylanase) from Streptomyces sp. using agrowaste mixture (Shrestha et al. 2022). Sinjaroonsak et al., recently optimized production of xylanase and cellulase by Streptomyces thermocoprophilus TC13W from pretreated empty fruit bunch of oil palm (Sinjaroonsak et al. 2020). Advantages of Streptomyces derived enzymes include their high level of extracellular activity, stability across a broad temperature (50–85 °C) and pH (pH 3–13) range (Kumar et al. 2019). Researchers are working to isolate novel Streptomyces strains from unexplored habitats for exploring the potential and application of enzymes secreted by them (Kumar et al. 2020).
Enzyme activity is influenced by numerous parameters such as pH, temperature, inducers, medium additives, and type of fermentation (Bhardwaj et al. 2019).
Furthermore, enzyme activation is dependent on various metal ions as activators or inhibitors. For this reason, optimization and in-depth analysis of these parameters are a must for maximizing enzyme activity. Here, we have employed submerged fermentation processes (SmF) for the production of xylanase and cellulase. Advantages of SmF include homogeneous conditions in the media, better biomass utilization, and easy scale-up. It has been reported that SmF enables better biomass utilization and therefore results in higher enzyme production (Hansen et al. 2015; Joshi et al. 2023).
This study aimed to study the influence of parameters such as pH, temperature, substrate and enzyme concentration for optimum enzyme production using response surface methodology (Siwach et al. 2024). Enzyme activity of crude and purified xylanase and cellulase produced from indigenously isolated Streptomyces lividans was studied using one factor at a time approach. Cultured broth media was filtered and taken as crude enzyme. Enzyme purification was carried out from broth media using spin column and gel electrophoresis (Boucherba et al. 2014; Joshi et al. 2023).
MATERIALS AND METHODS
Optimization using Response Surface Methodology :Response surface methodology was employed to get optimum conditions of significant variables. Three independent variables, namely, ammonium sulphate (g) and dextrose (g), and substrate (g) were assessed for the dependent response variable e., cellulase and xylanase enzyme production (U/mL), Table 1. State Ease Design-Expert V 10.0.1 software was used for multiple non-linear regression of response for each run to obtain the coefficient of the polynomial equation. Response surface methodology processed by use of central composite design (CCD). Statistical analysis of the model was performed to evaluate the analysis of variance (ANOVA). The statistical significance of the model was determined by the F-test and the goodness of fit of the estimated polynomial equation was assessed based upon the coefficient of determination (R2), response surface and contour plots (Vaishnav et al. 2022).
Table 1. RSM experimental runs and response
| Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 | ||
| Standard | Run |
A: Ammonium sulphate (g) |
B: Dextrose (g) | C: Substrate concentration (g) | Cellulase (U/mL) | Xylanase (U/mL) |
| 15 | 1 | 2.25 | 2.75 | 2 | 425.13 | 423.09 |
| 10 | 2 | 4.35224 | 2.75 | 2 | 356.06 | 364.08 |
| 1 | 3 | 1 | 1.5 | 1 | 372.09 | 305.79 |
| 16 | 4 | 2.25 | 2.75 | 2 | 429.45 | 419.98 |
| 6 | 5 | 3.5 | 1.5 | 3 | 418.42 | 438.84 |
| 8 | 6 | 3.5 | 4 | 3 | 453.55 | 460.32 |
| 13 | 7 | 2.25 | 2.75 | 0.318207 | 247.58 | 158.29 |
| 3 | 8 | 1 | 4 | 1 | 398.52 | 346.6 |
| 17 | 9 | 2.25 | 2.75 | 2 | 432.19 | 428.14 |
| 2 | 10 | 3.5 | 1.5 | 1 | 220.48 | 158.07 |
| 9 | 11 | 0.147759 | 2.75 | 2 | 393.47 | 396.11 |
| 20 | 12 | 2.25 | 2.75 | 2 | 421.78 | 417.69 |
| 14 | 13 | 2.25 | 2.75 | 3.68179 | 412.89 | 433.27 |
| 7 | 14 | 1 | 4 | 3 | 390.06 | 390.1 |
| 12 | 15 | 2.25 | 4.85224 | 2 | 471.02 | 428.95 |
| 5 | 16 | 1 | 1.5 | 3 | 430.38 | 432.79 |
| 18 | 17 | 2.25 | 2.75 | 2 | 440.52 | 432.28 |
| 4 | 18 | 3.5 | 4 | 1 | 353.64 | 297.28 |
| 19 | 19 | 2.25 | 2.75 | 2 | 423.98 | 420.34 |
| 11 | 20 | 2.25 | 0.647759 | 2 | 426.13 | 384.31 |
Bacterial strain: From our previous work we screened bacteria efficient in production of cellulase and xylanase using agricultural waste as substrate. Based on 16S rRNA analysis the indigenously isolated bacterium was identified as Streptomyces lividans. Gram’s staining was carried out to study cultural and morphological characteristics. Nutrient agar was used to study colony characteristics. The strain was maintained on nutrient agar slant and stored at 4 o
Crude enzyme and Purified enzyme: Sterilized basal salt media (yeast extract, K2HPO4, KH2PO4, MgSO4.7H2O, (NH2)2SO4, NaCl, CaCO3) containing 2 g of corn cob for xylanase or coconut husk for cellulase was inoculated with Streptomyces lividans and incubated for 72 hours at 37 o The broth was filtered and taken as crude enzyme. 100 mL of crude xylanase/cellulase enzyme was concentrated by spin column chromatography to give 10 mL of purified enzyme.
Protein (enzyme) characterization: Crude and purified protein (enzyme) was characterised for molecular weight by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using 3 mm gel slab. Coomassie brilliant blue G-250 was used as a stain. Broad range molecular weight marker was used for molecular weight determination. Crude and purified enzyme was characterised using SLS One Stop Protein Purification mini prep protocol.
The 10 mL of crude supernatant after xylanase/cellulase production was incubated at 37°C for 30 minute and mixed with equal volume of precipitation and binding buffer. The system was incubated on ice for 2-5 minute and then centrifuged for 10, 000 RPM for 5 minute.
The supernatant was removed and the pellet was resuspended in 500μl of resuspension buffer. The solution was transferred to SLS One-step protein Spin Column and centrifuged at 10,000 RPM for 1 minute.
The spin column was placed into a brand-new collecting tube, and 100μl of elution buffer was added to it. The tube was incubated at room temperature for 1 minute. and then centrifuge at 10,000 RPM for 1 minute. The eluted protein (1 mL) was collected for further analysis.
Effect of influencing parameter The effects of influencing parameters, such as temperature, pH, substrate concentration, and enzyme amount, were investigated. Enzyme activity of parameters was assayed by measuring released sugar using DNSA reagent (Miller 1959). Briefly, 1 mL of crude/purified enzyme was mixed with 1 mL of 1 % substrate (CMC for cellulase and Xylan for xylanase) in addition to 2 mL of buffer. After 10 minutes of incubation at ambient room temperature (̴ 30 + 3 oC), 1 mL DNSA was added Enzyme activity (µmol/minute) was calculated using equation 1.
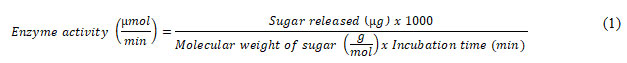
Here, incubation time was 10 minutes. Molecular weight of xylose and cellulose is 150.13 g/mol and 162.14 g/mol, respectively.
Enzyme activity was investigated for influencing parameters such as temperature, pH, substrate concentration and enzyme amount.
RESULTS AND DISCUSSION
Cultural and Morphological Characteristics
Table 2. Cultural Characteristics on Nutrient agar.
| Shape | Size | Colony appearance | Margin | Elevation | Texture | Gram’s reaction | Pigment |
| Round | Medium | Rough | Entire | Slightly Raised | Dry | Gram Positive | Nil |
Figure 1: Colony characteristics of KJB17 on A. Nutrient Agar plate, B. Basal Salt Agar plate
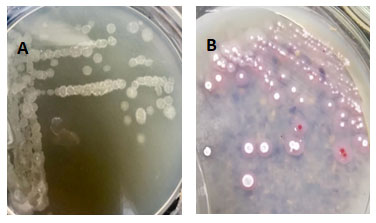
To verify the phenotypic characteristics of the KJB17 strain, the bacteria was grown on Nutrient Agar plate. The resulting colony was slightly raised, colourless colony with rough appearance on Nutrient Agar, Figure 1 A. Additionally, the KJB17 strain colony was also grown on Basal Salt Agar plate on which it gave distinctly pink colonies as can be seen in Figure 1 B. Furthermore, on Nutrient agar as well as Basal salt agar media plate, the KJB17 strain gave luxurious growth, Figure 1.

Based on microscopic observation of Gram staining, KJB17 strain is Gram positive, long rod and non-capsulating, Figure 2, based on Bergey’s Manual of Systematic Bacteriology (Beg et al. 2001; Abdulkhair et al. 2025).
Response surface methodology : For RSM analysis, twenty different experimental runs were design to study enzyme production response according to runs provided by minimum and maximum value of ammonium sulphate, dextrose, and substrate concentration, as listed in Table 1. Table 3 represents experimental design matrix with actual and predicted values of xylanase and cellulase production. Increasingly, RSM optimization is being studied for optimum enzyme production (Amadi et al. 2020; Siwach et al. 2024).
Table 3. Experimental design matrix of RSM with experimental and predicted values of xylanase and cellulase enzyme
| Xylanase | Cellulase | ||||
| Run | Actual
Value |
Predicted
Value |
Actual
Value |
Predicted
Value |
Standard |
| Order | Order | ||||
| 1 | 423.09 | 423.6934 | 425.13 | 428.9125 | 15 |
| 2 | 364.08 | 356.7232 | 356.06 | 347.927 | 10 |
| 3 | 305.79 | 301.1914 | 372.09 | 366.268 | 1 |
| 4 | 419.98 | 423.6934 | 429.45 | 428.9125 | 16 |
| 5 | 438.84 | 442.9705 | 418.42 | 423.1065 | 6 |
| 6 | 460.32 | 467.5572 | 453.55 | 461.124 | 8 |
| 7 | 158.29 | 161.3139 | 247.58 | 251.9438 | 13 |
| 8 | 346.6 | 345.1081 | 398.52 | 395.5854 | 3 |
| 9 | 428.14 | 423.6934 | 432.19 | 428.9125 | 17 |
| 10 | 158.07 | 166.6463 | 220.48 | 228.2753 | 2 |
| 11 | 396.11 | 399.7353 | 393.47 | 399.1254 | 9 |
| 12 | 417.69 | 423.6934 | 421.78 | 428.9125 | 20 |
| 13 | 433.27 | 426.5145 | 412.89 | 406.0486 | 14 |
| 14 | 390.1 | 384.1623 | 390.06 | 384.0167 | 7 |
| 15 | 428.95 | 433.5664 | 471.02 | 475.647 | 12 |
| 16 | 432.79 | 440.8606 | 430.38 | 437.0892 | 5 |
| 17 | 432.28 | 423.6934 | 440.52 | 428.9125 | 18 |
| 18 | 297.28 | 291.848 | 353.64 | 348.6827 | 4 |
| 19 | 420.34 | 423.6934 | 423.98 | 428.9125 | 19 |
| 20 | 384.31 | 375.9621 | 426.13 | 419.0253 | 11 |
Table 4. Analysis of variance (ANOVA) table for Response Surface Quadratic model for Xylanase enzyme
| Sum of | Mean | F | p-value | |||
| Source | Squares | df | Square | Value | Prob > F | |
| Model | 141127.8 | 9 | 15680.86 | 236.0699 | 1.90209E-10 | Significant |
| A-Ammonium sulphate | 2233.191 | 1 | 2233.191 | 33.61992 | 0.000173366 | Significant |
| B-Dextrose | 4005.483 | 1 | 4005.483 | 60.30115 | 1.52675E-05 | Significant |
| C-Substrate | 84897.47 | 1 | 84897.47 | 1278.102 | 6.96241E-12 | Significant |
| AB | 3303.626 | 1 | 3303.626 | 49.73493 | 3.48902E-05 | Significant |
| AC | 9337.295 | 1 | 9337.295 | 140.5697 | 3.27184E-07 | Significant |
| BC | 5061.689 | 1 | 5061.689 | 76.20196 | 5.44057E-06 | Significant |
| A2 | 3723.496 | 1 | 3723.496 | 56.05593 | 2.09372E-05 | Significant |
| B2 | 645.4682 | 1 | 645.4682 | 9.717298 | 0.010924847 | Significant |
| C2 | 30340.5 | 1 | 30340.5 | 456.7656 | 1.12078E-09 | Significant |
| Residual | 664.2466 | 10 | 66.42466 | |||
| Lack of Fit | 509.3735 | 5 | 101.8747 | 3.288972 | 0.108646708 | Not significant |
| Pure Error | 154.8731 | 5 | 30.97463 | |||
| Cor Total | 141792 | 19 |
Table 5. Analysis of variance (ANOVA) table for Response Surface Quadratic model for Cellulase enzyme
|
Source |
Sum of
Squares |
Df |
Mean
Square |
F
Value |
p-value
Prob > F |
|
| Model | 74609.29 | 9 | 8289.921 | 109.0996 | 8.64E-09 | Significant |
| A-Ammonium sulphate | 3164.153 | 1 | 3164.153 | 41.64189 | 7.32E-05 | Significant |
| B-Dextrose | 3870 | 1 | 3870 | 50.9312 | 3.15E-05 | Significant |
| C-Substrate | 28666.7 | 1 | 28666.7 | 377.2686 | 2.86E-09 | Significant |
| AB | 4148.694 | 1 | 4148.694 | 54.59896 | 2.34E-05 | Significant |
| AC | 7689.24 | 1 | 7689.24 | 101.1944 | 1.51E-06 | Significant |
| BC | 3394.056 | 1 | 3394.056 | 44.66753 | 5.48E-05 | Significant |
| A2 | 5526.093 | 1 | 5526.093 | 72.72623 | 6.7E-06 | Significant |
| B2 | 611.4555 | 1 | 611.4555 | 8.047071 | 0.017647 | Significant |
| C2 | 17983.98 | 1 | 17983.98 | 236.6785 | 2.74E-08 | Significant |
| Residual | 759.8486 | 10 | 75.98486 | |||
| Lack of Fit | 524.6043 | 5 | 104.9209 | 2.230041 | 0.199675 | Not significant |
| Pure Error | 235.2443 | 5 | 47.04886 | |||
| Cor Total | 75369.14 | 19 |
For determining regression model fitting was significant or not significant, ANOVA analysis was conducted for xylanase and cellulase which is summarised in Table 4 and Table 5, respectively. ANOVA of regression model demonstrated model was highly significant based on Fisher’s F-test with high F-value and p-value. The p-value checks significance of each coefficient, and indicates interaction strength between each independent parameter. F-value of 236.07 and 109.10 implies model is significant for xylanase and cellulase, respectively. Smaller p-value (here, p < 0.05 for xylanase and cellulase) indicates higher significance of corresponding coefficient. As can be seen from Table 4 and Table 5, A, B, C, AB, AC, BC, A2, B2, and C2 are significant for xylanase and cellulase production, respectively. The “Lack of Fit F-value” of 3.29 with corresponding p-value of 0.10 for xylanase (Table 4) and 2.23 with corresponding p-value of 0.19 for cellulase (Table 5) implies the Lack of Fit is not significant relative to the pure error. Insignificant lack of fit confirmed the validity of model.
Table 6. Statistical calculation of RSM experimental design for xylanase and cellulase
| Xylanase | Cellulase | |
| Std. Dev. | 8.150132 | 8.716929 |
| Mean | 376.816 | 395.867 |
| C.V. % | 2.162894 | 2.201984 |
| PRESS | 4321.114 | 4515.445 |
| R-Squared | 0.995315 | 0.989918 |
| Adj R-Squared | 0.991099 | 0.980845 |
| Pred R-Squared | 0.969525 | 0.940089 |
| Adeq Precision | 53.13943 | 40.13299 |
Furthermore, statistical calculation to determine the goodness of the model for experimetal desgin of xylanse and cellulase are depicted in Table 6. For xylanase, “Pred R-Squared” of 0.9695 is in reasonable agreement with the “Adj R-Squared” of 0.9911, i.e. the difference is less than 0.2. Likewise, for cellulase, “Pred R-Squared” of 0.9401 is in reasonable agreement with the “Adj R-Squared” of 0.9808, i.e. the difference is less than 0.2. “Adeq Precision” measures the signal to noise ratio and ratio greater than 4 is desirable. Adeq Precision value of 53.13943 and 40.13299 for xylanase and cellulase reveal an adequate signal. Thus, based on all these results, this model can be used to navigate xylanase and cellulase production.
Zhang et al. 2016 studied optimisation of xylanase and cellulase from Streptomyces griseorubens JSD-1 using RSM for improved enzymatic saccharification efficiency. Walia et al., 2015 (Walia et al. 2015) studied xylanase from Cellulosimicrobium cellulans CKMX1 and its improvement using RSM.
Figure 3: Esponse surface plots of xylanase and cellulase showing interaction of three
variables (Ammonium sulphate, dextrose, and, substrate concentration).
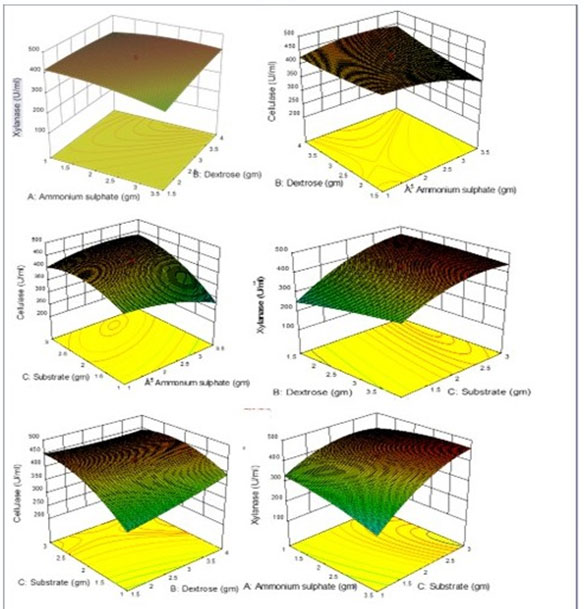
The three-dimension (3D) contour plots were prepared to understand the effect of three variables, i.e., ammonium sulphate, dextrose, and substrate concentration on xylanase and cellulase production, Figure 3. 3D contour plots visual interaction between two variables by fixing third variable at zero level. All graphs clearly reveal influence of substrate concentration is major contributor for xylanase and cellulase production.
Molecular identification by 16s rRNA gene technology
Figure 4: Phylogenetic tree of KJBs17 (Streptomyces lividans)

ABI 3730xl Genetic Analyzer was used to carry out the DNA sequencing reaction of the PCR amplicon with prime r27F using BDT v3.1 Cycle sequencing kit for molecular identification. The BLAST search of 16S rRNA gene sequence against sequences in nucleotide database showed 100% homology with Streptomyces lividans strain, Fig. 4. Thus, the new indigenously isolated strain was identified as Streptomyces lividans (Thomas et al. 2013). The genomic sequence is uploaded on National Center for Biotechnology Information (NCBI) portal with accession number of PP528163. The genomic sequence of identified Streptomyces lividans is
TGCTGGCAACACGGGACAAGGGTTGCGCTCGTTGCGGGACTTAACCCAACATCTCACGACACGAGCTGACGACAGCCATGCACCACCTGTACACCGACCACAAGGGGGGCACCATCTCTGATGCTTTCCGGTGTATGTCAAGCCTTGGTAAGGTTCTTCGCGTTGCGTCGAATTAAGCCACATGCTCCGCCGCTTGTGCGGGCCCCCGTCAATTCCTTTGAGTTT.
Streptomyces is described as a Gram positive, spore-forming, and aerobic actinobacterium, which has a high guanine-cytosine (GC) content in the genome and contains LL-diaminopimelic acid in the cell wall. Streptomyces are reported to produce amylase (Al-Dhabi et al. 2020; Lakshmi et al. 2020), protease (Sarkar and Suthindhiran 2020), cellulase (Danso et al. 2022), xyalanase (Porsuk 2013; Danso et al. 2022), nitrate reductase (Zhang et al. 2021), and catalase (Kim et al. 2013) to hydrolyze starch, protein, cellulose, xylan, nitrate, and hydrogen peroxide, respectively.
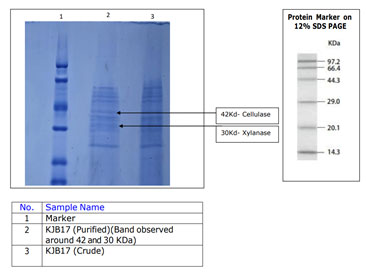
Crude and purified protein was characterised using the SLS one-stop Nontagged protein purification column per standard protocol. The column is specifically designed with combination of immobilized metal affinity chromatography and boronic acid-based resins for efficient protein purification. The molecular weight of crude and purified enzyme was determined using SDS-PAGE. As can be seen from Figure 5, there are several fragments of crude and purified protein. Molecular mass of denatured protein was between 66.4 to 20.1 kDa as measured by comparative movement of proteins on SDS-PAGE (Wu et al. 2018). The obtained results are in consistent with that obtained by (Waheeb et al. 2021b; Abdulkhair et al. 2025).
Figure 5: Electrophoresis on agar gel
Enzyme activity and its influencing parameter
Enzyme activity was investigated for influencing parameters such as temperature, pH, substrate concentration and enzyme amount. Enzyme activity was measured using DNSA method (Miller 1959; Islam and Roy 2018). Several studies have studied optimization of influencing parameters for enzyme activity for xylanase (Techapun et al. 2002; Khurana et al. 2007; Fatokun et al. 2016b; Sinjaroonsak et al. 2020) and cellulase (Fatokun et al. 2016b; Sinjaroonsak et al. 2020).
Figure 6: Temperature effect on enzyme activity of crude and purified enzyme. A. Xylanase B. Cellulase
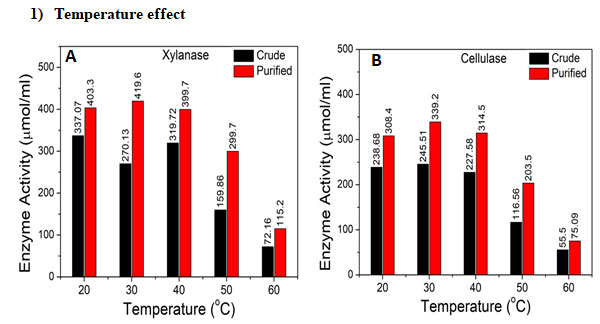
The effect of temperature in the range of 20 to 60 oC on enzyme activity of crude and purified xylanase and cellulase was determined, Figure 6. As can be seen purified enzyme of xylanase and cellulase reported higher activity than crude enzyme for all studied temperature, Figure 6 Xylanase and cellulase show good activity at within temperature range of 20 to 40oC, Figure 5. Maximum activity of 419.6 µmol/mL (Figure 6A) and 339.2 µmol/mL (Figure 6B) for purified xylanase and cellulase respectively was reported at 30oC. Maximum activity for crude xylanase of 337.07 µmol/mL (Figure 6A) and cellulase of 245.51 µmol/mL (Figure 6B) was reported at 20oC and 30oC, respectively. Similar results have been reported by Leya et al., for Streptomyces sp. (Thomas et al. 2013; Sinjaroonsak et al. 2020). Temperature is important parameter for optimum enzyme production due to alterations in microbial protein structure and its properties with variations in temperature (Juturu and Wu 2014; Li et al. 2025).
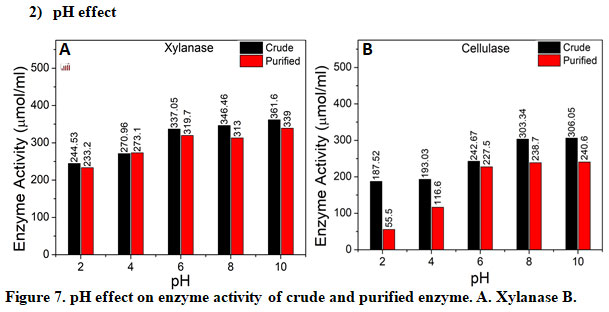
Cellulase: The effect of initial pH in the range of 2 to 10 were investigated to understand its influence on enzyme activity of crude and purified xylanase and cellulase, Figure 7. pH of growth media was regulated with 0.1 N HCl/NaOH. From the results it can be seen that pH 8 and 10 are optimum for xylanase, Figure 7 A and cellulase, Figure 7 B, with respect to crude as well as purified. In addition, clearly for all pH studied crude for cellulase reported better activity than purified, Figure 7 B. Interestingly for xylanase, no significant change in enzyme activity of crude and purified was reported, Figure 7 A. It is noted that synthesis and expression of certain genes, and microbial metabolic activities, are influenced by the internal pH, in response to that of the external environment (Fatokun et al. 2016b). In addition, many enzyme systems and the transport of several enzyme species across the cell membrane are influenced by the initial pH of the cultivation medium (Fatokun et al. 2016b). Thus, it is crucial to maintain optimum pH.
Figure 8: Substrate concentration effect on enzyme activity of crude and purified
enzyme. A. Xylanase B. Cellulase

Effect of substrate concentration in the range of 5 to 25 mg was studied on enzyme activity of crude and purified xylanase and cellulase, Figure 8. As can be seen for xylanase optimum enzyme activity of 486.2 µmol/mL was reported for crude and purified enzyme at substrate concentration of 20 mg and 25 mg, Figure 8 A. Interestingly, for purified cellulase optimum enzyme concentration of 400 µmol/mL was reported at 25 mg and crude cellulase enzyme activity of 395 µmol/mL was optimum at 20 mg and 25 mg of substrate concentration, Figure 8 B. Since xylanase and cellulase are inducible extracellular enzyme, substrate concentration plays an important role (Ortiz-Cortés et al. 2021). Overall, substrate concentration exhibited positive linear behaviour on enzyme activity as expected. However, higher concentration of substrate is also reported to adversely affect the growth and enzyme activity as seen in case of purified cellulase enzyme at 25 mg (Bajar et al. 2020).
Figure 9: Enzyme volume effect on enzyme activity of crude and purified enzyme. A. Xylanase B.
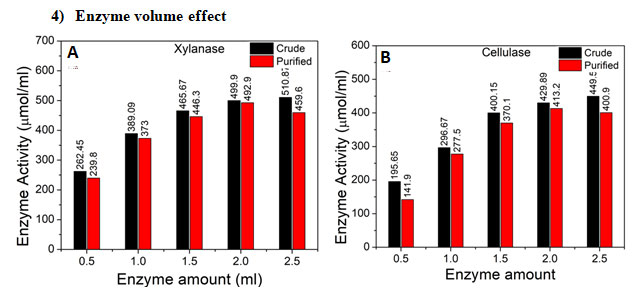
Cellulase : The effect of enzyme amount in the range of 0.5 to 2.5 mL was studied on enzyme activity of crude and purified xylanase and cellulase, Figure 9. As can be seen, Figure 9 A and B, enzyme activity of crude was optimum than purified for xylanase as well as cellulase at enzyme amount of 2.5 mL. Enzyme activity of 510.87 µmol/mL was optimum for xylanase and enzyme activity of 449.5 µmol/mL was optimum for cellulase. The results are as expected that with increase in enzyme amount enzyme activity would show an increase.
Figure 10: Influence of optimized parameters on enzyme activity of
crude and purified Xylanase and Cellulase
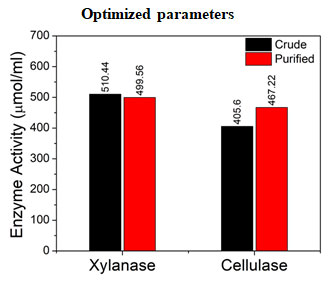
From the above results (Figure 10), optimized parameters for xylanase were 30oC temperature, 10 pH, 20 mg substrate concentration, and 2.5 mL enzyme volume. These parameters reported enzyme activity of 510 µmol/mL and 499.56 µmol/mL for crude and purified xylanase respectively. Likewise, optimised parameters for cellulase were 30oC temperature, 8 pH, 20 mg substrate concentration, and 2.5 mL enzyme volume. These optimized influencing parameters reported enzyme activity of 405.6 µmol/mL and 467.22 µmol/mL for crude and purified enzyme respectively. By exploiting the potential of Streptomyces lividans and optimizing the fermentation process, we can enhance the production of cellulase and xylanase for its applications.
CONCLUSION
Overall, we used response surface methodology to investigate the influence of three variables (ammonium sulfate, dextrose, and substrate concentration) on the activity profile of xylanase and cellulase production. Through experimental design and three-dimensional contour plots, we found that substrate concentration was the major contributor to the production of both enzymes (xylanase and cellulase). We evaluated the potential of indigenous Streptomyces lividans isolated from agrowaste for xylanase and cellulase production using substrate fermentation. Furthermore, we examined the influence of various parameter, i.e., temperature (20-60oC), pH (2-8), substrate concentration (5-25 mg), and enzyme volume (0.5-2.5 mL) on enzyme activity of xylanase and cellulase. The specific enzyme activity under optimum parameters conditions was found to be 4.21 µmol/mg-minute for xylanase and 3 µmol/mg-minute for cellulase, respectively. By exploring the potential of Streptomyces lividans and optimizing the fermentation process, we can enhance the production of these important industrial enzymes. Furthermore, future research should focus on the potential enhancement of enzyme activity through genetic manipulation, thereby improving the applicability of xylanase and cellulase.
ACKNOWLEDGMENTS
We extend our heartfelt gratitude to Dr. Meenu Sarraf, Head Department of Microbiology M G Science Institute, DadaSaheb Mavlankar Campus Opposite Gujarat University, Navrangpura, Ahmedabad 380009, Gujarat.
Author conflict statement: All authors confirm that there are no known conflicts of interest associated with this publication.
Funding: Research work received no external funding. Kinjal Joshi received financial support from SHODH fellowship
Data Availability Statement: Data will be made available on request
REFERENCES
Abena T, Simachew A (2024) Production and characterization of acidophilic xylanase from wood degrading white rot fungus by solid-state fermentation of wheat straw. Heliyon 10:e35496. https://doi.org/10.1016/J.HELIYON.2024.E35496/ASSET/F50A38E6-F039-4289-B374-AC32DF514D2A/MAIN.ASSETS/GR9.JPG
Alazaiza MYD, Bin Mokaizh AA, Baarimah AO, Al-Zghoul T (2025) From agro-waste to bioactive wealth: Analyzing nutraceutical extraction and applications. Case Studies in Chemical and Environmental Engineering 11:101066. https://doi.org/10.1016/J.CSCEE.2024.101066
Amadi OC, Egong EJ, Nwagu TN, Okpala G, Onwosi CO, Chukwu GC, Okolo BN, Agu RC, Moneke AN (2020) Process optimization for simultaneous production of cellulase, xylanase and ligninase by Saccharomyces cerevisiae SCPW 17 under solid state fermentation using Box-Behnken experimental design. Heliyon 6:e04566. https://doi.org/10.1016/j.heliyon.2020.e04566
Bajar S, Singh A, Bishnoi NR (2020) Exploration of low-cost agro-industrial waste substrate for cellulase and xylanase production using Aspergillus heteromorphus. Appl Water Sci 10:153. https://doi.org/10.1007/s13201-020-01236-w
Basit A, Jiang W, Rahim K (2021) Xylanase and Its Industrial Applications. In: Biotechnological Applications of Biomass. IntechOpen
Beg Q, Kapoor M, Mahajan L, Hoondal GS (2001) Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol 56:326–338
Bhardwaj N, Kumar B, Verma P (2019) A detailed overview of xylanases: an emerging biomolecule for current and future prospective. Bioresour Bioprocess 6:40. https://doi.org/10.1186/s40643-019-0276-2
Boucherba N, Gagaoua M, Copinet E, Bettache A, Duchiron F, Benallaoua S (2014) Purification and Characterization of the Xylanase Produced by Jonesia denitrificans BN-13. Appl Biochem Biotechnol 172:2694–2705. https://doi.org/10.1007/s12010-013-0709-x
Chakdar H, Kumar M, Pandiyan K, Singh A, Nanjappan K, Kashyap PL, Srivastava AK (2016) Bacterial xylanases: biology to biotechnology. 3 Biotech 6:150. https://doi.org/10.1007/s13205-016-0457-z
Chaudhary R, Kuthiala T, Singh G, Rarotra S, Kaur A, Arya SK, Kumar P (2023) Current status of xylanase for biofuel production: a review on classification and characterization. Biomass Convers Biorefin 13:8773–8791. https://doi.org/10.1007/s13399-021-01948-2
Danso B, Ali SS, Xie R, Sun J (2022) Valorisation of wheat straw and bioethanol production by a novel xylanase- and cellulase-producing Streptomyces strain isolated from the wood-feeding termite, Microcerotermes species. Fuel 310:122333. https://doi.org/10.1016/j.fuel.2021.122333
Devi S, Dwivedi D, Bhatt AK (2022) Utilization of Agroresidues for the Production of Xylanase by Bacillus safensis XPS7 and Optimization of Production Parameters. Fermentation 8:221. https://doi.org/10.3390/fermentation8050221
Fatokun E, Nwodo U, Okoh A (2016a) Classical Optimization of Cellulase and Xylanase Production by a Marine Streptomyces Species. Applied Sciences 6:286. https://doi.org/10.3390/app6100286
Fatokun E, Nwodo U, Okoh A (2016b) Classical Optimization of Cellulase and Xylanase Production by a Marine Streptomyces Species. Applied Sciences 6:286. https://doi.org/10.3390/app6100286
Freitas LC, Barbosa JR, da Costa ALC, Bezerra FWF, Pinto RHH, Carvalho Junior RN de (2021) From waste to sustainable industry: How can agro-industrial wastes help in the development of new products? Resour Conserv Recycl 169:105466. https://doi.org/10.1016/j.resconrec.2021.105466
Grand View Research (2023) Enzymes Market Size, Share, Trends & Growth Report, 2030. https://www.grandviewresearch.com/industry-analysis/enzymes-industry. Accessed 2 Nov 2023
Gudynaite-Savitch L, White TC (2016) Fungal Biotechnology for Industrial Enzyme Production: Focus on (Hemi)cellulase Production Strategies, Advances and Challenges. pp 395–439
Hansen GH, Lübeck M, Frisvad JC, Lübeck PS, Andersen B (2015) Production of cellulolytic enzymes from ascomycetes: Comparison of solid state and submerged fermentation. Process Biochemistry 50:1327–1341. https://doi.org/10.1016/j.procbio.2015.05.017
Islam F, Roy N (2018) Screening, purification and characterization of cellulase from cellulase producing bacteria in molasses. BMC Res Notes 11:445. https://doi.org/10.1186/s13104-018-3558-4
Javed Z, Tripathi GD, Mishra M, Dashora K (2021) Actinomycetes – The microbial machinery for the organic-cycling, plant growth, and sustainable soil health. Biocatal Agric Biotechnol 31:101893. https://doi.org/10.1016/j.bcab.2020.101893
Joshi K, Goyal Noopur, Patwa Rupal (2023) Production, Optimization, and Purification of Xylanase from agricultural waste by indigenously isolated Bacillus paralicheniformis (KJB3). Adv Biores 228–236
Juturu V, Wu JC (2014) Microbial cellulases: Engineering, production and applications. Renewable and Sustainable Energy Reviews 33:188–203. https://doi.org/10.1016/j.rser.2014.01.077
Kaur G, Kaur P, Kaur J, Singla D, Taggar MS (2024) Xylanase, xylooligosaccharide and xylitol production from lignocellulosic biomass: Exploring biovalorization of xylan from a sustainable biorefinery perspective. Ind Crops Prod 215:118610. https://doi.org/10.1016/j.indcrop.2024.118610
Khangwal I, Shukla P (2022) A Comparative Analysis for the Production of Xylooligosaccharides via Enzymatic Hydrolysis from Sugarcane Bagasse and Coconut Coir. Indian J Microbiol 62:317–321. https://doi.org/10.1007/s12088-022-01010-3
Khushboo, Kumar P, Dubey KK, Usmani Z, Sharma M, Gupta VK (2022) Biotechnological and industrial applications of Streptomyces metabolites. Biofuels, Bioproducts and Biorefining 16:244–264. https://doi.org/10.1002/bbb.2294
Kumar L, Kumar D, Nagar S, Gupta R, Garg N, Kuhad RC, Gupta VK (2014) Modulation of xylanase production from alkaliphilic Bacillus pumilus VLK-1 through process optimization and temperature shift operation. 3 Biotech 4:345–356. https://doi.org/10.1007/s13205-013-0160-2
Kumar M, Kumar P, Das P, Kapur MK (2019) Draft genome of Streptomyces sp. strain 130 and functional analysis of extracellular enzyme producing genes. Mol Biol Rep 46:5063–5071. https://doi.org/10.1007/s11033-019-04960-y
Kumar M, Kumar P, Das P, Solanki R, Kapur MK (2020) Potential applications of extracellular enzymes from Streptomyces spp. in various industries. Arch Microbiol 202:1597–1615. https://doi.org/10.1007/s00203-020-01898-9
Li S, Yang X, Yang S, Zhu M, Wang X (2012) Technology Prospecting on Enzymes: Application, Marketing and Engineering. Comput Struct Biotechnol J 2:e201209017. https://doi.org/10.5936/csbj.201209017
Li T, Wang R, Hua B, Cao L, Zhang Q, Zhai Y, Ling S, Wang M, Li E (2025) Improving the Thermal Stability of GH11 Xylanase XynASP through Cord Region Engineering. J Agric Food Chem 73:1516–1528. https://doi.org/10.1021/acs.jafc.4c10256
Menendez E, Garcia-Fraile P, Rivas R (2015) Biotechnological applications of bacterial cellulases. AIMS Bioeng 2:163–182. https://doi.org/10.3934/bioeng.2015.3.163
Miller GL (1959) Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Ortiz-Cortés LY, Ventura-Canseco LMC, Abud-Archila M, Ruíz-Valdiviezo VM, Velázquez-Ríos IO, Alvarez-Gutiérrez PE (2021) Evaluation of temperature, pH and nutrient conditions in bacterial growth and extracellular hydrolytic activities of two Alicyclobacillus spp. strains. Arch Microbiol 203:4557–4570. https://doi.org/10.1007/s00203-021-02332-4
Panda SK, Mishra SS, Kayitesi E, Ray RC (2016) Microbial-processing of fruit and vegetable wastes for production of vital enzymes and organic acids: Biotechnology and scopes. Environ Res 146:161–172. https://doi.org/10.1016/j.envres.2015.12.035
Pandey C, Sharma P, Gupta N (2022) Engineering to enhance thermostability of xylanase: For the new era of biotechnology. J Appl Biol Biotechnol. https://doi.org/10.7324/JABB.2023.110204
Phiri R, Mavinkere Rangappa S, Siengchin S (2024) Agro-waste for renewable and sustainable green production: A review. J Clean Prod 434:139989. https://doi.org/10.1016/J.JCLEPRO.2023.139989
Rodrigues VJ, Odaneth AA (2021) Industrial application of cellulases. In: Current Status and Future Scope of Microbial Cellulases. Elsevier, pp 189–209
Sadh PK, Duhan S, Duhan JS (2018) Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioprocess 5:1. https://doi.org/10.1186/s40643-017-0187-z
Sarangi A, Thatoi H (2024) Xylanase as a Promising Biocatalyst: A Review on Its Production, Purification and Biotechnological Applications. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences. https://doi.org/10.1007/s40011-024-01567-7
Shrestha S, Chio C, Khatiwada JR, Kognou ALM, Qin W (2022) Formulation of the agro-waste mixture for multi-enzyme (pectinase, xylanase, and cellulase) production by mixture design method exploiting Streptomyces sp. Bioresour Technol Rep 19:101142. https://doi.org/10.1016/j.biteb.2022.101142
Singh A, Bajar S, Devi A, Bishnoi NR (2023) Adding value to agro-industrial waste for cellulase and xylanase production via solid-state bioconversion. Biomass Convers Biorefin 13:7481–7490. https://doi.org/10.1007/s13399-021-01503-z
Singh R, Das R, Sangwan S, Rohatgi B, Khanam R, Peera SKPG, Das S, Lyngdoh YA, Langyan S, Shukla A, Shrivastava M, Misra S (2021) Utilisation of agro-industrial waste for sustainable green production: a review. Environmental Sustainability 4:619–636. https://doi.org/10.1007/s42398-021-00200-x
Sinjaroonsak S, Chaiyaso T, H-Kittikun A (2020) Optimization of Cellulase and Xylanase Productions by Streptomyces thermocoprophilus TC13W Using Low Cost Pretreated Oil Palm Empty Fruit Bunch. Waste Biomass Valorization 11:3925–3936. https://doi.org/10.1007/s12649-019-00720-y
Siwach R, Sharma S, Khan AA, Kumar A, Agrawal S (2024) Optimization of xylanase production by Bacillus sp. MCC2212 under solid-state fermentation using response surface methodology. Biocatal Agric Biotechnol 57:103085. https://doi.org/10.1016/j.bcab.2024.103085
Talamantes D, Biabini N, Dang H, Abdoun K, Berlemont R (2016) Natural diversity of cellulases, xylanases, and chitinases in bacteria. Biotechnol Biofuels 9:133. https://doi.org/10.1186/s13068-016-0538-6
Thomas L, Sindhu R, Pandey A (2013) Identification and characterization of a highly alkaline and thermotolerant novel xylanase from Streptomyces sp. Biologia (Bratisl) 68:1022–1027. https://doi.org/10.2478/s11756-013-0248-5
Tingthong S, Suwanakood P, Rattanachaikunsopon P, Sangswan J (2021) Production of Endoglucanases by Streptomyces thermocoprophilus CP1 using Rice Straw as a Substrate. J Pure Appl Microbiol 15:1963–1975. https://doi.org/10.22207/JPAM.15.4.18
Vaishnav A, Upadhyay K, Koradiya M, Tipre D, Dave S (2022) Valorisation of fruit waste for enhanced exopolysaccharide production by Xanthomonas campestries using statistical optimisation of medium and process. Food Biosci 46:101608. https://doi.org/10.1016/j.fbio.2022.101608
Velvizhi G, Goswami C, Shetti NP, Ahmad E, Kishore Pant K, Aminabhavi TM (2022) Valorisation of lignocellulosic biomass to value-added products: Paving the pathway towards low-carbon footprint. Fuel 313:122678. https://doi.org/10.1016/j.fuel.2021.122678
Waheeb MS, Elkhatib W, Yassien M, Hassouna N (2021) Production of Cellulase by Soil Isolated Streptomyces sp. Archives of Pharmaceutical Sciences Ain Shams University 5:225–233. https://doi.org/10.21608/aps.2021.92280.1067
Walia A, Mehta P, Chauhan A, Shirkot CK (2013) Optimization of cellulase-free xylanase production by alkalophilic Cellulosimicrobium sp. CKMX1 in solid-state fermentation of apple pomace using central composite design and response surface methodology. Ann Microbiol 63:187–198. https://doi.org/10.1007/s13213-012-0460-5
Walia A, Mehta P, Guleria S, Shirkot CK (2015) Improvement for enhanced xylanase production by Cellulosimicrobium cellulans CKMX1 using central composite design of response surface methodology. 3 Biotech 5:1053–1066. https://doi.org/10.1007/s13205-015-0309-2
Wu H, Cheng X, Zhu Y, Zeng W, Chen G, Liang Z (2018) Purification and characterization of a cellulase-free, thermostable endo-xylanase from Streptomyces griseorubens LH-3 and its use in biobleaching on eucalyptus kraft pulp. J Biosci Bioeng 125:46–51. https://doi.org/10.1016/j.jbiosc.2017.08.006
Yadav P, Anu, Kumar Tiwari S, Kumar V, Singh D, Kumar S, Manisha, Malik V, Singh B (2022) Sugarcane bagasse: an important lignocellulosic substrate for production of enzymes and biofuels. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02791-9
Yafetto L (2022) Application of solid-state fermentation by microbial biotechnology for bioprocessing of agro-industrial wastes from 1970 to 2020: A review and bibliometric analysis. Heliyon 8:e09173. https://doi.org/10.1016/j.heliyon.2022.e09173
Zhang D, Luo Y, Chu S, Zhi Y, Wang B, Zhou P (2016) Biological pretreatment of rice straw with Streptomyces griseorubens JSD-1 and its optimized production of cellulase and xylanase for improved enzymatic saccharification efficiency. Prep Biochem Biotechnol 46:575–585. https://doi.org/10.1080/10826068.2015.1084932


