1Key Laboratory of Chemical Biology and Molecular Engineering of Ministry of Education, Institute of Biotechnology, Shanxi University, Taiyuan 030006, PR China
2Key Laboratory of Biomedical Engineering & Technology of Shandong High School, Qilu Medical University, Zibo, 255213, China
Article Publishing History
Received: 02/09/2016
Accepted After Revision: 25/09/2016
This study aims to obtain a phytase with thermal stability and acid resistance for potential industrial production. The phytase gene appA was optimized according to Pichia pastoris and modified by site-directed mutagenesis, which replaces specific residues with another amino acid. Escherichia coli DH5á cells (cultured at 37 °C in Luria broth media) were the host strain for recombinant DNA manipulation. P. pastoris GS115 and the plasmid pPIC9 were the host strain and expression vector, respectively, for heterologous phytase gene expression. Through site-directed mutagenesis, we obtained six mutants, namely, M1, C2, K24E, K43E, M2, and M4 of which the mutants M2 and M4 maintained higher activity in a wider reaction temperature range than other mutants. The mutants M1 and K24E showed strong thermostability and retained more than 60% activity after heat treatment for 20 min (even at 90 °C). We screened mutants that expressed phytase, which can withstand the high-temperature feed pelleting process and retain a high level of phytase activity at the low pH of the monogastric gut environment.
Phytase; Designated Mutations; Thermal Stability; Escherichia Coli
Zhang Y, Wang Q, Liang A. Site-Directed Mutagenesis and Thermal Stability Analysis of Phytase from Escherichia Coli. Biosc.Biotech.Res.Comm. 2016;9(3).
Zhang Y, Wang Q, Liang A. Site-Directed Mutagenesis and Thermal Stability Analysis of Phytase from Escherichia Coli. Biosc.Biotech.Res.Comm. 2016;9(3). Available from: https://bit.ly/2BrFjGw
Introduction
Phytases (myo-inositol-hexaphosphate phosphohydrolase) catalyze the hydrolysis of phytate, the major form of phosphorus storage in cereals and legumes (Pandee, et al., 2011), into inositol and phosphoric acid in a stepwise manner (Greiner, et al., 2001, Kim, et al., 2008), thus increasing the available phosphorus content and decreasing the affinity of phytate to minerals and proteins (Pandee, et al., 2011, Zhang, et al., 2007). The addition of phytase in animal feed could not only improve the utilization rate of phytate phosphorus in feed efficiently but also significantly reduce the environmental pollution from phytate phosphorus excreted by swine and poultry, (Haefner, et al., 2005, Leytem, et al., 2008and Kim, et al., 2010).Research on phytases has recently focused on its application to human food and the synthesis of lower inositol phosphates (Guerrero-Olazaran, et al., 2010). Commercially produced phytases are currently used worldwide. However, one of the problems that phytase still faces is its poor thermal stability. Heat resistance of the phytase enzymes are desirable during the pelleting process of phytase additives, which can allow it to withstand high temperatures of 60 °C to 95 °C lasting at least 30 s, (Pandee, et al., 2011).
However, this characteristic is rarely found among naturally occurring sources (Zhang, et al., 2007). Therefore, site-directed mutagenesis (Kim, et al., 2008, Tran, et al., 2011), error-prone PCR (Liao, et al., 2012), DNA shuffling, exon shuffling, stagger extension processes (Zhu, et al., 2010), and substituting residues (Zhang, et al., 2007) have been used to enhance the thermal stability of phytase.Phytases are diffusely distributed among plants, animal tissues, and microorganisms (Guerrero-Olazaran, et al., 2010, Liao, et al., 2012).
The most prevalent phytases are from bacteria, yeast, and fungi (Liao, et al., 2012). Among many phytases, Escherichia coli phytase AppA as a bifunctional enzyme (Greiner, et al., 1993) has a great potential for industrial applications due to its wide range of active acid pH, high specific activity for phytate, and resistance to pepsin digestion (Greiner, et al., 1993, Luo, et al., 2007).Thus, the product of the appA gene was chosen as a candidate to enhance the thermal stability of phytase and to promote the residual activity of phytase during the pelleting process, which can reach temperatures as high as 70 °C to 90 °C (Zhang, et al., 2007). The phytase gene has 1233 bp and the protein it encodes has 410 amino acids. The deduced amino acid sequence of appA contains an RHGXRXP motif in the N-terminal and HD motif in the C-terminal, which are characteristics in common with histidine acid phosphatases, (Kim, et al., 2006, Pandee, et al., 2011, Yao, et al., 2012, Fan, et al., 2013 and Roy, et al., 2016).
Pichia pastoris is a eukaryotic expression system that was proposed in the 1980s and has since been widely used for the production of various recombinant heterogeneous proteins (Chang, 2008). By 2005, P. pastoris had been used to successfully express more than 500 exogenous proteins (Cereghino, et al., 2000), many of which had reached 10 mg·mL−1 (Cregg, et al., 2000). As one of the most ideal exogenous protein expression systems, P. pastoris has the advantage of being simple to operate, being highly stable, having high expression levels, and having large quantities of secretion (Li, et al., 2001).
Based on the previous reports of enhancing the thermal stability of phytase, (Kim, et al., 2008, Zhang, et al., 2007, Zhu, et al., 2010, Liao, et al., 2012, Tran, et al., 2011, Noorbatcha, et al., 2013, Rocky-Salimi, et al., 2016 and Tan et al., 2016), we chose six amino acid residues sites which maybe change thermal stability by changing residual charge. In this study, phytase gene appA was optimized according to P. pastoris and was modified by site-directed mutagenesis. Site-directed mutagenesis of the cloned appA gene was used to replace specific residues with another amino acid. The mutant genes were expressed in P. pastoris GS115, and the recombinant phytases were subjected to detailed assays of their thermostability, pH dependence of enzyme activity, and their Michaelis constant. The biochemical characteristics of these recombinant phytases were compared to obtain a phytase with excellent quality, which may have potential for industrial production.
Material And Methods
- coliDH5ácells (TaKaRa, Japan) were the host strain for recombinant DNA manipulation and were cultured at 37 °C in Luria broth media. P. pastoris GS115 and the plasmid pPIC9 (Invitrogen, USA) was the host strain and expression vector, respectively, for heterologous phytase gene expression. Plasmid vector pGEM-T Easy (Promega, USA) containing a fusion gene of the synthetic phytase gene appA and the synthetic signal peptide (designated MF4I; maintained in our laboratory) was used as a PCR template for site-directed mutagenesis. Oligonucleotide primers for gene cloning and site-directed mutagenesis were designed and synthesized by TaKaRa (Japan).
The phytate substrate (sodium salt, P0109) was purchased from Sigma (USA). EcoRI, NotI, BamHI, BglII, Taq DNA polymerase, T4 ligase, and dNTP were purchased from Promega (USA). Yeast extract–peptone–dextrose medium, minimal dextrose (MD) medium, buffered glycerol complex (BMGY) medium, and buffered methanol complex (BMMY) medium were prepared according to the P. pastoris Expression Kit manual (Invitrogen 2002). All other chemicals were of analytical grade and were commercially available.
Site-Directed Mutagenesis
Double-stranded methylated plasmid pGEM-T Easy containing the appA gene was utilized to encode the mutant phytases. Six pairs of complementary primers (Table 1) containing the desired point mutation were employed to generate the necessary mutants (K24E, K43E, W46E, Q62W, A73P, and K75C). The many mutants of Citrobacter braakii phytase in Patent PCT/EP2011/054639 were analyzed, and the part of the same site also generated in phytase AppA to know their effect on Escherichia coli phytase. The mutagenesis primers were extended by PrimeSTAR®HS DNA polymerase in a thermocycling process (94 °C for 4 min, 20 cycles at 94 °C for 10 s, 55 °C for 15 s, and 72 °C for 4 min). The product was treated with DpnI at 37 °C for 1 h to remove methylated and hemimethylated DNA template strands. The nicked plasmid DNA containing the desired mutations was then conveyed into E. coli DH5á cells, where the nick was repaired by the cell.
| Table 1: Primers used for site-directed mutagenesis | |
| Primer Name | Primer Sequence (5’→3’) |
| AppA (W46E)-Reverse | CCTCTAGGTGTCAACTCACCCAGCTTGACTGGC |
| AppA (W46E)-Forward | GCCAGTCAAGCTGGGTGAGTTGACACCTAGAGG |
| AppA (Q62W)-Reverse | GCAACAAGACGCTGTCTCCAGTAGTGAC |
| AppA (Q62W)-Forward | GTCACTACTGGAGACAGCGTCTTGTTGC |
| AppA (A73P)-Reverse | GTGGACAACCCTTCTTGGGCAACAATCC |
| AppA (A73P)-Forward | GGATTGTTGCCCAAGAAGGGTTGTCCAC |
| AppA (K75C)-Reverse | GATTGTGGACAACCACACTTGGGCAACAATCC |
| AppA (K75C)-Forward | GGATTGTTGCCCAAGTGTGGTTGTCCACAATC |
| AppA (K24E)-Reverse | GTTGGGTGGCCTCGGTTGGTGCTCTAAC |
| AppA (K24E)-Forward | GTTAGAGCACCAACC GAGGCCACCCAAC |
| AppA (K43E)-Reverse | CAACCCAGCTCGACTGGCCAGGTTG |
| AppA (K43E)-Forward | CAACCTGGCCAGTCGAGCTGGGTTG |
Construction Of Expression Vector
Expression vectors pPIC9-appAm(X) were constituted by digesting the recombinant plasmids pGEM-T Easy containing mutant genes with NotI-EcoRI and EcoRI-BamHI, respectively, obtaining appAm (X) and the synthetic signal peptide, and then inserting it into pPIC9 (Invitrogen) digested with NotI-BamHI. The recombinant plasmids pPIC9-appAm(X) were linearized with BglII and transformed into P. pastoris GS115 (Invitrogen) using a LiCl method.
Screening And Expression
The transformants were screened using SDS-PAGE and the phytase activity assay. After being grown on the MD plate and cultured in BMGY/BMMY medium at 30 °C, six positive transformants containing different mutations were successfully obtained (M1, K24E, K43E, C2, M2, and M4). To generate the enzyme, P. pastoris GS115 was cultivated at 30 °C, 200 rpm, and pH 6.0 in 50 mL BMGY for proliferation in an Erlenmeyer flask for 48 h; P. pastoris GS115 was then cultivated at 30 °C, 200 rpm, and pH 6.0 in 50 mL BMMY for the expression of phytases in an Erlenmeyer flask for 48 h.
Purification Of Recombinant Phytases
Purifying the recombinant proteins involves the precipitation of ammonium sulfate followed by two chromatographic steps. Ammonium sulfate precipitation of the recombinant proteins was achieved by first saturating the crude culture filtrate to 80%; the precipitate is subsequently collected by centrifugation and concentrated at a speed of 10,000 rpm for 10 min. The precipitate was subsequently dissolved in acetate buffer (pH 4.5). The traces of ammonium sulfate in the resuspended phytase solution were removed by dialysis (2 h, three acetate buffer washings).
A 1 mL Resource S column was equilibrated in the acetate buffer, and the dialyzed protein samples were applied at a flow rate of 0.6 mg·mL−1. A linear sodium chloride gradient (0.2–1.0 M) was developed in 10 min by using the same buffer. The activity was eluted as a single component. In the final chromatographic step, samples from the previous step were passed through the Superdex 75 column at a flow rate of 0.6 mg·mL−1. Activity was eluted as a single peak (at peak), and the active fractions were pooled in the following test.
Enzyme Activity Assay
Phytase activity was assayed using the molybdenum blue method. The enzyme reaction was performed in 1 mL of 0.1 M sodium acetate buffer using sodium phytate (6.25 mM) as a substrate at 37 °C. The reaction was terminated by adding 1 mL of 10% (w/v) trichloroacetic acid. One unit of phytase activity was defined as the amount of enzyme required to liberate 1 μmol of phosphate per minute under assay conditions. Protein was quantified using the BCA assay (Fermentas). All determinations were performed three times.
Optimum Ph And Ph Stability
The optimum pH was determined by incubating the purified phytase with sodium phytate in the following buffers: pH 2.0–3.5 (0.05 M glycine-HCl), pH 4.0–6.0 (0.05 M sodium acetate-acetic acid), and pH 6.5–8.0 (0.05 M Tris-HCl). These assays were performed at 37 °C for 5 min. For assays of pH stability, the enzyme was incubated at 37 °C in the same buffers over the range of pH 2.0–8.0 for 2 h, and residual enzyme activity was measured under standard conditions (optimum pH, 37 °C, 30 min).
Optimum Temperature And Thermal Stability
Enzyme activity was measured at nine different temperatures ranging from 37–80°C to determine optimal temperature. The temperatures chosen were 37, 40, 45, 50, 55, 60, 65, 70, and 80 °C. The tests were performed using standard buffers. Thermal stability was measured by assessing residual enzyme activity under standard conditions (optimum pH, 37 °C, 30 min) following incubation of the enzyme at six different temperatures. The enzyme samples were incubated at 37, 50, 60, 70, 80, or 90 °C for 20 min, respectively. The enzyme samples were then chilled on melting ice for 1 h before the test.
Kinetic Measurements
Vmax and Km values for each phytase were determined at 37 °C in 0.05 M sodium acetate (pH 4.0 to 4.5, according to the optimum pH of each enzyme) with 0.125–5.0 mM sodium phytate as substrate. The initial reaction rates were assayed for a 7 min period. The Vmax and Km were estimated by Lineweaver–Burk plots.
Structural Analysis Of Mutant Phytase
Basing on the crystal structure of phytase AppA (Golovan, et al., 2000), structure analysis was applied to the mutant that showed the most potential as a phytase-producing strain. Three-dimensional structure prediction was done using the program Swiss-PdbViewer 3.7.
Results And Discussion
Site-Directed Mutagenesis And Construction Of Expression Vector
Using a site-directed mutagenesis method, a total of six mutants were obtained, including four single mutants (K24E, K43E, W46E, and Q62W), one double mutant (W46E and Q72W), and one quadruple mutant (W46E, Q62W, A73P, and K75C; Table 2). Mutants were generated by introducing residual substitutions (K24E, K43E, W46E, Q62W, A73P, and K75C) into the previously synthetic phytase gene appA. The names of the mutants, including the mutant sites, are presented in Table 2. The designated mutations in each variant were verified by DNA sequencing.
| Table 2: Name of the mutant including the mutant site | |
| Name | Mutant site |
| M1 | W46E |
| C2 | Q62W |
| K24E | K24E |
| K43E | K43E |
| M2 | W46E, Q62W |
| M4 | W46E, Q62W, A73P, K75C |
Screening And Expression
The genes coding the protein without a native signal sequence were inserted into vector pPIC9. Similar to homologous recombination, the mutant genes with the signal sequence of yeast á-mating factor were integrated into the genome of P. pastoris so that proteins could be expressed stably. After transforming the resultant recombinant plasmids, six positive transformants that expressed phytase were screened by phytase activity assay and SDS-PAGE. The positive transformant for each mutant, which was grown on the MD plate, was transferred to BMGY and proliferated in an Erlenmeyer flask for 48 h. After continuous cultivation in BMMY for 48 h, the supernatant protein had reached 1.0–1.3 mg·mL−1. The crude enzyme solution was produced after centrifuging supernatant protein in the culture medium (10,000 g for 10 min). The crude enzyme solution of recombinant proteins from M1, C2, K24E, K43E, M2, and M4 were finally obtained using the same method.
Purification Of Recombinant Phytases
The phytases obtained from the supernatants were purified to homogeneity by ammonium sulfate precipitation, anion exchange chromatography, and gel filtration on Superdex 75 column ( Fig. 1). Only one peak with high phytase activity was obtained when samples were chromatographed on gel filtration. After three purification steps, the recombinant proteins were purified 28.6-fold from the crude extract with a final yield of 17%. The purification factor and yield are related to elution procedure (Zhang, et al., 2010). The purification scheme of the phytase is summarized in Table 3.
| Table 3: Purification scheme of phytase | |||||
| Step | Total activities (ë/U·mL−1) | Total protein (m/mg) | Specific activity (ë/U·mg−1) | Purification (fold) | Recovery (%) |
| Crude enzymes | 93,866 | 19.56 | 4800 | 1 | 100 |
| (NH4)2SO4 (80%) | 91,096 | 11.16 | 8157 | 1.7 | 97 |
| Resource S | 4561 | 1.53 | 27,818 | 5.8 | 46 |
| Superdex-75 | 17,486 | 0.13 | 134,508 | 28.6 | 17 |
| The every assay value was averaged from three different experiments. | |||||
Optimum Ph And Ph Stability
As shown in Fig. 2a, the optimal reaction pH for the mutant K24E was 4.0, whereas that of WT and other mutant phytases was 4.5. All phytase activities showed one sharp peak at the optimal pH, and the relative activities of the phytases decreased significantly when the pH value exceeded the optimal pH. After incubating in the same buffer system at different pH levels for 2 h, the enzymes were purified and the phytase activity was tested under standard conditions (optimum pH, 37 °C, 30 min). The effects of pH on the stability of phytase are shown in Fig. 2b. Phytase AppA and its mutants had excellent pH stability (except for mutants K43E and C2), and after incubation at 37 °C in different buffers ranging from pH 2.0 to 8.0 for 2 h, the residual enzyme activity was kept above 80%. A buffer of pH 2.0 or pH 8.0 might not affect the enzyme activity significantly, but for the mutants C2 and K43E, enzyme activity residue was less than 30% after incubating with buffer of pH 8.0.
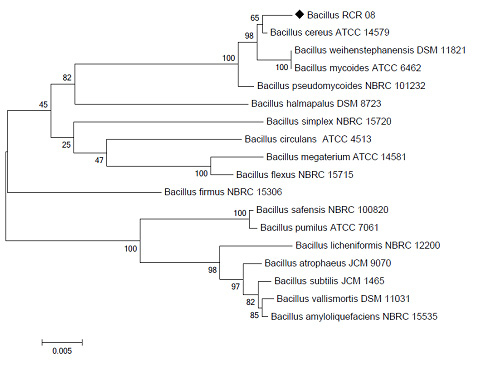 |
Figure 2: Optimal reaction pH and pH stability of phytase AppA and its mutants |
A: Optimal reaction pH of Mutant K24E is a round 4.0; Optimal reaction pH of AppA and other mutants (M1, C2, M2, M4 and K43E) is a round 4.5. The every assay value was averaged from three different experiments. B: Except for mutant K43E and C2, Phytase appA and mutants (M1, M2, M4 and K43E) all had a pH stability and maintained more than 80% enzymatic activity after 2 hours when they were place in pH 2.0-8.0 buffer solution except for K43E and C2. The every assay value was averaged from three different experiments.
Optimum Temperature And Thermal Stability
The optimal reaction temperature was 60 °C for the mutants M1, C2, K24E, and K43E as well as for the phytase AppA; however, the optimal temperature was 65 °C for the mutants M2 and M4. For the mutants M1, C2, K24E, and K43E, and the phytase AppA, the enzyme activity decreased significantly when the reaction temperature was lower than 55 °C or higher than 60 °C. For the mutants M2 and M4, the enzyme activity decreased distinctly when the reaction temperature was lower or higher than 65 °C. As shown in Fig. 3, the mutants M2 and M4 maintained higher activity at a wider reaction temperature range than any other mutants (M1, C2, K24E, or K43E) including the wild-type phytase AppA. The phytase activity of wild-type AppA decreased sharply when incubated at 50 °C for 20 min, and when incubated at 70, 80, or 90 °C for 20 min, the phytase activity practically did not exist. Furthermore, the mutant phytases exhibited improved thermostability compared with AppA. The phytase activity remained above 30% after heat treatment at 70, 80, to 90 °C for 20 min, among which the mutants M1 and K24E showed strong thermostability and retained more than 60% activity after heat treatment for 20 min even at 90 °C.
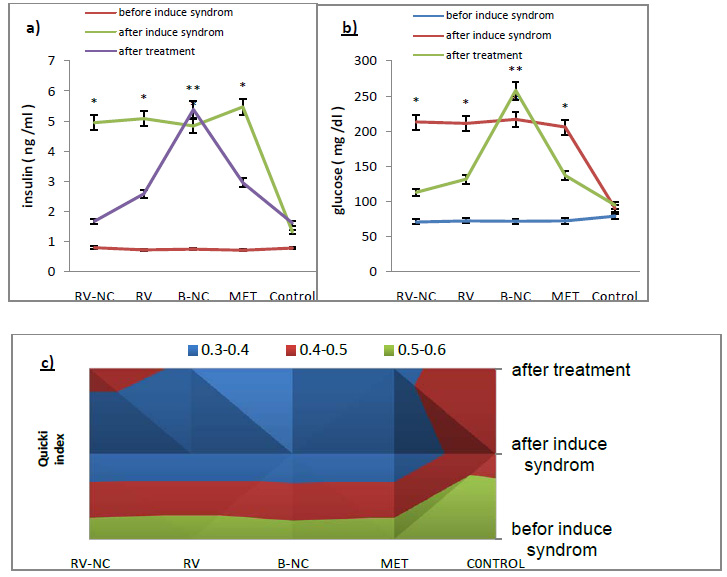 |
Figure 3: Optimal reaction temperature and thermal stability of phytase AppA and its mutants. |
A: Optimal reaction temperature of phytase AppA mutants (M2 and M4) is a round 65 °C; AppA and other mutant is a round 60 °C. The every assay value was averaged from three different experiments. B: The activity of phytase AppA decreased by 50% under 60 °C for 20 minutes and was almost entirely lost under 70 °C, 80 °C and 90 °C for 20 minutes; mutant K43E and C2 decreased by 70% under 90 °C for 20 minutes; mutant M1, M2, M4 and K24E decreased by 40% under 80 °C for 20 minutes. The every assay value was averaged from three different experiments.
Kinetic Measurements
Under standard conditions (optimum pH, 37 °C, 7 min), the Km and Vmax values of WT enzymes and its mutants were calculated using sodium phytate as the substrate and based on the Lineweaver–Burk method (see Table 4). As indicated in Table 4, the Km and Vmax of AppA were 0.45 mM·L−1 and 3.85 mmol·m−1·mg−1, respectively, whereas the Km of mutant K24E was 0.32 mM·L−1, which was the lowest, and the Vmax of K24E was 4.10 mmol·(m·mg)−1, which was the highest. This finding indicated that K24E has the most affinity and the most catalytic efficiency to sodium phytate.
| Table 4: Km and Vmax of phytase AppA and its mutants | |||||||
| Sample | AppA | M1 | C2 | K24E | K43E | M2 | M4 |
| Km (mM/L) | 0.45 | 0.38 | 0.43 | 0.32 | 0.37 | 0.35 | 0.47 |
| Vmax (mmol·m−1·mg−1) | 3.85 | 2.86 | 2.16 | 4.10 | 4.04 | 3.18 | 2.04 |
| The Km and Vmax value was averaged from three different experiments. | |||||||
Structural Analysis Of Mutant Phytase
An analysis of all the previous experimental results and data shows that the mutant K24E outstrips all the other mutants because of its low optimal reaction pH and high optimal reaction temperature, preferable pH stability, and outstanding thermostability. Therefore, the mutant K24E was chosen to demonstrate structural analysis by using 3D conformation. Using SWISS-MODEL protein data analysis (template: 1dkq.1.A), the three-dimensional conformations of mutant K24E and AppA were found to have the same structure, and their structural domains and active sites were not different. As shown in Fig. 4, the crystal structure of AppA consisted of an á-helix, a â-sheet, and an irregular coil. In addition, a deep concave pocket harboring the active enzyme site within the surface of the domains was found. Lys24 substituted by Glu was proximal to the concave pocket and was located at the surface region of the pocket structure’s edge and was close to the active center site.
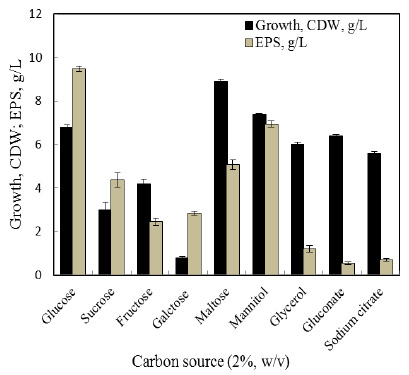 |
Figure 4: Model of mutant K24E A: Model of mutant K24E; B: Model of AppA |
Some research confirmed that Escherichia coli AppA phytase’s C-terminal plays an important role in its thermostability by the thermostable mutants Q307D, Y311K, and I427L (Fei, et al., 2012). Subsequently, the team also found that the salt bridge subtraction mutant E31Q showed 13.96% thermostability decreasement, and the salt bridge addition mutant Q307D showed 9.15% thermostability enhancement than the wild-type both without the pH and temperature optimum changed (Fei, et al., 2013). Wu et al. found that Escherichia coli phytase mutants V89T, one from eleven mutants, exhibited 17.5% increase in catalytic activity (Wu, et al., 2014).
In this study, we sought methods that reformed the phytase AppA to enable it to withstand the high-temperature feed pelleting process and retain a high level of phytase activity at the low pH of the monogastric gut environment (Yao, et al., 2013).
Using site-directed mutagenesis, we successfully screened six mutants (M1, C2, K24E, K43E, M2, and M4). Phytase AppA was purified to homogeneity by ammonium sulfate precipitation, anion exchange chromatography, and gel filtration on a Superdex 75 column. Using the same strategy, we also obtained purified protein from the six mutants. Subsequently, we carried out experiments on optimal reaction pH and pH stability, optimal reaction temperature, thermostability, and the Michaelis constant. The six mutant enzymes showed varying degrees of improvement in thermostability compared with the wild-type enzyme, among which the mutant K24E was the most stable even after being heated to 80 °C or 90 °C for 20 min. As shown by the results of the Michaelis constant, the Km of mutant K24E was lower and the Vmax was higher than wild-type AppA. The optimal reaction pH of K24E was 4.0 and the residual activity of K24E remained above 90% after treatment in the pH range of 3.0–8.0 for 2 h. The optimal reaction temperature of K24E was 60 °C, and the residual activity of K24E remained above 60% after treatment in 70, 80, or 90 °C for 20 min. Using the Lineweaver–Burk method, the Km and Vmax values of mutant K24E were calculated to be 0.32 mM·L−1 and 4.10 mmol·(m·mg)−1, whereas Km and Vmax of AppA were 0.45 mM·L−1 and 3.85 mmol·(m·mg)−1, respectively. The above results showed the thermal resistance of mutant phytase AppA at 70-90 is higher than previously reported, (Liao, et al., 2013, Xu et al., 2015 and Tan et al., 2016).
This result indicated that K24E has more affinity and more catalytic efficiency toward sodium phytate compared with AppA. We applied a 3D structure analysis on mutant K24E. SWISS-MODEL was employed for model building of mutant K24E by using the X-ray structure of phytase AppA as a template.
The validated mutant structure was aligned with the wild-type structure by using SWISS-MODEL protein data analysis. As shown in Fig. 4, the 3D conformations of mutant K24E and AppA were found to have the same structure, and a deep concave pocket harboring the enzyme active site in the inner surface of the domains was found. Mutant K24E was located at a supporting point on the surface of the pocket edge structure and close to the active center site. The replacement of Lys with Glu, which has a smaller side chain, might reduce structural hindrances when combined with the substrate. This phenomenon might be the reason behind the increased affinity of enzyme and substrate and the decreased Km of enzymatic reactions. The electrostatic interaction between enzyme and substrate increased as the positively charged Lys mutated into the negatively charged Glu. This interaction might be another reason for the increase of affinity between phytase and its substrate. The isoelectric point of Lys and Glu are pH 9.74 and 3.22, respectively. Substitution of Lys for Glu presumably contributes to the decrease of the optimum reaction pH. Our results have showed that phytase AppA mutant had excellent pH stability (Keeping 80% residual enzyme activity from pH 2.0 to 8.0 for 2 h at 37° C), which is more favorable to apply to animal feed than wild-type AppA and other phytase (Rocky-Salimi, et al., 2016).
Acknowledgments
This work was supported by grants from the Natural Scientific Foundation of Shandong Province, China (ZR2014CM046, ZR2010CQ031 and ZR2015CL019) and Collaborative Innovation Center of Chinese medicine antivirus in Shandong University of Tranditional Chinese Medicine (XTCX2014B01-07).
References
Cereghino, J. L. and Cregg, J. M. (2000): Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol Rev, 24, 45-66
Cregg, J. M., Cereghino, J. L., Shi, J. and Higgins, D. R. (2000): Recombinant protein expression in Pichia pastoris. Mol Biotechnol, 16, 23-52
Fan, C., Wang, Y., Zheng, C. and Fu, Y. (2013): Fingerprint motifs of phytases. African Journal of Biotechnology, 12, 1138-1147
Fei, B., Cao, Y., Xu, H., Li, X., Song, T., Fei, Z. and Qiao, D. (2013): AppA C-terminal plays an important role in its thermostability in Escherichia coli. Curr Microbiol, 66, 374-378
Fei, B., Xu, H., Zhang, F., Li, X., Ma, S., Cao, Y., Xie, J., Qiao, D. and Cao, Y. (2012): Relationship between Escherichia coli AppA phytase’s thermostability and salt bridges. J Biosci Bioeng, 115, 623-627
Golovan, S., Wang, G., Zhang, J. and Forsberg, C. W. (2000): Characterization and overproduction of the Escherichia coli appA encoded bifunctional enzyme that exhibits both phytase and acid phosphatase activities. Can J Microbiol, 46, 59-71
Greiner, R., Carlsson, N. and Alminger, M. L. (2001): Stereospecificity of myo-inositol hexakisphosphate dephosphorylation by a phytate-degrading enzyme of Escherichia coli. J Biotechnol, 84, 53-62
Greiner, R., Konietzny, U. and Jany, K. D. (1993): Purification and characterization of two phytases from Escherichia coli. Arch Biochem Biophys, 303, 107-113
Guerrero-Olazaran, M., Rodriguez-Blanco, L., Carreon-Trevino, J. G., Gallegos-Lopez, J. A. and Viader-Salvado, J. M. (2010): Expression of a Bacillus phytase C gene in Pichia pastoris and properties of the recombinant enzyme. Appl Environ Microbiol, 76, 5601-5608
Haefner, S., Knietsch, A., Scholten, E., Braun, J., Lohscheidt, M. and Zelder, O. (2005): Biotechnological production and applications of phytases. Appl Microbiol Biotechnol, 68, 588-597
Kim, M. S. and Lei, X. G. (2008): Enhancing thermostability of Escherichia coli phytase AppA2 by error-prone PCR. Appl Microbiol Biotechnol, 79, 69-75
Kim, M. S., Weaver, J. D. and Lei, X. G. (2008): Assembly of mutations for improving thermostability of Escherichia coli AppA2 phytase. Appl Microbiol Biotechnol, 79, 751-758
Kim, O. H., Kim, Y. O., Shim, J. H., Jung, Y. S., Jung, W. J., Choi, W. C., Lee, H., Lee, S. J., Kim, K. K., Auh, J. H., Kim, H., Kim, J. W., Oh, T. K. and Oh, B. C. (2010): beta-propeller phytase hydrolyzes insoluble Ca(2+)-phytate salts and completely abrogates the ability of phytate to chelate metal ions. Biochemistry, 49, 10216-10227
Kim, Y. O., Kim, H. W., Lee, J. H., Kim, K. K. and Lee, S. J. (2006): Molecular cloning of the phytase gene from Citrobacter braakii and its expression in Saccharomyces cerevisiae. Biotechnol Lett, 28, 33-38
Leytem, A. B., Widyaratne, G. P. and Thacker, P. A. (2008): Phosphorus utilization and characterization of ileal digesta and excreta from broiler chickens fed diets varying in cereal grain, phosphorus level, and phytase addition. Poult Sci, 87, 2466-2476
Li, Z., Xiong, F., Lin, Q., d’Anjou, M., Daugulis, A. J., Yang, D. S. and Hew, C. L. (2001): Low-temperature increases the yield of biologically active herring antifreeze protein in Pichia pastoris. Protein Expr Purif, 21, 438-445
Liao, Y., Li, C. M., Chen, H., Wu, Q., Shan, Z. and Han, X. Y. (2013): Site-directed mutagenesis improves the thermostability and catalytic efficiency of Aspergillus niger N25 phytase mutated by I44E and T252R. Appl Biochem Biotechnol, 171, 900-915
Liao, Y., Zeng, M., Wu, Z. F., Chen, H., Wang, H. N., Wu, Q., Shan, Z. and Han, X. Y. (2012): Improving phytase enzyme activity in a recombinant phyA mutant phytase from Aspergillus niger N25 by error-prone PCR. Appl Biochem Biotechnol, 166, 549-562
Luo, H., Huang, H., Yang, P., Wang, Y., Yuan, T., Wu, N., Yao, B. and Fan, Y. (2007): A novel phytase appA from Citrobacter amalonaticus CGMCC 1696: gene cloning and overexpression in Pichia pastoris. Curr Microbiol, 55, 185-192
Ming-Hui CHANG, C.-C. Y., Shiuan-Yuh CHIEN, A.B. ARUN (2008): Expression of recombinant Pichia pastoris X33 phytase for dephosphorylation of rice bran fermented liquid. Annals of Microbiology, 58, 6
Noorbatcha, I. A., Sultan, A. M., Salleh, H. M. and Amid, A. (2013): Understanding thermostability factors of Aspergillus niger PhyA phytase: a molecular dynamics study. Protein J, 32, 309-316
Pal Roy, M., Mazumdar, D., Dutta, S., Saha, S. P. and Ghosh, S. (2016): Cloning and Expression of Phytase appA Gene from Shigella sp. CD2 in Pichia pastoris and Comparison of Properties with Recombinant Enzyme Expressed in E. coli. PLoS One, 11, e0145745
Pandee, P., Summpunn, P., Wiyakrutta, S., Isarangkul, D. and Meevootisom, V. (2011): A Thermostable phytase from Neosartorya spinosa BCC 41923 and its expression in Pichia pastoris. J Microbiol, 49, 257-264
Rocky-Salimi, K., Hashemi, M., Safari, M. and Mousivand, M. (2016): A novel phytase characterized by thermostability and high pH tolerance from rice phyllosphere isolated Bacillus subtilis B.S.46. J Adv Res, 7, 381-390
Tan, H., Miao, R., Liu, T., Cao, X., Wu, X., Xie, L., Huang, Z., Peng, W. and Gan, B. (2016): Enhancing thermal resistance of a novel Acidobacteria-derived phytase by engineering of disulfide bridges. J Microbiol Biotechnol,
Tran, T. T., Mamo, G., Buxo, L., Le, N. N., Gaber, Y., Mattiasson, B. and Hatti-Kaul, R. (2011): Site-directed mutagenesis of an alkaline phytase: influencing specificity, activity and stability in acidic milieu. Enzyme Microb Technol, 49, 177-182
Wu, T. H., Chen, C. C., Cheng, Y. S., Ko, T. P., Lin, C. Y., Lai, H. L., Huang, T. Y., Liu, J. R. and Guo, R. T. (2014): Improving specific activity and thermostability of Escherichia coli phytase by structure-based rational design. J Biotechnol, 175, 1-6
Xu, W., Shao, R., Wang, Z. and Yan, X. (2015): Improving the neutral phytase activity from Bacillus amyloliquefaciens DSM 1061 by site-directed mutagenesis. Appl Biochem Biotechnol, 175, 3184-3194
Yao, M. Z., Wang, X., Wang, W., Fu, Y. J. and Liang, A. H. (2013): Improving the thermostability of Escherichia coli phytase, appA, by enhancement of glycosylation. Biotechnol Lett, 35, 1669-1676
Yao, M. Z., Zhang, Y. H., Lu, W. L., Hu, M. Q., Wang, W. and Liang, A. H. (2012): Phytases: crystal structures, protein engineering and potential biotechnological applications. J Appl Microbiol, 112, 1-14
Zhang, G. Q., Dong, X. F., Wang, Z. H., Zhang, Q., Wang, H. X. and Tong, J. M. (2010): Purification, characterization, and cloning of a novel phytase with low pH optimum and strong proteolysis resistance from Aspergillus ficuum NTG-23. Bioresour Technol, 101, 4125-4131
Zhang, W., Mullaney, E. J. and Lei, X. G. (2007): Adopting selected hydrogen bonding and ionic interactions from Aspergillus fumigatus phytase structure improves the thermostability of Aspergillus niger PhyA phytase. Appl Environ Microbiol, 73, 3069-3076
Zhu, W., Qiao, D., Huang, M., Yang, G., Xu, H. and Cao, Y. (2010): Modifying thermostability of appA from Escherichia coli. Curr Microbiol, 61, 267-273