1Department of Life Sciences, School of Basic Sciences & Research, Sharda
University, Greater Noida, Uttar Pradesh-201310, India.
2School of Medical Sciences & Research, Sharda ,Hospital, Sharda University
Campus, Greater Noida, Uttar Pradesh-201310, India.
3Shri Maneklal M Patel Institute of Science & Research, Kadi Sarva
Vishwavidhyalaya, Gandhinagar, Gujrat-382024, India.
4Virology Laboratory, Department of Microbiology, All India Institute of
Medical Sciences (AIIMS), New Delhi 110029, India.
Corresponding author email: vinodjoshidmrc@gmail.com
Article Publishing History
Received: 15/08/2021
Accepted After Revision: 24/11/2021
Clinical management of COVID-19 patients through a robust protocol is key to the good recovery and reduced mortality of patients. Efficient kidney functions during treatment period can contribute for improvised recovery and reduced mortality of patients. Analysis of the kidney function among Recovered and Dead cases of COVID-19 was made to reveal the degree of association of kidney functions with the two categories of patients. 83.4% of recovered patients did not show hyper values of blood urea whereas 72.5% of dead patients showed hyper-urea level in blood. 88.8% of survivors showed non-hyper creatinine level of blood whereas only 40% of dead cases showed hyper creatine level. Strong degree of association of blood urea with recovery/mortality was observed.
Sodium levels were seen to be low while potassium and chloride ions were seen to be high in COVID-19 individuals. Our preliminary study suggests that kidney functions especially the value of blood urea and creatinine need to be addressed during COVID-19 patients to ensure the best recovery and reduced mortality. After more number of case studies, the present observation could sensitize consideration for inclusion of addressal and treatment of kidney functions into treatment protocol against COVID-19. It was also interesting to observe that levels of sodium and potassium ions among Survivors and Dead cases have impacted function of the essential ion channels in patient’s physiology.
Covid-19, Electrolytes, Kidney, Sars-Cov2, Urea
Angel B, Angel A, Joshi V, Jindal M, Rastogi P, Peer N, Shareef B.M, Sadhu S, Singh S, Sharma S, Priya K, Chouhan R, Jethani J. Significance of addressal of clinical investigations of Kidney functions in recovery/mortality of COVID-19 patients: A preliminary study. Biosc.Biotech.Res.Comm. 2021;14(4).
Angel B, Angel A, Joshi V, Jindal M, Rastogi P, Peer N, Shareef B.M, Sadhu S, Singh S, Sharma S, Priya K, Chouhan R, Jethani J. Significance of addressal of clinical investigations of Kidney functions in recovery/mortality of COVID-19 patients: A preliminary study. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/3Ec2p1v“>https://bit.ly/3Ec2p1v</a>
Copyright © Angel et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Severe Acute Respiratory Syndrome-CoronaVirus-2 (SARS-COV2) has now taken the shape of one of the most rampant human pandemics the world has ever faced. In India, there prevails the second wave of pandemic starting from 11th February 2021 with a total of 178360849 cases and 3869384 as of today as updated on W.H.O’s Coronavirus (COVID-19) dashboard (https://covid19.who.int/ ), (Ranjan et al. 2021).
Due to huge number of cases being reported to hospitals every day, the treating clinicians are compelled to focus aggressively only on the anti-viral/anti-bacterial treatments to the patients with parallel attention on normalcy in vital levels of pulmonary oxygen saturation level, cardiac parameters and Blood Pressure levels. However, although many other parameters such as Liver Function Tests, Hematological parameters and Kidney Function Tests etc.
are being measured and recorded essentially among all the admitted patients, yet kidney function parameters are not paid attention to address them in the corresponding treatment protocols. It is well known that the human cell receptor of SARS-COV2 is Angiotensin-Converting Enzyme-2 (ACE-2) and it is very important enzyme of the RAS pathway (Renin-Angiotensin System) i.e. in regulating the blood pressure homeostasis of the body as well as in maintaining the fluid and salt balance in the body (https://www.rndsystems.com/resources/articles/ace-2-sars-receptor-identified), Acc Apr 2021)
This ACE receptor is expressed specifically on the lungs and also on the kidney, gastrointestinal cells, vascular epithelial cells, kidney and Leydig cells. The IFFC (International Federation of Clinical chemistry and Laboratory Medicine) Guidelines on COVID-19 highlights the need of monitoring creatinine levels in critical COVID-19 patients so as to diagnose any injury to kidney at an early stage (IFCC guide on COVID-19, 2020).. The observations reported in the present study are in concurrence with the IFFC guidelines. Previous studies have reported that in the SARS-COV 1, 2003 strain and the Middle East respiratory syndrome (MERS) infection, there were cases with Acute kidney injury (AKI) and subsequent mortality of cases (Elias & Benito, 2018, Cheng et al. 2020; Naicker et al. 2020).
The present study highlights the association between the recovery/mortality of Covid-19 patients with their Kidney Function parameters and reports that surviving patients showed normal and not surviving patients showed abnormal kidney function parameters. Crucial kidney function parameters viz; Blood urea and creatinine level needs addressal and after more number of studies their treatment could be included in treatment protocols of COVID-19 patients. Similarly, the observations reported of Sodium and potassium levels among “survivors” and “dead” sensitized further clinic-basic studies on roles of SARS-COV2 in blocking essential ion channels of human physiological system.
MATERIAL AND METHODS
A study of association between Kidney Function Tests and survival/mortality of COVID-19 patients was undertaken among patients reporting in Noida and Greater Noida, UP, India. Surviving and Dead cases were chosen at random for the study. Few investigations were done in the residential society as per the information of COVID-19 patients obtained from the society notifications/news board. Few samples were also studied among students, staff and faculties in Sharda University who had either been a patient of COVID-19 or had any family members infected with COVID-19 in the past.
Some information was collected from the patients records of Sharda hospital, Greater Noida, UP, India after obtaining appropriate permission from the Hospital administration. The contact details of the COVID-19 patients obtained from the above survey was tabulated and patients were contacted telephonically. The aims and objectives of this study were telephonically conveyed to the patients or their family members (who so ever were available on phone for the conversation). After taking their verbal consent for participation in the study, their Kidney function tests parameters were noted down along with other clinical parameters.
RESULTS AND DISCUSSION
A total of 76 patients were included in the study which comprised of 36 “Recovered” patients and 40 “Dead” cases (Table 1 &2). Association of Kidney Function parameter with the Recovery from COVID-19 infection: Of the 36 study patients, 5.5% (2 patients; 1 Male, 1 Female) were in the age group of 0-20 years; 33.3% (12 patients; 7 Males, 5 Female) were of the age 21-40 years; 27.7 % (10 patients; 5 Males, 5 Females) were of the age 41-60 years and 30.55 % ( 11 males & 2 Females) were above 61years of age. (Table 1)
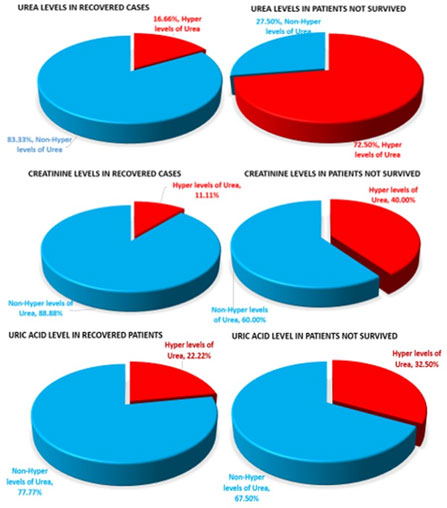
Association between Recovery of patients and Blood Urea level: It was observed that of the total 36 “Recovered” patients, 30 patients (83.3%) did not have hyper levels of urea (non-hyper level) in their blood establishing the optimum functioning of kidney with respect to Urea extraction from blood. Only 6 patients (16.6%) who recovered had hyper/higher levels of blood urea. (Fig. 1).
Association between Recovery of patients and Blood Creatinine level:Of the total 36 patients studied who recovered from infection, 32 patients (88.8%) had non-hyper levels of Creatinine in their blood establishing the optimum functioning of kidney with respect to Creatinine extraction from blood. Only 4 patients (11.11%) who recovered had higher levels of blood creatinine. This comprised of 2 females (56& 65years respectively) and 2 males (65& 78 years respectively). (Fig.1).
Association between Recovery of patients and Blood Uric acid level: Out of 36 patients who recovered from COVID-19, 8 (22.2%) patients had hyper levels of Uric acid whereas 28 patients (77.8%) showed non-hyper levels of Uric acid in the blood. The 8 patients included 4 males (between 46-65 years) and 4 females (between 56-75 years) (Fig. 1).
Figure 1: Schematic presentation of degree of association between kidney function values
(Urea, Creatinine and Uric Acid) and recovery/mortality of COVID-19 patients
Association between Recovery of patients and Blood Sodium level: The values observed in the recovered patients indicated that 12 (33.3%) patients (8 males and 4 female patients of age 41 to 78 years) had low levels i.e. between 130 to 136mg/Eq/L. Higher values were observed in 2 (5.55%) patients (1 male patient of 46 years and 1 female of age 75years) the value being 148 and 150 mgEq/L respectively i.e. 18 patients (61.11%) had normal sodium levels (Fig. 2).
Figure 2: Schematic presentation of degree of association between Electrolyte values
(Sodium, Potassium and Chloride) and recovery/mortality of COVID-19 patients
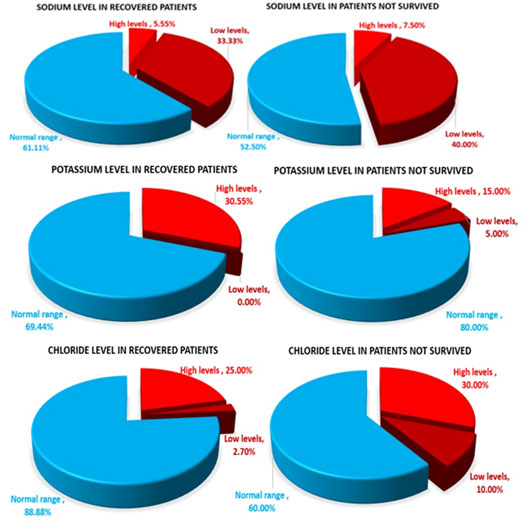
Association between Recovery of patients and Blood Potassium level: Higher values of Potassi:um were observed in 11 (30.55%) patients (six female of 23-59 years; 5 males of 23-65 years), the value was found to be between 5.3 to 10.0 mgEq/L. No low values were observed in the recovered patients, the remaining 25 patients had normal levels of potassium account to 69.44%. (Fig. 2).
Association between Recovery of patients and Blood Chloride level:The values observed in the recovered patients indicated that one male patient (2.7%) had low level (age 78 years) of Chloride while Higher values were observed in 9 (25%) patients; six females (age 23-75 years), 3 male patients (age 46-65 years). The remaining 26 (88.88%) had normal chloride levels (Fig. 2).
Table 1. Values of Kidney function parameters obtained from patients recovered from COVID-19 infection.
| S.No | Patient code
|
Age
|
Sex
|
Day of recovery/ discharge after hospitalization | Kidney Function Test | Electrolytes | ||||
| Urea (mg/dl)
Ref level: 20.0-43.0 mg/dl |
Creatinine (mg/dl)
Ref level: 0.52-1.04 mg/dl |
Uric acid (mg/dl)
Ref level: 2.50-6.20 mg/dl |
Sodium (mEq/L)
Ref Level: 137.0-145.0 mgEq/L |
Potassium (mgEq/L)
Ref Level: 3.50-5.10mgEq/L |
Chloride (mgEq/L)
Ref level: 98.0-107.0mgEq/L |
|||||
| 1. | SCR 5 | 41 | M | 8th | 38.5 | 0.5 | 2.2 | 136 | 3.7 | 98 |
| 2. | SCR 7 | 56 | F | 9th | 99.7 | 1.1 | 7.1 | 138 | 4.6 | 103 |
| 3. | SCR 9 | 35 | M | 10th | 19.2 | 0.8 | 5.6 | 142 | 4.2 | 105 |
| 4. | SCR 13 | 62 | M | 10th | 30.90 | 0.70 | 4.0 | 139 | 4.4 | 103 |
| 5. | SCR 15 | 23 | F | 11th | 19.20 | 0.4 | 3.5 | 140 | 4.4 | 105 |
| 6. | SCR 16 | 31 | F | 10th | 16.2 | 0.5 | 1.7 | 139 | 4.4 | 108 |
| 7. | SCR 17 | 25 | F | 11th | 14.8 | 0.6 | 2.9 | 140 | 3.8 | 104 |
| 8. | SCR 18 | 35 | F | 9th | 22.0 | 0.5 | 2.3 | 139 | 4.1 | 104 |
| 9. | SCR 19 | 5 | F | 6th | 20.5 | 0.2 | 3.0 | 140 | 4.1 | 105 |
| 10. | SCR 20 | 65 | M | 8th | 20.8 | 0.9 | 5.2 | 141 | 4.2 | 109 |
| 11. | SCR 21 | 29 | M | 10th | 25.1 | 0.7 | 5.2 | 145 | 5.0 | 107 |
| 12. | SCR 22 | 37 | M | 3rd | 23.1 | 0.5 | 4.4 | 138 | 4.8 | 102 |
| 13. | SCR 25 | 46 | M | 8th | 24.7 | 0.9 | 6.7 | 148 | 4.9 | 109 |
| 14. | SCR 26 | 48 | F | 5th | 29.7 | 0.5 | 1.9 | 136 | 5.3 | 109 |
| 15. | SCR 27 | 65 | M | 14th | 125.3 | 3.7 | 9.6 | 132 | 7.7 | 109 |
| 16. | SCR 28 | 51 | M | 7th | 23.4 | 0.8 | 7.1 | 141 | 5.1 | 110 |
| 17. | SCR 31 | 78 | M | 13th | 61.4 | 1.1 | 5.2 | 135 | 4.1 | 97 |
| 18. | SCR 32 | 58 | F | 11th | 30.4 | 0.6 | 7.3 | 135 | 4.0 | 101 |
| 19. | SCR 35 | 62 | M | 8th | 25.6 | 0.9 | 5.8 | 140 | 5.0 | 102 |
| 20. | SCR 37 | 65 | M | 11th | 26.5 | 1.0 | 7.0 | 133 | 5.4 | 102 |
| 21. | SCR 41 | 23 | M | 7th | 20.9 | 0.8 | 3.9 | 139 | 5.8 | 104 |
| 22. | SCR 44 | 23 | F | 13th | 17.5 | 0.6 | 3.9 | 145 | 5.9 | 110 |
| 23. | SCR 45 | 21 | M | 13th | 17.9 | 0.6 | 5.2 | 139 | 10.0 | 105 |
| 24. | SCR 53 | 59 | F | 18th | 35.0 | 0.7 | 10.0 | 133 | 5.8 | 98 |
| 25. | SCR 54 | 62 | M | 3rd | 24.3 | 0.6 | 5.1 | 135 | 5.4 | 101 |
| 26. | SCR 55 | 74 | M | 9th | 40.0 | 0.9 | 5.1 | 134 | 4.3 | 98 |
| 27. | SCR 56 | 75 | F | 9th | 41.4 | 3.0 | 10.0 | 150 | 5.3 | 112 |
| 28. | SCR 60 | 52 | M | 7th | 82.8 | 0.9 | 5.0 | 137 | 4.3 | 104 |
| 29. | SCR 61 | – | F | 2nd | 21.3 | 0.6 | 5.9 | 139 | 5.9 | 106 |
| 30. | SCR 62 | 41 | F | 8th | 40.1 | 0.4 | 5.8 | 144 | 5.2 | 108 |
| 31. | SCR 64 | 35 | M | 30th | 53.1 | 0.8 | 4.5 | 131 | 5.1 | 100 |
| 32. | SCR 65 | 62 | M | 4th | 34.2 | 0.7 | 3.9 | 133 | 4.1 | 100 |
| 33. | SCR 67 | 72 | F | 4th | 24.5 | 0.5 | 5.5 | 130 | 3.5 | 99 |
| 34. | SCR 72 | 14 | M | 10th | 25.9 | 0.3 | 3.6 | 138 | 4.4 | 105 |
| 35. | SCR 73 | 40 | M | 8th | 22.9 | 0.7 | 5.2 | 139 | 5.0 | 103 |
| 36. | SCR 74 | 49 | M | 12th | 59.4 | 1.0 | 5.4 | 138 | 4.3 | 105 |
Association of Kidney Function parameters with the Mortality of patients from COVID-19 infection: Of the total 40 patients who did not survive, their age ranged from 23 to 99 years. 7.5% were of age between 21-40 years (one female & 2 male), 30% were of the age 41-50 years (5 females & 7 males) and 55 % were above 61 years of age (7 females; 15 males) (Table 2).
Association between Mortality of patients and Blood Urea level: Referring to the level of urea to be 20.0-43.0 mg/dl in a healthy person, the values were analyzed among 40 “Dead” patients. It was found that hyper values of blood urea were observed in 29 patients (72.5%) out of the 40 patients (8 females of age 24 to 99 years and 19 males of age 46 to 82 years). The values ranged from 43.2. to as high as 221.8mg/dl. Only 11 (27.5%) “Dead” patients had non-hyper levels of urea (Fig. 1).
Association between Mortality of patients and Blood Creatinine: The values observed for blood creatinine in the “Dead” cases indicated that 16 patients (40%) (3 females and 13 males) had hyper values of creatinine ranging from 1.1 to 7.0mg/dl while 24 patients (60%) had non-hyper levels of creatinine (Fig. 1).
Association between Mortality of patients and Blood Uric acid: The values observed in the dead patients indicated that 13 patients (32.5%) (2 females, 10 males and 1 UnK case) had hyper values. The age group was 24 -63 years in female patients, while in male patients it ranged from 57 to 82 years (within 6.2 to 13.9mg/dl range). However, there were 27 (67.5%) of “Dead” patients who had non-hyper levels of uric acid (Fig. 1).
Association between Mortality of patients and Blood Sodium:The values of blood sodium observed in the patients who did not survive indicated 16 (40%) patients with low Sodium values (5 females, 11males; age group 24-66 years and 53 to 82 years respectively). The values were found to be between 115-136mgEq/L. Higher values were observed in three (7.5%) male patients, of 40, 65 and 67 years with values between 149-153mgEq/L. Remaining 21 (52.5%) had normal levels of sodium (Fig. 2)
Association between Mortality of patients and Blood Potassium:The Potassium levels observed in the patients who did not survive, indicated that two (5%) male patients of age 71 & 80 years had low levels of potassium (2.6 & 2.8mgEq/L respectively). Higher values were observed in six (15%) patients; 5 males & 1 UnK of age between 58-82years, the values were between 5.1-6.5 mgEq/L. Remaining 32 (80%) patients showed normal levels (Fig. 2).
Association between Mortality of patients and Blood Chloride: The Chloride values observed in the patients who did not survive indicated low values (94-96mEq/L) in 4 (10%) patients i.e. two females and two male patients (age between 57-66years). Higher values were observed in 12 (30%) patients; one UnK with 108mEq/L; three females (age 24-74 years), had values between 110-114mgEq/l. The 6 male patients (age 23-80 years) had values between 114-117mgEq/l. Normal values were seen on 24 (60%) patients (Fig. 2).
Table 2. Values of Kidney function parameters obtained from patients who did not survive the COVID-19 infection.
| S.No. | Patient code
|
Age
|
Sex
|
Day till hospitalized | Kidney Function Test | Electrolytes | ||||
| Urea (mg/dl)
Ref level: 20.0-43.0 mg/dl |
Creatinine (mg/dl)
Ref level: 0.52-1.04 mg/dl |
Uric acid (mg/dl)
Ref level: 2.50-6.20 mg/dl |
Sodium (mEq/L)
Ref Level: 137.0-145.0 mgEq/L |
Potassium (mgEq/L)
Ref Level: 3.50-5.10mgEq/L |
Chloride (mgEq/L)
Ref level: 98.0-107.0mgEq/L |
|||||
| 1. | SCD 1 | 60 | M | 5th | 234.9 | 7.0 | 13.3 | 124 | 5.6 | 98 |
| 2. | SCD 2 | 63 | F | 6th | 52.6 | 0.8 | 13.7 | 142 | 5.0 | 99 |
| 3. | SCD 3 | 80 | M | 5th | 86.6 | 0.8 | 4.3 | 136 | 3.8 | 104 |
| 4. | SCD 4 | 70 | M | 4th | 221.8 | 4.4 | 10.0 | 137 | 6.2 | 105 |
| 5. | SCD 5 | 51 | F | 3rd | 39 | 0.8 | 3.5 | 140 | 3.6 | 107 |
| 6. | SCD 7 | 52 | F | 9th | 114.8 | 3.4 | 2.5 | 141 | 4.1 | 106 |
| 7. | SCD 8 | 24 | F | 4th | 63.5 | 0.6 | 6.4 | 134 | 3.9 | 110 |
| 8. | SCD 9 | 80 | F | 4th | 52.4 | 0.6 | 4.8 | 145 | 2.8 | 107 |
| 9. | SCD 10 | 46 | M | 6th | 123.4 | 3.3 | 3.2 | 143 | 5.1 | 116 |
| 10. | SCD 11 | 63 | M | 5th | 116.3 | 1.0 | 3.9 | 144 | 4.8 | 117 |
| 11. | SCD 19 | 23 | M | 9th | 36.1 | 0.7 | 5.5 | 140 | 5.0 | 113 |
| 12. | SCD 22 | 67 | M | 8th | 29.7 | 0.8 | 5.9 | 137 | 4.2 | 103 |
| 13. | SCD 23 | 57 | F | 7th | 36.3 | 1.1 | 5.0 | 135 | 4.3 | 96 |
| 14. | SCD 24 | 68 | F | 2nd | 47.8 | 0.5 | 5.1 | 140 | 3.5 | 98 |
| 15. | SCD 26 | 67 | M | 5th | 70.3 | 0.9 | 6.3 | 135 | 3.5 | 99 |
| 16. | SCD 27 | 70 | M | 8th | 112.7 | 10.6 | 4.8 | 123 | 4.1 | 94 |
| 17. | SCD 28 | 67 | M | 6th | 87.0 | 3.0 | 5.8 | 149 | 3.6 | 115 |
| 18. | SCD 29 | 53 | M | 3rd | 23.5 | 0.8 | 4.5 | 135 | 5.0 | 106 |
| 19. | SCD 30 | 71 | M | 6th | 55.2 | 1.5 | 7.8 | 141 | 2.6 | 97 |
| 20. | SCD 31 | 65 | M | 10th | 74.1 | 0.9 | 3.8 | 147 | 3.5 | 102 |
| 21. | SCD 32 | 58 | M | 4th | 85.1 | 0.8 | 7.2 | 138 | 5.7 | 99 |
| 22. | SCD 33 | 67 | M | 5th | 35.5 | 1.1 | 6.6 | 133 | 5.6 | 97 |
| 23. | SCD 34 | 54 | M | 5th | 23.2 | 0.6 | 3.1 | 128 | 4.5 | 98 |
| 24. | SCD 38 | 57 | M | 2nd | 43.2 | 1.5 | 6.6 | 137 | 4.4 | 95 |
| 25. | SCD 42 | 60 | F | 3rd | 67 | 2.2 | 5.1 | 134 | 4.7 | 110 |
| 26. | SCD 45 | – | – | 5th | 112 | 1.0 | 13.9 | 145 | 6.5 | 108 |
| 27. | SCD 46 | 54 | M | 4th | 36 | 1.5 | 4.2 | 133 | 4.6 | 105 |
| 28. | SCD 49 | 61 | M | 1st | 55.7 | 0.82 | – | 133 | 4.5 | 101 |
| 29. | SCD 52 | 66 | F | 8th | 45.2 | 0.9 | 5.4 | 127 | 3.6 | 94 |
| 30. | SCD 53 | 66 | M | 3rd | 75.7 | 1.5 | 6.9 | 139 | 4.4 | 104 |
| 31. | SCD 55 | 40 | M | 6th | 42.3 | 0.7 | 2.8 | 153 | 4.2 | 116 |
| 32. | SCD 57 | 78 | M | 2nd | 91.8 | 5.7 | 5.3 | 139 | 4.3 | 104 |
| 33. | SCD 58 | 59 | F | 2nd | 25.6 | 0.6 | 5.1 | 136 | 5.0 | 105 |
| 34. | SCD 60 | 74 | F | 6th | 86.4 | 0.8 | 4.8 | 145 | 3.3 | 114 |
| 35. | SCD 61 | 80 | M | 5th | 47.7 | 0.7 | 3.3 | 139 | 3.1 | 115 |
| 36. | SCD 62 | M | 9th | 44.8 | 0.7 | 2.4 | 137 | 4.2 | 98 | |
| 37. | SCD 64 | 99 | F | 3rd | 70.7 | 0.9 | 4.8 | 144 | 3.9 | 107 |
| 38. | SCD 67 | 82 | M | 2nd | 207.7 | 1.6 | 7.3 | 115 | 5.6 | 111 |
| 39. | SCD 68 | M | 4th | 54.3 | 0.6 | 4.3 | 133 | 4.7 | 99 | |
| 40. | SCD 69 | 82 | M | 4th | 133.9 | 3.0 | 7.8 | 145 | 4.2 | 112 |
Relative association of Blood Urea, Creatinine and Uric acid with survival/mortality of patients: Blood Urea: It was observed that average percentage of the association between hyper blood urea and recovery/mortality of the cases was 77.9%. As 83.4% of “Recovered” patients did not show hyper urea level whereas 72.5% of “Dead” patient showed hyper urea levels.
Blood Creatinine: It was observed that average percentage of the association between hyper blood creatinine and recovery/mortality of the cases was 64.4%. 88.8% of “Recovered” patients did not show hyper creatinine level whereas 40.0% of “Dead” patient showed hyper creatinine levels.
Uric Acid: It was observed that average percentage of the association between hyper blood uric acid and recovery/mortality of the cases was observed to be 55.1%. 77.7% of “Recovered” patients did not show hyper uric acid levels whereas 32.5% of “Dead” patient showed hyper creatinine levels.
Association of Electrolyte levels in survival/mortality in COVID-19 patients: Sodium & Potassium level: An inverse association of Sodium and Potassium levels were observed in study subjects. Of the total number of 36 patients who recovered, it was observed that maximum patients were having Low levels of sodium and high levels of potassium (33.33% and 30.55% respectively) when compared to the high and normal values. Similarly, of the total number of 40 patients who died, maximum patients were reported with Low levels of sodium and high levels of potassium (40% and 15% respectively) when compared with their high and normal values. Chloride level: Chloride levels also had trend like that of potassium levels. Maximum number of patients who survived and who died were comparatively more (25% and 30% respectively)
Present work has been undertaken to study association of kidney function parameters with the survival/mortality of COVID-19 patients. After careful observations and analysis of Kidney Function parameters among the categories of “Recovered” and “Dead” cases, we observed that most crucial of these KFT parameters is the level of Blood Urea followed closely by Blood Creatinine level among the two categories of COVID-19 patients. The degree of association observed between blood urea level versus survival/mortality of patients emerged to be the most significant observation.
Our preliminary study reveals that need for addressal and/or correction of blood urea level among patients may be considered for possible inclusion into the COVID-19 TREATMENT protocol through appropriate clinical interventions. Fundamentally, when protein is broken down in the body, then urea is made in the liver and is then passed out of the body in the form of urine, which indirectly amounts to the level of nitrogen in the body. So if there is elevated levels of Urea in the blood it is as indication of inefficient kidney functions, which may impact the blood chemistry to lead to cardiac and/or pulmonary problems enhancing mortality of COVID-19 patients.
The second important observation emerged out of present study is a high degree of association of Serum Creatine level with the survival and mortality of patients. The degree of association between creatinine and survival/mortality of patients was computed to be 64.4 which reveals that treatment to keep normal creatinine after studying the observed value of this parameter among patients has to be the part of COVID-19 treatment protocol. Creatine is yet an another amino acid which we get through dietary intake. This is also made in the liver and provides energy to the body. It is also present in muscles and when the body utilizes creatine as an energy source, then creatinine is expelled out of the body as waste material (https://www.medicalnewstoday.com/articles/322380) (Acc May 2021).
Elevated levels of creatinine in blood may be correlated with decreased excretion and besides this Creatinine is also measured to correlate the Glomerular filtration rate (GFR) in our body (Thomas 2005). The IFFC (International Federation of Clinical chemistry and Laboratory Medicine) Guidelines on COVID-19 highlights the need of monitoring creatinine levels in critical COVID-19 patients so as to diagnose any injury to kidney at an early stage (IFCC guide on COVID-19, 2020).. The observations reported in the present study are in concurrence with the IFFC guidelines. Previous studies have reported that in the SARS-COV 1, 2003 strain and the Middle East respiratory syndrome (MERS) infection, there were cases with Acute kidney injury (AKI) and subsequent mortality of cases (Cheng et al. 2020; Naicker et al. 2020).
Many viruses including SARS-COV2 have been reported to show ‘viroporin’ activity for which it depends on the ion channels present on the host cells (Charlton et al. 2020; Neiva et al. 2012; Neiva et al. 2015; Royle et al. 2015) as it does for its replication and dissemination activities. As it causes infection and complication when it enters specific cell or tissue, similarly it may also cause impaired ion-channels and may contribute to internal imbalance or disturbances.
Of the whole array of 300 ions channels known in humans (Yu et al. 2005), there are many channels which have been studied for their involvement either in viral entry or in replication or in dissemination and also in the involvement in dysfunctions generalized as ‘Channelopathies’ and hence causing chaos in the ion channels associated with the nervous, musculoskeletal, cardiovascular, immune systems, (Kim, 2014; Elias & Benito, 2018; Vaeth & Feske, 2018, Charlton et al. 2020).
It has also been known that the E protein of the SARS-COV2 virus has more affinity for sodium ions than potassium ions (Kai et al. 2016; Melton et al. 2002), while the 3a protein have a tendency to form ion channel and thus aid in virus dissemination (Lu et al. 2006). The low levels of sodium as seen in the patients who did not survive may indicate utility of ion channels by the virus and thus leading to decrease in the sodium channels availability for the human system to perform its normal functioning such as disturbance in the RAS/homeostasis pathway.
The fact that low levels of sodium had been observed in recovered patients also can be supported by the fact that may be use of ion channel blockers are hypertensive medication may have contributed as a modulator of this viral activity. This necessitate further studies of adding ion channel modulators in the treatment protocol (only after careful analysis of KFT test reports) in complicated cases. The Dysregulation of sodium channels specifically of the respiratory tract has been observed in Human Respiratory Syncytial virus (Chen et al. 2009). This observation also focuses studies to be extended on understanding role of SARS-COV2 with chloride and potassium levels too.
CONCLUSION
All the parameters i.e. urea, creatinine, uric acid and electrolytes are very important and speak for their individual existence and persistence within the human body, but the fact needs to be considered whether these are symptoms prior to viral infection or whether these are the post effects of COVID-19 infection. The findings of the present study thus highlight an important observation which needs to be considered to improvise renal treatment regime of the COVID-19 patients. Preliminary observations reported in the present paper sensitizes to further understand and confirm the reported association of kidney function and recovery/mortality of patients and if proved the treatment protocol of COVID-19 may be considered for possible modification.
Disclosure: The authors declare that there are no conflicts of interest in this work.
ACKNOWLEDGEMENTS
We thank Dr Ashutosh Niranjan, Medical Superintendent, Sharda Hospital for kindly providing us with data for analysis of this work and also to the staff and students who have worked in providing us the data.
REFERENCES
ACE-2: The receptor for SARS-COV2 https://www.rndsystems.com/resources/articles/ace-2-sars-receptor-identified [accessed 19 May 21]
Charlton FW, Pearson HM, Hover S, et al. (2020). Ion Channels as Therapeutic Targets for Viral Infections: Further Discoveries and Future Perspectives. Viruses. 12(8):844.
Chen L, Song W, Davis IC, et al. (2009). Inhibition of Na+ transport in lung epithelial cells by respiratory syncytial virus infection. Am J Respir Cell Mol Biol, 40: 588–600.
Cheng Y, Luo R, Wang K, et al. (2020). Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Internat. 97: 829–38.
Garcia-Elias A, Benito B. (2018). Ion channel disorders and sudden cardiac death. Int J Mol Sci. 19, 692.
IFCC Information Guide on COVID-19 [Internet]. IFCC Information Guide on COVID-19. 2020. Available from: www.ifcc.org/ifcc-news/2020-03-26-ifcc-information-guide-on-covid-19/ https://covid19.who.int/ [accessed 22 June 2021].
Kai W, Shiqi X, Bing S. (2011). Viral proteins function as ion channels. Biochim et Biophys Acta (BBA) – Biomem. 1808 (2): 510-515.
Kim JB. (2014) Channelopathies. Korean J Pediat, 57, 1–18.
Lu W, Zheng BJ, Xu K, et al. (2006). Severe acute respiratory syndrome-associated coronavirus 3a protein forms an ion channel and modulates virus release. Proc Natl Acad Sci USA. 103:12540-12545.
Melton JV, Ewart JD, Weir RC, et al. (2002). Alphavirus 6 K proteins form ion channels. J Biol Chem, 277: 46923-46931
Naicker S, Yang C-W, Hwang S-J, et al. (2020). The Novel Coronavirus 2019 epidemic and kidneys. Kidney Internat. 97:824–8.
Nieva JL, Carrasco L. (2015) Viroporins: Structures and functions beyond cell membrane permeabilization. Viruses, 7, 5169–5171.
Nieva JL, Madan V, Carrasco L. (2012). Viroporins: Structure and biological functions. Nat Rev Genet. 10, 563–574.
Normal range for Creatinine blood test? https://www.medicalnewstoday.com/articles/322380 [accessed on 30 April 21].
Ranjan R, Sharma A, Verma MK (2021). Characterization of second wave of COVID-19 in India. MedRxiv; doi: https://doi.org/10.1101/2021.04.17.21255665.
Royle J, Dobson SJ, Müller M, et al. (2015). Emerging roles of viroporins encoded by DNA viruses: Novel targets for antivirals? Viruses, 7, 5375–5387.
Thomas L, editor. (2005). Labor und Diagnose: Indikation und Bewertung von Laborbefunden für die medizinische Diagnostik. 6. Aufl. Frankfurt/Main: TH-Books-Verl.-Ges.
Vaeth, M, Feske, S. (2018). Ion channelopathies of the immune system. Curr Opin Immunol, 52, 39–50.
Yu FH, Yarov-Yarovoy V, Gutman GA, et al (2005). Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev. 57, 387–395.


