1, 2Shri M. M. Patel Institute of Science and Research, Kadi Sarva Vishwavidyalaya, Gandhinagar, Gujarat, India.
Corresponding author email: rajeshri.patel0417@gmail.com
Article Publishing History
Received: 09/05/2020
Accepted After Revision: 15/06/2020
Peyronellaea pinodella BL-3/4, an ascomycete was isolated from the humus of municipal solid waste. The novelty regarding the present study is that, to date the isolated fungal strain has not been explored for laccase production and statistical optimization of medium parameters for enhanced laccase production. Efficient laccase production from this fungal strain was carried out by optimizing fermentation medium using the design of experiments through submerged fermentation. Initially, the medium components were screened using Plackett Burman design. A five-level-four factor central composite design was applied to statistically specify the effect of important process variables, namely glucose, orange peelings, peptone and copper sulphate. The significance of the factors and their interactions were verified by using the analysis of variance with 95% confidence level (p<0.05). Among the variable screened, orange peelings, glucose, peptone and copper sulfate were found significant in laccase production.
The central composite design of response surface methodology revealed that the best combination of fermentation medium for maximum laccase production is 2% glucose, 1% orange peelings, 0.5% peptone and 0.001 mg% copper sulphate with maximum laccase production of 151.5 U/mL. Statistical optimization leads to 2 fold higher laccase production than the unoptimized media in the present study. Purification by ammonium sulphate precipitation followed by dialysis and gel filtration chromatography leads to 17.5 fold purification with 14.1% yield of pure laccase. Purified enzyme was identified as 60 kDa monomeric protein by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The production of laccase by P.pinodella BL-3/4 was also confirmed by the evaluating presence of copper in the purified fraction. Presence of copper in structure of purified laccase was confirmed by UV-visible spectroscopy, atomic absorption spectroscopy and scanning electron microscopy coupled with energy dispersive X-ray analysis. Use of orange peelings as valuable substrate by P. pinodella, make the fungi a better candidate for large scale production of laccase as well as for bioremediation, when compared to all other reported fungi.
Central composite design, Laccase, Peyronellaea pinodella, Plackett Burman design, Statistical Optimization
Patel R. J, Bhaskaran L. Statistical Optimization and Partial Purification of Laccase from A Novel Fungal Strain Peyronallaea pinodella BL-3/4. Biosc.Biotech.Res.Comm. 2020;13(2).
Patel R. J, Bhaskaran L. Statistical Optimization and Partial Purification of Laccase from A Novel Fungal Strain Peyronallaea pinodella BL-3/4. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/2UXQzVb
Copyright © Patel and Bhaskaran This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The major structural component of all plant is a renewable organic material, lignocelluloses (Dashtban et al., 2009). Many industries like forestry, pulp and paper, agriculture, and food generates lignocellulosic waste during processing. Such wastes are also present in municipal solid waste (MSW), and animal wastes (Kim and Dale, 2004, Kalogo et al., 2007, Batista Meneses et al., 2020). Among all three different components of lignocelluloses, lignin is a natural heterogeneous biopolymer and highly recalcitrant in nature (Wong 2009, Anwar et al., 2014, Brenelli et al., 2018, Polo et al., 2020)
Due to complexity in structure, enzymes of most microorganisms are not able to degrade lignin. Ligninolytic enzymes are the group of enzymes that degrade lignin efficiently. Laccase (EC 1.10.3.2, para-diphenol: oxygen oxidoreductase) is one of the most important enzyme among group of ligninolytic enzymes. Having diversity in substrate specificity as well as catalytic active site of copper atom (Pointek et al., 2002), laccases non specifically catalyze oxidation of wide range of phenolic compounds, aromatic amines as well as non phenolic compounds with the four-electron reduction of molecular oxygen to water (Vishwanath et al., 2014, Jaber et al., 2017, Agrawal et al., 2018, Janusz et al., 2020).
The non specific catalytic ability makes laccase highly suitable biocatalysts for various Biotechnological applications. Such application includes effluents treatment and waste detoxification, food industry, paper and pulp industry, textile industry, synthetic chemistry, cosmetics, soil bioremediation, pesticide or insecticide degradation, organic synthesis, biosensor and analytical applications. Fungal laccases also play an important role in spore formation, pigment production, fruiting body formation, and plant pathogenesis (Sadhasivam et al., 2008).Laccase was first extracted and described by Yoshida (1883) from the sap of the Japanese lacquer tree, Rhus vernicifera. Laccases mostly been isolated and described from the white rot fungi, including Trametes versicolor, Agaricus bisporus, Coriolus spp., Pleurotus ostreatus, Phlebia radiata, Pycnoporus cinnabarinus and Coprinus cinereus. and few from the ascomycete group. Basidiomycetes are known laccase producers under both sub merged fermentation (SmF) (El-Batal et al., 2015) as well as solid state fermentation (SSF) (Patel and Gupte, 2016). Though SSF is preferred over SmF in terms of higher production yield, robust control of physical process parameters is difficult thus imposing problem in product recovery and scale up of laccase production.
Scale up needs right choice of the nutritive substrate in the culture medium that significantly decreases the total production costs and reduces the time period for expression of enzyme. Carbon, nitrogen and copper sources are the main nutritional parameters that regulate the level of gene transcription for laccase expression. Strain improvement to obtain higher laccase yield by single parameter approach is simple but laborious and time consuming and often do not tell about interaction effects between the medium parameters. Statistical optimization by design of experiments (DOE) concepts is the only solution to search such key factors and study interaction between medium parameters in a very few experiments.
Plackett-Burman design (PBD) [Plackett and Burman, 1946] is most widely used experimental design for initial screening of such significant factors from multiple nutritional parameters and optimizes only the positive and main effects on laccase production. The important and positive factors obtained from the screening experiments could be further optimized by employing response surface methodology (RSM) that enables the study of interaction effects between different variables.
Few reports are available on the statistical optimization of media components for the production of laccase in different fungal strain of division ascomycota i.e. Trichoderma harzianum strain (Gao et al, 2013, Bagewadi et al., 2017), Aspergillus flavus (Ghosh and Ghosh, 2017). Moreover, laccase production during dye degradation has only been reported from Peyronellaea prosopidis (Bankole et al., 2018).
To the best of our knowledge there are no reports on laccase production and use of statistical approach for its optimization from novel fungal strain Peyronellaea pinodella BL-3/4. The main objective of the study is to statistically optimize laccase production by fungal strain Peyronellaea pinodella BL-3/4 using DOE concept.
MATERIAL AND METHODS
A newly isolated fungal strain Peyronalleea pinodella BL-3/4 (Gene bank Accession Number: KT833620) prescreened (using lignin model compounds) from the humus soil of composted MSW was used in this study. This genus of ascomycetes has not been explored for any enzyme production specifically laccase production. The fungal strain was grown and maintained on Mineral salt- glucose peptone (MS-GP) medium according to the method of Patel and Bhaskaran, (2020). Ground orange peelings were employed as support-substrates as it is the best source for improving laccase productivity during single parameter optimization studies performed with Peyronellaea pinodella BL-3/4.
The enzyme production was performed in MS-GP medium supplemented with 0.5% orange peelings (Patel and Bhaskaran, 2020). The enzyme was extracted by filtering fermentation broth and the filtrate was used as the crude enzyme preparation. Extracellular laccase activity of crude enzyme preparation was determined spectrophotometrically with 2.5 mM 2, 2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (SIGMA) by method of Silva et al. (2007) and Patel and Bhaskaran, (2020).
Screening of factors by PBD is commonly employed to select significant factors in a production medium with lesser experimentations (Rajendran et al., 2007). For laccase optimization by Peyronellaea pinodella BL-3/4, factors considered for screening by PBD were orange peelings (A), glucose (B), peptone (C), ammonium acetate (D), KH2PO4 (E), MgSO (F), CaCL2 (G) MnSO4(H) and CuSO4 (J). Selected factors were experimentally screened with 12 trials in triplicates at 2 stages, high (+1) and low (- 1) (Table 1). The laccase activity is mean of 3 independent experiments. PBD is based on the first-order polynomial model shown in equation 1.
Y = β0+∑ βiXi ………………………………………………………………………… (1)
Where Y is the response (laccase production U/mL), β0 is the model intercept, βi is the linear coefficient, and Xi is the level of the independent factor (i = A, B, C, D, E, F, G, H, J).
The significant factors were identified by the analysis of the PBD experiments and their levels were further optimized by Central Composite Design under RSM. Each selected factor was studied at five different levels coded as –α, −1, 0, +1, and +α in a total of (α= (24)1/4 = 2.000) 30 runs, with two blocks (Bhamare et al., 2018). The laccase yield U/mL as the measured response (Y) was fitted by second-order polynomial equation 2.
Y = βo+β1A+β2B+β3C +β4D
+β11A2 +β22B2 +β33C2+β44D2
+β12AB+β13AC +β14AD+β23BC +β24BD+ β34CD ……………………………………… (2)
Where, Y is the measured response (laccase production U/mL), A, B, C and D are independent factors, β1, β2, β3, β4, are linear coefficients, β 11, β 22, β 33, β 44 are quadratic coefficients and β 12, β 13, β 14, β 23, β 24, β 34 are cross product coefficients of the model.
This design was used to evaluate the main effects, interaction effects and quadratic effects to optimize the levels of parameters for enhancing laccase production. The fitted polynomial equation was expressed as three-dimensional response surface plots and counter plots to find the concentration of each factor for maximum laccase production (Sondhi and Saini, 2019). The statistical significance of the model terms was studied using analysis of variance (ANOVA). The significance of the model was assessed using Fisher’s ‘F’ test and its corresponding probability ‘p’. Design-Expert Design-Expert version 10.0.6.0 software Version 10.0.6.0, Stat-Ease, Minneapolis, USA. was used as a tool to design experiments of statistical optimization and all statistical analysis.
Purification of laccase was carried out by growing the fungal culture in statistically optimized medium. The fermentation broth was centrifuged at 3000 x g for 10 min at 4°C for crude laccase preparation. Obtained supernatant was precipitated by ammonium sulphate in the range of 0-70% (w/v) at low temperature. Precipitated protein was dialyzed overnight with 0.1 M sodium acetate buffer using dialysis membrane of 10 kDa (Hi-Media Laboratories, India). Total protein content (method of Lowry et al., 1951) and laccase activity of the precipitated samples and dialyzed samples were determined according to method mention in laccase enzyme assay.
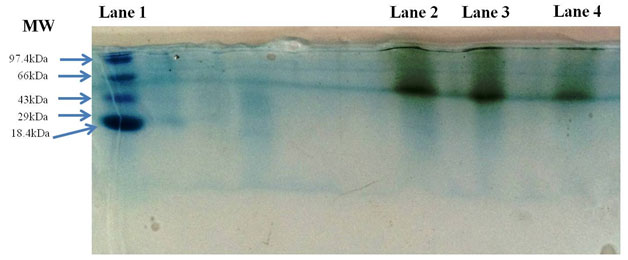
Concentrated dialyzed protein sample (1.5 mL) was applied to sephacryl s-100 HR (Amersham biosciences, USA) column (1.8×30 cm) pre-equilibrated with sodium phosphate buffer (pH 6.0). Protein was eluted with the same buffer having 0.15 M NaCl at a flow rate of 0.4 ml/min. A total of 30 fractions were collected and assayed for protein content and laccase activity. The purity of the laccase enzyme was confirmed on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS- PAGE) according to the method of Laemmli (1970). Prestained protein molecular weight marker (Genei, Bangalore, India) [Phosphorylase b (97.4 kDa), bovine serum albumin (66 kDa), ovalbumin (43 kDa), carbonic anhydrase (29 kDa), lacto globulin (18.4 kDa)] was loaded along with crude, dialyzed and gel filtered protein samples to know the approximate molecular mass of laccase enzyme. Protein bands on SDS-PAGE gels were stained with coomassie brilliant blue G-250 and compared with standard protein.
The presence of Cu2+, Zn2+, Fe2+ and Mn2+ in purified laccase were determined and quantified by Atomic absorption spectroscopy (AAS) (SL 194; ELICO, India). Spectroscopic characterization (Schimadzu UV 1800) of purified laccase was performed to confirm type of Cu centers. The presence of Cu2+ in purified laccase was confirmed by scanning electron microscopy coupled with energy dispersive X-ray (SEM/EDAX) analysis (Model: ESEM EDAX XL-30; Philips, Netherlands).
RESULTS AND DISCUSSION
The design matrix generated by design expert statistical software for the screening of variables and corresponding responses in terms of laccase enzyme yield is shown in Table 1. Highest laccase production (132.77 U/mL) was observed in a 9th run with a high level of glucose. Variation in laccase production among the different combinations occurred due to the influence of the factors at high and low levels as shown in Table 1. Parameters with statistically significant effects were identified using Fisher’s test for ANOVA. ANOVA for laccase production indicated ‘F-value’ of 95.33, which implied that the model was appropriate. Model terms having ‘Prob>F’ values less than 0.05 are considered to be significant and Prob>F’ values greater than 0.1 indicates the insignificant model terms (Niladevi et al., 2006, Sondhi and Saini, 2019).
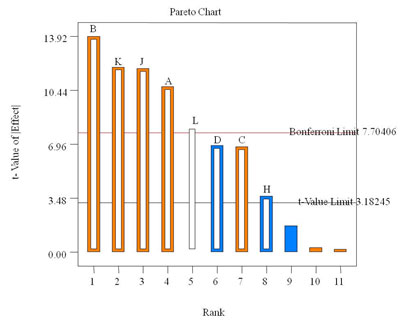
Factors having a confidence level greater than 95% were considered to have a significant effect on the response and were selected for further studies. In present study glucose was found to be the most influencing factor (p<0.0008), followed by CuSO4 (p<0.0013), orange peelings (p<0.0018) and peptone (p<0.0065) in to the medium (Table 2). Positive effect of glucose and copper on laccase production has been reported recently ( Karp et al., 2015, Ghosh and Ghosh, 2017, Bhamare et al., 2018). Although ammonium acetate and MnSO4 were significant model terms, they exerted a negative effect on laccase response. According to model KH2PO4, MgSO4 and CaCl2 had no significant effect on laccase production as shown in Figure 1.
The first-order model was fitted to the experimental results with the following final equation 3 in terms of coded factors:
Laccase activity = +65.91 +9.97* A +13.00* B +6.35* C -6.42* D – 3.37* H +11.05 * J ….. (3)
Where A, B, C, D, H and J are coded value of orange peelings, glucose, peptone, ammonium acetate, MnSO4 and CuSO4 respectively.
Table 1. PBD matrix of nine variables (A-H and J) and two dummy variables (K and L) along with observed response.
| Factor
A |
Factor
B |
Factor
C |
Factor
D |
Factor
E |
Factor F | Factor
G |
Factor
H |
Factor
J |
Factor
K |
Factor
L |
Response | |
| Run | Orange Peel (gm%) | Glucose (gm%) | Peptone (gm%) | Ammonium Acetate (gm%) | KH2PO4 (gm%) | MgSO4 (mg%) | CaCl2 (mg%) | MnSO4 (mg%) | CuSO4 (mg%) | Dummy | Dummy | Laccase activity |
| 1 | 0.2 | 1 | 0.5 | 0.1 | 0.05 | 0.5 | 0.5 | 0.2 | 0.25 | 1 | 1 | 59.16 |
| 2 | 0.2 | 0.5 | 0.5 | 0.05 | 0.1 | 1 | 0.5 | 0.2 | 0.5 | 1 | -1 | 84.16 |
| 3 | 0.2 | 0.5 | 0.2 | 0.05 | 0.05 | 0.5 | 0.5 | 0.1 | 0.25 | -1 | -1 | 32.91 |
| 4 | 0.5 | 1 | 0.2 | 0.05 | 0.05 | 1 | 0.5 | 0.2 | 0.5 | -1 | 1 | 79.58 |
| 5 | 0.5 | 0.5 | 0.2 | 0.05 | 0.1 | 0.5 | 1 | 0.2 | 0.25 | 1 | 1 | 50.41 |
| 6 | 0.5 | 0.5 | 0.5 | 0.1 | 0.05 | 1 | 1 | 0.2 | 0.25 | -1 | -1 | 43.33 |
| 7 | 0.2 | 0.5 | 0.2 | 0.1 | 0.05 | 1 | 1 | 0.1 | 0.5 | 1 | 1 | 46.66 |
| 8 | 0.5 | 1 | 0.2 | 0.1 | 0.1 | 1 | 0.5 | 0.1 | 0.25 | 1 | -1 | 89.16 |
| 9 | 0.5 | 1 | 0.5 | 0.05 | 0.05 | 0.5 | 1 | 0.1 | 0.5 | 1 | -1 | 132.77 |
| 10 | 0.2 | 1 | 0.2 | 0.1 | 0.1 | 0.5 | 1 | 0.2 | 0.5 | -1 | -1 | 58.6 |
| 11 | 0.5 | 0.5 | 0.5 | 0.1 | 0.1 | 0.5 | 0.5 | 0.1 | 0.5 | -1 | 1 | 59.99 |
| 12 | 0.2 | 1 | 0.5 | 0.05 | 0.1 | 1 | 1 | 0.1 | 0.25 | -1 | 1 | 54.16 |
Figure 1. Positive and negative effect of different factors on laccase production by Peyronellaea pinodella BL-3/4 as screened with a PBD.
Table 2. ANOVA table for selected factorial model in PBD.
| F | p-value | ||||
| Source | Value | Prob > F | |||
| Model | 95.33 | 0.0016 | Significant | ||
| A-Orange Peel | 113.89 | 0.0018 | |||
| B-Glucose | 193.72 | 0.0008 | |||
| C-Peptone | 46.30 | 0.0065 | |||
| D-Ammonium Acetate | 47.32 | 0.0063 | |||
| H-MnSO4 | 13.00 | 0.0366 | |||
| J-CuSO4 | 140.08 | 0.0013 | |||
| K-Dummy 1 | 142.46 | 0.0013 | |||
| L-Dummy 2 | 65.90 | 0.0039 | |||
The model R2 Value: 0.9961, The Predicted R2 Value: 0.9373 and the adjusted R2 value: 0.9856; Coefficient of Variance (CV): 4.91
The positive effects of the four factors namely glucose, orange peelings, peptone and CuSO4 on laccase production were studied using CCD of RSM to optimize their levels for maximum enzyme yield. The levels of other factors kept constant during experiments. Experimental study based on a CCD experimental design was performed according to Table 3 and 4. These factors and their levels were chosen based on the preliminary experiments. The 30 experimental trials reveal the different combinations of the factors. Maximum laccase response (125.0 U/mL) was obtained in run 30 having a maximum concentration of glucose (2.9%). Fisher’s F test for the analysis of variance of data indicated that the model was highly significant with Prob>F’ value of less than 0.0001 and F-value of 17.14 (Table 5). A not significant lack of fit showed that the quadratic model was valid for the present study. Among all factors and interactions considered in the experimental design, A, B, D2 and AD were statistically significant at 95% confidence level. The value of R2 (0.8903) indicated a good agreement between the experimental and predicted values of laccase yield.
To evaluate the main effects, interaction effects and quadratic effects of the selected factors on the laccase yield, second-order polynomial equation was derived equation 4:
Y= 58.8112 + 15.7583 A + 19.825 B + 1.39917 C + -4.15833 D + 2.60875 AB + -11.4312 AD + -0.18 BD + -0.9975 CD + -10.1603 D2
Where Y is the predicted response and A, B, C, D are coded factors.
The equation can be used to make predictions about the response for given levels of each factor. A positive linear coefficient value for A and B indicates laccase production was increased with increased concentrations of orange peelings (up to 1%) and glucose (up to 2.9%) as shown in Table 3 and 4. The response obtained at different level of orange peelings clearly indicate that growth and enzyme yield was high at high concentration of orange peelings (1%) and very low when orange peelings concentration was low (0.1%) or absent in the medium. However results of present study disagree with report of the Ire and Ahuekwe (2016) on use of 0.1% orange peelings for maximum laccase production by Pleurotus ostreatus. Moreover, laccase activity was high at high level of glucose (2.9%) and low when glucose concentration was low (0.2%) Ghosh and Ghosh (2017) studies have revealed that although glucose supports the highest specific growth rate, specific rate of laccase production was significantly reduced. This is not supported by the findings of the present study where maximum laccase activity was obtained at 2.9% glucose with good mycelial growth. Bhamare et al. (2018) reported less laccase production even at at the 9th day of incubation. More yield in less incubation period ( 4 days) indicated the metabolic potential of fungus and its suitability for cost efective and economized production of enzyme at industrial scale.
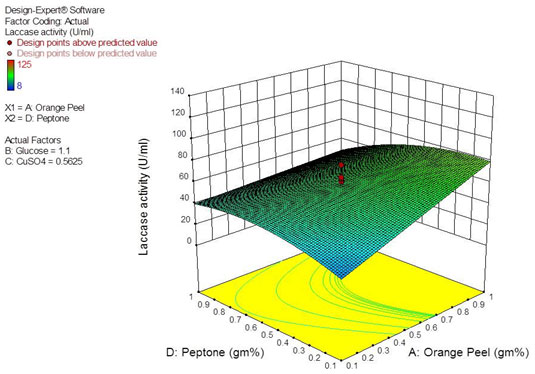
The interactive effect of various factors on laccase production by Peyronellaea pinodella BL-3/4 was investigated by plotting the contour plots and three-dimensional response surface curves against any two independent variables while keeping the third independent variable at the ’0’ level. Studying the interaction among two variables provides knowledge of the optimum concentration of individual factor for highest laccase yield. The interactive effect of response surface quadratic model reveals that interaction among factors AB, BD and CD are insignificant whereas AD is significant (Table 5). The response surface curve for interactive effect of AD is shown in Figure 2. The yield was found to be increasing with the increase in orange peelings (A) concentration with limited level of peptone (D). But increasing the concentration of peptone inhibits laccase production even in the presence of higher concentration of orange peelings. This is in accordance with Hammel (1997), who confirmed that the ligninolytic enzymes are produced during the secondary metabolism under conditions of limited nitrogen. Optimum concentration of each factor was revealed by performing confirmation run in triplicates. Actual mean laccase activity of 151.5 U/mL was obtained with optimum concentration of orange peelings-1.0%, Glucose-2.0%, Peptone-0.5% and CuSO4-1.0 mg% against predicted laccase activity (139.0 U/mL) by design expert software.
Table 3. Central composite experiments design matrix (Block 1) for laccase production from Peyronellaea pinodella BL-3/4.
| Factor A | Factor B | Factor C | Factor D | Actual Response | ||
| Block | Run | Orange Peel | Glucose | CuSO4 | Peptone | Laccase activity |
| gm% | gm% | mg% | gm% | U/ml | ||
| Block 1 | 1 | 0.55 | 1.1 | 0.5625 | 0.55 | 52 |
| Block 1 | 2 | 1 | 2 | 1 | 1 | 56 |
| Block 1 | 3 | 1 | 2 | 0.125 | 1 | 51.24 |
| Block 1 | 4 | 0.1 | 0.2 | 1 | 0.1 | 1.33 |
| Block 1 | 5 | 0.1 | 2 | 0.125 | 0.1 | 27.49 |
| Block 1 | 6 | 0.55 | 1.1 | 0.5625 | 0.55 | 50.83 |
| Block 1 | 7 | 1 | 0.2 | 0.125 | 1 | 14.16 |
| Block 1 | 8 | 1 | 0.2 | 1 | 0.1 | 45 |
| Block 1 | 9 | 0.1 | 0.2 | 1 | 1 | 11.8 |
| Block 1 | 10 | 0.1 | 2 | 1 | 0.1 | 35 |
| Block 1 | 11 | 0.55 | 1.1 | 0.5625 | 0.55 | 53.74 |
| Block 1 | 12 | 0.1 | 2 | 1 | 1 | 45 |
| Block 1 | 13 | 1 | 0.2 | 1 | 1 | 17.49 |
| Block 1 | 14 | 0.1 | 0.2 | 0.125 | 1 | 9 |
| Block 1 | 15 | 0.1 | 2 | 0.125 | 1 | 45.41 |
| Block 1 | 16 | 1 | 2 | 1 | 0.1 | 99 |
| Block 1 | 17 | 0.1 | 0.2 | 0.125 | 0.1 | 8 |
| Block 1 | 18 | 1 | 2 | 0.125 | 0.1 | 86.66 |
| Block 1 | 19 | 0.55 | 1.1 | 0.5625 | 0.55 | 43.33 |
| Block 1 | 20 | 1 | 0.2 | 0.125 | 0.1 | 48.74 |
Table 4. Central composite experiments design matrix (Block 2) for laccase production from Peyronellaea pinodella BL-3/4.
| Block | Run | Orange Peel | Glucose | CuSO4 | Peptone | Laccase activity |
| gm% | gm% | mg% | gm% | U/ml | ||
| Block 2 | 21 | 0.55 | 1.1 | 0.5625 | 0.55 | 76.66 |
| Block 2 | 22 | 1.45 | 1.1 | 0.5625 | 0.55 | 99.16 |
| Block 2 | 23 | 0.55 | 1.1 | 0.5625 | -0.35 | 35.83 |
| Block 2 | 24 | -0.35 | 1.1 | 0.5625 | 0.55 | 39.19 |
| Block 2 | 25 | 0.55 | 1.1 | 1.4375 | 0.55 | 64.16 |
| Block 2 | 26 | 0.55 | -0.7 | 0.5625 | 0.55 | 23.74 |
| Block 2 | 27 | 0.55 | 1.1 | -0.3125 | 0.55 | 75.83 |
| Block 2 | 28 | 0.55 | 1.1 | 0.5625 | 0.55 | 64.99 |
| Block 2 | 29 | 0.55 | 1.1 | 0.5625 | 1.45 | 24.99 |
| Block 2 | 30 | 0.55 | 2.9 | 0.5625 | 0.55 | 125 |
Table 5. Analysis of variance for response surface quadratic model
| F | p-value | ||
| Source | Value | Prob > F | |
| Model | 17.14 | < 0.0001 | significant |
| A-Orange Peel | 43.68 | < 0.0001* | |
| B-Glucose | 69.13 | < 0.0001* | |
| C-CuSO4 | 0.34 | 0.5643a | |
| D-Peptone | 3.04 | 0.0973a | |
| AB | 0.80 | 0.3829a | |
| AD | 15.32 | 0.0009* | |
| BD | 3.799E-003 | 0.9515a | |
| CD | 0.12 | 0.7364a | |
| D2 | 21.79 | 0.0002* | |
| Lack of Fit | 0.83 | 0.6517 | not significant |
R2 = 0.8903; adjusted R2 = 0. 8384; Predicted R2=0.7630; probability P *(P<0.05) corresponds to Significance; Pa corresponds to insignificance
Figure 2: Response surface curve showing the interactive effect of orange peel (A) and peptone (D) on laccase production.
Extracellular laccase produced by Peyronellaea pinodella BL-3/4 was purified by ammonium sulphate precipitation followed by dialysis and gel filtration chromatography. Laccase purification at different steps is summarized in Table 5. Enzyme was purified to 6.01 fold with 85.1% yield after dialysis. Final purification with sephacryl s-100 HR gel filtration chromatography leads to 17.5 fold purification with 14.1% yield of pure laccase.
Table 6. Purification of laccase from Peyronellaea pinodella BL-3/4.
| Purification step | Total activity (U) | Total protein (mg) | Specific activity (U/mg) | Purification fold | Yield (%) |
| Crude filtrate | 13500 | 633 | 21.3 | 1 | 100 |
| Ammonium sulfate precipitation and Dialysis | 11491 | 89.7 | 128.1 | 6.01 | 85.1 |
| Gel filtration chromatography | 1900 | 0.85 | 2235.3 | 17.5 | 14.1 |
Laccase purified by gel filtration chromatography was resolved on SDS-PAGE Gels. Results on gels showed a single band of monomeric protein with a molecular mass of 60 kDa relative to the standard protein molecular weight marker (Figure 3). Molecular masses of the most of the fungal laccases reported between 55-97 kDa (Patel and Gupte 2016; Irshad et al., 2011)
Figure 3: SDS-PAGE of purified laccase from Peyronellaea pinodella BL-3/4. Lane 1: Standard Protein molecular weight marker, Lane 2: Crude Laccase, Lane 3: Dialyzed Laccase and Lane 4: Purified Laccase.
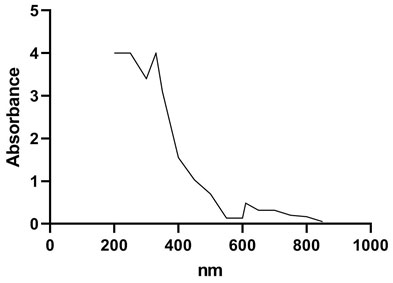
AAS studies showed presence of copper in laccase active fraction (6.8 mg/mL) where as iron, zinc and manganese were absent. The type of copper catalytic centre was investigated spectrophotometrically by UV-Visible spectrum. The UV-Visible spectrum (Figure 4) shows presence of a shoulder at 330 nm. Shoulder at 330 nm indicates type III binuclear copper (Solomon et al., 1994, Solomon et al., 1996) having two electron accepting site, which is characteristic to the yellow laccases. Absorption peak around 600 nm confirms presence of type 1 copper, which is characteristic of blue laccases (Bertrand et al., 2002, Morozova et al., 2007, Madhavi and Lele 2009). Type III Cu exhibits a weak absorption at 600 nm (Palmieri et al., 1997). In present study, absence of peak around 600 nm (Figure 4) conferred absence of type 1 copper in purified laccase and presence of type III copper in purified laccase.
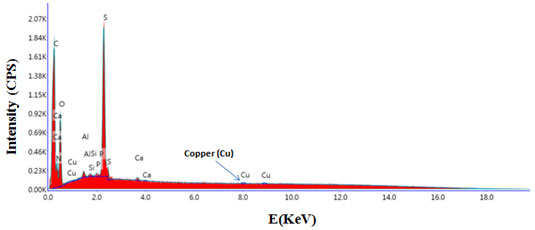
Evidence was also provided for instability of type I copper in all fungal laccases (Rogalski and leonowicz, 2004). Most of the laccases are blue containing four copper atoms per enzyme molecule. Reports of Giardina et al. (2009) suggests that formation of yellow laccase is due to altered oxidation state of active copper centre during binding of the lignin degradation aromatic products which in turn results in the reduction of type 1 copper and loss of the characteristic blue copper of laccase. SEM-EDAX analysis was performed to confirm presence of copper. Figure 5 shows scanning electron microscopic image of purified laccase. Peak at 8 KeV in SEM-EDAX spectrum confirmed presence of copper in structure of laccase (Figure 6).
Figure 4: UV-Visible spectrum of purified laccase.
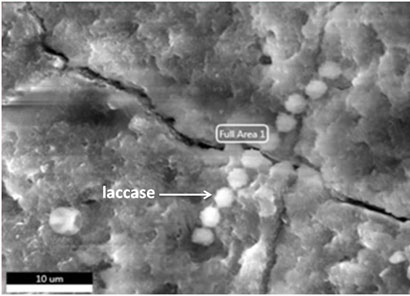
Figure 5: Scanning electron microscopy of purified laccase from Peyronellaea pinodella BL-3/4.
Figure 6: Energy dispersive X-ray (SEM/EDAX) spectrum showing presence of copper in purified laccase from Peyronellaea pinodella BL-3/4.
CONCLUSION
The present study has explored the potential of Peyronellaea pinodella BL-3/4, a newly isolated ascomycetes to produce laccase under optimal medium components designed by statistical software through submerged fermentation. Statistical optimization has provided best combinations of medium components while considering interaction between medium components studied. The usage of design expert software reduces the resources required and also saved time. Optimization leads to two fold increases in laccase production compared to control using orange peelings (1%) as a lignocellulosic substrate. Laccase yield of 14.1% was achieved in final purification with sephacryl s-100 HR gel filtration chromatography. The production of laccase by Peyronellaea pinodella BL-3/4 was also confirmed by the evaluating presence of copper in the purified fraction. SEM-EDAX analysis confirms the presence of copper in purified laccase. Further research on Peyronellaea pinodella BL-3/4 can be explored to scale up the laccase production for its vivid industrial applications.
ACKNOWLEDGEMENTS
The authors are thankful to the Shri M. M. Patel Institute of Sciences and research, Gandhinagar, Gujarat for providing laboratory facilities. The authors also wish to acknowledge SICART, V.V. Nagar, Gujarat, for doing SEM-EDAX Analysis.
Conflict of Interests:None
REFERENCES
Agrawal K Chaturvedi V Verma P ( 2018) Fungal laccase discovered but yet undiscovered Bioresources and Bioprocessing Vol 5 No 4
Anwar Z Gulfraz M Irshad M (2014) Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review Journal of Radiation Research and Applied Sciences Vol 7 Pages 163–173
Bagewadi ZK Mulla SI and Ninnekar HZ (2017) Optimization of laccase production and its application in delignification of biomass International Journal of Recycling of Organic Waste in Agriculture Springer Berlin Heidelberg Vol 6 No 4 Pages 351–365
Bankole PO Adekunle AA Obidi OF et al (2018) Biodegradation and detoxification of Scarlet RR dye by a newly isolated filamentous fungusPeyronellaea prosopidis Sustainable Environment Research Vol 28 No 5 Pages 214–222
Batista Meneses D Montes de Oca-Vásquez G Vega-Baudrit JR et al (2020) Pretreatment methods of lignocellulosic wastes into value-added products: recent advances and possibilities Biomass Conversion and Biorefinery
Bertrand T Jolivalt C Briozzo P et al (2002) Crystal structure of a four-copper laccase complexed with an arylamine: Insights into substrate recognition and correlation with kinetics Biochemistry Vol 41 No 23 Pages 7325–7333
Bhamare HM Jadhav HP Sayyed RZ (2018) Statistical optimization for enhanced production of extracellular laccase from Aspergillus sp HB_RZ4 isolated from bark scrapping Environmental Sustainability Vol 1 Pages 159–166
Brenelli L Squina FM Felby C Cannella D (2018) Laccase-derived lignin compounds boost cellulose oxidative enzymes AA9 Biotechnology Biofuels Vol 11 No 10
Dashtban M Schraft H and Qin W (2009) Fungal bioconversion of lignocellulosic residues; Opportunities & perspectives International Journal of Biological Sciences Vol 5 No 6 Pages 578–595
El-Batal AI ElKenawy NM Yassin AS et al (2015) Laccase production by Pleurotus ostreatus and its application in synthesis of gold nanoparticles Biotechnology Reports Vol 5 No 1 Pages 31–39
Gao H Chu X Wang Y et al (2013) Media Optimization for Laccase Production by Trichoderma harzianum ZF-2 Using Response Surface Methodology Journal of Microbiology and Biotechnology Vol 23 No 12 Pages 1757–1764
Ghosh P and Ghosh U (2017) Statistical optimization of laccase production by Aspergillus flavus PUF5 through submerged fermentation using agro-waste as cheap substrate Acta Biologica Szegediensis Vol 61 No 1 Pages 25–33
Giardina P Faraco V Pezzella C et al (2010) Laccases: a never-ending story Cellular and Molecular Life Sciences Vol 67 No 3 Pages 369–385
Hammel KE (1997) Fungal Degradation of Lignin in: Cadisch G and Giller KE Eds Driven by Nature: Plant Litter Quality and Decomposition CAB International Wallingford Pages 33-45
Ire F and Ahuekwe E (2016) Production of Fungal Laccase Using Orange Peelings as Substrate by Submerged Static Fermentation British Microbiology Research Journal Vol 15 No 5 Pages 1–19
Irshad M Asgher M Sheikh MA Nawaz H (2011) Purification and characterization of laccase produced by Schyzophylum commune IBL-06 in solid state culture of banana stalks BioResources Vol 6 No 3 Pages 2861–2873
Jaber SM Md Shah UK Mohamed Asaari AZ Ariff AB (2017) Optimization of Laccase Production by Locally Isolated Trichoderma muroiana IS1037 Using Rubber Wood Dust as Substrate BioResources Vol 12 Pages 3834–3849
Janusz G Pawlik A Świderska-Burek U et al (2020) Laccase Properties Physiological Functions and Evolution Internation journal of molecular sciences Vol 21 Pages 966
Kalogo Y Habibi S MacLean HL Joshi SV (2007) Environmental Implications of Municipal Solid Waste-Derived Ethanol Environmental Science and Technology Vol 41 Pages 35–41
Karp SG Faraco V Amore A et al (2015) Statistical Optimization of Laccase Production and Delignification of Sugarcane Bagasse by Pleurotus ostreatus in Solid-State Fermentation BioMed Research International Vol 2015 Pages 1–8
Kim S and Dale B E (2004) Global potential bioethanol production from wasted crops and crop residues Biomass and Bioenergy Vol 26 No 4 Pages 361–375
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4 Nature Vol 227 Pages 680–685
Lowry OH Rosebrough NJ Farr AL et al (1951) Protein measurement with the folin phenol reagent The Journal of Biological Chemistry Vol 193 Pages 265–275
Madhavi V and Lele SS (2009) laccase: properties and applications Bioresources Vol 4 No 4 Pages 1694-1717
Morozova OV Shumakovich GP Gorbacheva et al (2007) Blue laccases Biochemistry Moscow Vol 72 No 10 Pages 1136–1150
Niladevi KN Sukumaran RK Jacob N Anisha GS Prema P (2006) Optimization of laccase production from a novel strain—Streptomyces psammoticus using response surface methodology Microbiological Research Vol 164 Pages 105–113
Palmieri G Giardina P Bianco C Scaloni A Capasso A Sannia G (1997) A Novel White Laccase from Pleurotus ostreatus The Journal of Biological Chemistry Vol 272 Pages 31301–31307
Patel H and Gupte A (2016) Optimization of different culture conditions for enhanced laccase production and its purification from Tricholoma giganteum AGHP Bioresources and Bioprocessing Springer Berlin Heidelberg Vol 3 No 1
Patel RJ and B Lakshmi (2020) Orange peel as an inducer for Laccase production in a novel fungal strain Peyronellaea pinodella BL-3/4 and optimization of its cultural parameters by single parameter approach Indian journal of science and technology Vol 13 Pages 1656–1667
Piontek K Antorini M Choinowski T (2002) Crystal Structure of a Laccase from the Fungus Trametes versicolor at 190-Å Resolution Containing a Full Complement of Coppers Journal of Biological Chemistry Vol 277 No 40 Pages 37663–37669
Plackett RL and Burman JP (1946) The Design of Optimum Multifactorial Experiments Biometrika Vol 33 No 4 Pages 305-325
Polo CC Pereira L Mazzafera P et al (2020) Correlations between lignin content and structural robustness in plants revealed by X-ray ptychography Scientific Report Vol 10 Pages 6023
Rajendran A Sundaramurthy A B and Thangavelu V (2007) Statistical evaluation of medium components using plackett-burman experimental design and kinetic modeling of lipase production by Bacillus sphaericus Chemical and Biochemical Engineering Quarterly Vol 21 No 2 Pages 181–188
Rogalski J Janusz G Legiec D et al (2011) Purification of extracellular lacease from Rhizoctonia praticola Journal of the Faculty of Agriculture Kyushu University Vol 56 No 1 Pages 1–7
Sadhasivam S Savitha S Swaminathan K Lin FH (2008) Production purification and characterization of mid-redox potential laccase from a newly isolated Trichoderma harzianum WL1 Process Biochemistry Vol 43 No 7 Pages 736–742
Sánchez C (2009) Lignocellulosic residues: Biodegradation and bioconversion by fungi Biotechnology Advances Elsevier Inc Vol 27 No 2 Pages 185–194
Silva C Silva CJ Zille A et al (2007) Laccase immobilization on enzymatically functionalized polyamide 66 fibres Enzyme and Microbial Technology Vol 41 No 6–7 Pages 867–875
Solomon EI Sundaram U M and Machonkin T E (1996) Multicopper oxidases and oxygenases Chemical Reviews Vol 96 No 7 Pages 2563–2605
Solomon EI Tuczek F Root DE Brown CA et al (1994) Spectroscopy of Binuclear Dioxygen Complexes Chemical Reviews Vol 94 N0 3 Pages 827–856
Sondhi S Saini K (2019) Response surface based optimization of laccase production from Bacillus sp MSK-01 using fruit juice waste as an effective substrate Heliyon 5 e01718
Viswanath B Rajesh B Janardhan A Kumar AP Narasimha G (2014) Fungal Laccases and Their Applications in Bioremediation Enzyme Research Vol 2014 Pages 1–21
Wong DWS (2009) Structure and action mechanism of ligninolytic enzymes Applied Biochemistry and Biotechnology Vol 157 Pages 174–209
Yoshida H (1883) Chemistry of Lacquer (Urushi) Journal of the society Transactions Vol 43 No 47 Pages 472–486