School of Life Sciences, Jawaharlal Nehru University, New Delhi 110067, India
Corresponding author Email: baishnabtripathy@yahoo.com
Article Publishing History
Received: 15/04/2019
Accepted After Revision: 20/06/2019
Exposure of plants to extreme environmental conditions greatly reduces their growth by increasing the concentration of reactive oxygen species (ROS). The elevation in the ROS accumulation directly impacts the photosynthetic machinery of the plant. Among the ROS, singlet oxygen (1O2) cannot be detoxified by any antioxidative enzyme-mediated reactions. Therefore, 1O2 is the major cause of damage to plants during day time. Spraying plants with a chlorophyll precursor 5- aminolevulinic acid (ALA) increases the accumulation of Chl biosynthetic intermediate protochlorophyllide (Pchlide) which acts as a photosensitizer. Upon light exposure of ALA-treated plants, overaccumulated Pchlide in chloroplasts gets excited and transfer their absorbed energy to oxygen to generate 1O2 via type-II photosensitization reaction. The 1O2 immediately damages thylakoid membranes and induces wilting in the whole plant. The porC-2 mutant that lacks the photoenzyme protochlorophyllide oxidoreductase C (PORC) fail to photo-transform overaccumulated Pchlide to Chlide upon light exposure therefore, leading to the excess generation of 1O2. This leads to severe damage to photosynthetic apparatus that destroys chlorophylls and the photosynthetic reactions as indicated from Chl a fluorescence imaging, reduced Fv/Fm and Fv/Fo ratio, the quantum yield of PSII and electron transport rate. Conversely, plants overexpressing protochlorophyllide oxidoreductase C (PORCx) are capable of efficiently photo-converting photodynamic Pchlide to Chlide that reduces the 1O2 production in ALA-treated and light-exposed plants. Reduced 1O2 produced in PORCx plants causes less damage to the photosynthetic machinery and plants do not bleach. Results demonstrate the pivotal role of protochlorophyllide oxidoreductase C in the protection of plants from 1O2-induced oxidative stress during day time.
Arabidopsis thaliana · Imaging PAM · Oxidative stress · Protochlorophyllide oxidoreductase C · Singlet oxygen
Chauhan G, Tripathy B. C. Role of Protochlorophyllide Oxidoreductase C in Protection of Plants from Singlet Oxygen-Induced Oxidative Stress. Biosc.Biotech.Res.Comm. 2019;12(2).
Chauhan G, Tripathy B. C. Role of Protochlorophyllide Oxidoreductase C in Protection of Plants from Singlet Oxygen-Induced Oxidative Stress. Biosc.Biotech.Res.Comm. 2019;12(2). Available from: https://bit.ly/30kmob5
Copyright © Chauhan and Tripathy, This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
In a cell, metabolic reactions are tightly regulated to minimize endogenous ROS production. Several antioxidant enzymes and antioxidant molecules work in coordination to regulate the redox status in plants. However, this balance gets disrupted when plants are exposed to extreme environmental conditions (Dat et al., 2000). Excess light exposure over reduces the photochemical electron transport chain, thereby reducing the overall photosynthetic efficiency of the plant (Nishiyama et al., 2001). Apart from chlorophyll molecules present in the antenna and reaction center complex, the membrane-bound chlorophyll (Chl) biosynthetic intermediates can also absorb excess excitation energy and gets excited. Since they are not directly connected to the reaction centers, the energy absorbed by the intermediates cannot be channeled to the photochemical reaction (Tripathy, Mohapatra and Gupta, 2007). Instead, they return to their ground state by transferring their energy to molecular oxygen to give rise to 1O2 via type II photosensitization reaction (Foote, 1991; Montoya et al., 2005). Accumulation of 1O2 results into peroxidation of membrane lipids (Triantaphylides et al., 2008), the irreversible oxidation of D1 proteins (Krieger-Liszkay, Fufezan and Trebst, 2008) and gravely damages PS I and PS II (Tripathy and Pattanayak, 2010). Experiments conducted on chlorina1 mutant (lack chlorophyll b) shows severe phenotypic damage when exposed to light (Ramel et al., 2013). In the absence of chlorophyll b, the photosystem II chlorophyll protein antenna complex becomes nonfunctional and produces 1O2 at the natural site of their production due to naked PS II centers. HPLC analysis of enzymatic and nonenzymatic mediated lipid peroxidation products such as octadecatrienoic hydroxyl acids (HOTEs) increased when exposed to high light for 2 days (Ramel et al., 2013; Yang et al., 2019).
Carotenoids are the natural quencher of singlet oxygen, which are present in close abundance in the antenna molecule to dissipate the excess energy absorbed as heat (Baroli et al., 2000). However, in the reaction center, the distance between the reaction center and beta carotene molecule is too large to allow energy transfer, therefore results in the formation of 1O2. (Laloi and Havaux, 2015). The Chl biosynthetic intermediates are bound to the plastidic membrane (Mohapatra and Tripathy, 2007). The carotenoids present in the thylakoid and envelope membranes are not in close proximity of Chl biosynthetic intermediates. Therefore, carotenoids fail to quench the 1O2 from the excited states of Chl biosynthetic tetrapyrroles and allow the production of intermediate derived 1O2 in response to high light stress. Instead, 1O2 oxidized carotenoid metabolites actively participate in downstream regulation of gene expression in response to high light stress, (D’Alessasndro and Havaux 2019).
Application of tetrapyrrole precursor, ALA induce accumulation of chlorophyll biosynthetic intermediate, Pchlide (Tripathy and Chakraborty, 1991; Chakraborty and Tripathy, 1992; Fujii et al., 2017). Its conversion into nonphotodynamic intermediate is catalyzed by a light-dependent enzyme, Protochlorophyllide oxidoreductase (POR) which requires light and NADPH to reduce Pchlide to Chlide at the C17 and C18 on the D ring (Armstrong et al., 1995; Oosawa et al., 2000; Pattanayak and Tripathy, 2002). The evolution of POR enzyme occurred very early among oxygenic photosynthetic organisms (Vedalankar and Tripathy 2019). External application of ALA induces production of Pchlide which cannot be converted into Chlide due to the limited supply of POR in WT plants. In rice plants, two isoforms of POR are present, in which POR A is expressed during early development whereas, PORB is present throughout maturity (Kwon et al., 2017).
In the model plant Arabidopsis thaliana, multiple isoforms of POR enzyme exist, namely POR A, POR B, and POR C (Armstrong et al., 1995; Reinbothe et al., 1996; Oosawa et al., 2000; Su, Armstrong and Apel, 2001; Pattanayak and Tripathy, 2002). Among them, POR C is highly expressed in response to light and is chiefly present in the photosynthesizing tissues (Oosawa et al., 2000; Masuda et al., 2003). Therefore, overexpression of POR C rapidly converts the accumulated Pchlide into Chlide and thereby reducing the possibility of Pchlide derived 1O2 oxygen production (Pattanayak and Tripathy, 2011). In contrast, POR C knock-down mutants (porC-2) (Masuda et al., 2003) have a limited supply of PORC. We have taken the mutant to modulate Pchlide derived 1O2 generation in plants to ascertain the role of PORC during oxidative stress. In the present study, WT, PORC overexpressors (PORCx) (Pattanayak and Tripathy, 2011) and porC-2 mutants were used to modulate the Pchlide sensitized 1O2 production to study its impact on the photosynthetic efficiency of plants. We have demonstrated the pivotal role of protochlorophyllide oxidoreductase C in the protection of plants from 1O2-induced oxidative stress in light.
Materials and Methods
Plant material and growth conditions
Arabidopsis seeds were soaked in double distilled water for 48h at 4°C for seed scarification. Seeds were sterilized with 4% sodium hypochlorite solution and washed 5 times with autoclaved double distilled water. The sterilized seeds were plated on half-strength Murashige and Skoog media (Sigma). After 12 days of plating, the seedlings were transferred to autoclaved agropete and vermiculite potting mixture (6:1) and grown at 100 μmol photons m-2 s-1 at 21°C under 14 h light/ 10 h dark photoperiod.
ALA treatment
1 mM aqueous ALA solution was used to spray on three-week-old pot grown Arabidopsis plants. Later, plants were covered with aluminum foil and kept in the dark for 12 hours to accumulate Pchlide. Control plants were sprayed with distilled water.
Light treatment
After 12 h of dark incubation, ALA-treated and untreated plants were exposed to low light (75 μmol photons m-2 s-1) for different time intervals.
Imaging PAM
After completion of light treatment, plants were again incubated in the dark for 20 min to open all the reaction centers. Images were captured from IMAGING PAM MAXI chlorophyll fluorometer (Walz, Germany) and the fluorescence parameters were calculated from ImagingWin software (Walz, Germany). Chlorophyll fluorescence parameters such as the maximum quantum yield of PS II (Fv/Fm) was determined by the equation Fv/Fm= (Fm-Fo )/Fm where Fo is the dark fluorescence yield, Fm is the maximum fluorescence yield and (Fm-Fo) is the variable fluorescence (Fv). Maximum efficiency of the water diffusion reaction is analyzed from the ratio of variable to minimum fluorescence yield (Fv/Fo). Other parameters such as Electron transport rate of photosystem II was calculated from the equation ETR (II) = ø PS II x PAR x 0.5 x 0.84 where ø PS II is effective PSII quantum yield (calculated by (Fʹm –Ft)/ Fʹm=ΔF/ Fʹm where Fʹm is referred as the maximum fluorescence yield when the samples are illuminated, and Ft is the fluorescence yield at any given time (t). PAR abbreviates for photosynthetically active radiation, 0.5 is the factor of the ratio of PS II and PS I (1:1), 0.84 is the value that correlates with the percentage of incident photons are absorbed by the leaf to drive photosynthesis. The nonphotochemical quenching of the maximum fluorescence was calculated by (Fm- Fʹm)/ Fʹm. Quantum yield of nonregulated energy dissipation Y(NO) is calculated by the equation 1/(NPQ+1+qL (Fm/Fo-1)), where qL represents the fraction of reaction centers that are open according to the lake model (Baker, 2008).
Results and Discussion
Overexpression of PORC protect plants from 5-Aminolevulinic acid (ALA) induced oxidative stress: WT, PORCx, and porC-2 plants were grown under the low light regime (100 μmol photons m-2 s-1). After three weeks, plants were sprayed with 1 mM aqueous ALA solution and covered with aluminum foil and kept in the dark for 12 h to allow phototransformation were kept under low light (75 μmol photons m-2 s-1) for 1 or 2 h. Upon exposure to light, due to the limited presence of POR enzyme, plants failed to photo-transform overaccumulated Pchlide to Chlide which resulted in the build-up of excess Pchlide pool that acted as a photosensitizer. The excess Pchlide absorbed a supra-optimal amount of light that could not be transferred to the photosynthetic reaction centers to synthesize reducing equivalents. Instead, the absorbed light energy was transferred to molecular oxygen to generate 1O2 via type II photosensitization reaction of tetrapyrroles (Tripathy and Chakraborty, 1991; Chakraborty and Tripathy, 1992).
The severity of 1O2-induced damage to plants was monitored by IMAGING PAM that measured Chl a fluorescence. Figure 1 shows false color images of chlorophyll fluorescence at a time t (Ft), from WT, PORC overexpressor, and its knock-down mutant porC-2 plants treated without and with 1 mM ALA. In ALA-treated porC-2 mutants, the fluorescence was seen from only a few partially green patches of bleached leaves. The porC-2 mutant plants due to lack of PORC enzyme were the most affected as they failed to photo-transform overaccumulated Pchlide to Chlide. Under identical conditions, the overexpressor plants had a lot of fluorescence emanating from greener leaves whose images were recorded by the fluorometer.
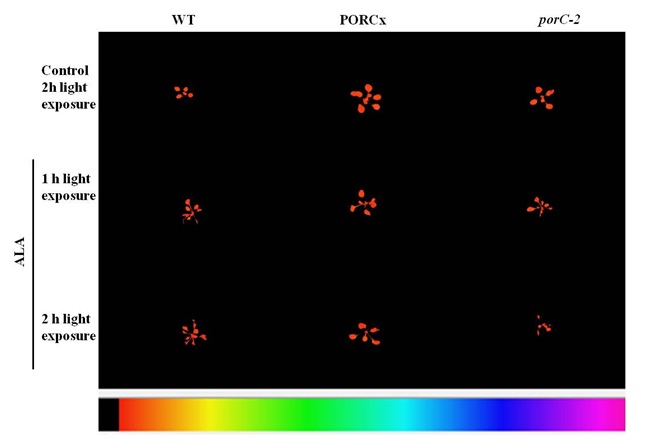 |
Figure 1: Overexpression of PORC protect plants from 5-Aminolevulinic acid (ALA) induced oxidative stress |
WT, PORC overexpressor and its knock-down mutant (porC-2) plants of three week stage was sprayed with 1mM ALA or distilled water and kept in the dark for 12 h to accumulate photosensitizer Pchlide. Chlorophyll a fluorescence images at time t (Ft) was imaged by using Imaging-PAM (Walz, Germany) after 1 h and 2 h of light exposure. The color code beneath the image depict values from 0 to 1.
Singlet oxygen accumulation decreases chlorophyll fluorescence yield: External application of tetrapyrrole precursor ALA to three-week-old WT, PORCx and porC-2 plants induced oxidative stress when ALA-treated dark incubated plants were exposed to low light (75 μmol photons m-2 s-1), i.e., lower than the intensity at which they were grown (100 μmol photons m-2 s-1). To understand if ALA-induced oxidative stress influence the maximum PS II quantum yield in dark incubated plants, the Fv/Fm ratio was analyzed in both control and ALA-treated samples after 1 h and 2 h of light exposure (Fig.2a). The Fv/Fm ratio of dark incubated ALA-treated WT and porC-2 plants was reduced by 8% and 14% after 1 h of light treatment. Under identical conditions, the Fv/Fm ratio of PORCx plants was unaffected. After 2 h of light treatment, the Fv/Fm ratio declined by 14% in WT, 20% in porC-2 and to a small extent (5%) in PORC overexpressors.
To ascertain the intensity of damage caused by 1O2 to the oxygen evolution complex, the Fv/Fo ratio in ALA-treated dark incubated plants was monitored upon light exposure (Fig.2b). The Fv/Fo ratio denotes the activity of water splitting complex of PSII. Due to the limited availability of PORC enzyme in porC-2 and WT plants, the accumulation of Pchlide caused a burst in 1O2 production that significantly damages the oxygen-evolving complex. The maximum decrease in Fv/Fo ratio was observed in porC-2 plants followed by WT whereas PORCx plants had smaller impairment of photosynthetic oxygen evolution machinery. The Fv/Fo ratio declined by 23% and 18% after 2 h of light exposure in porC-2 and WT plants respectively. However, under identical conditions, minimal damage was observed with respect to the Fv/Fo ratio of PORCx plants.
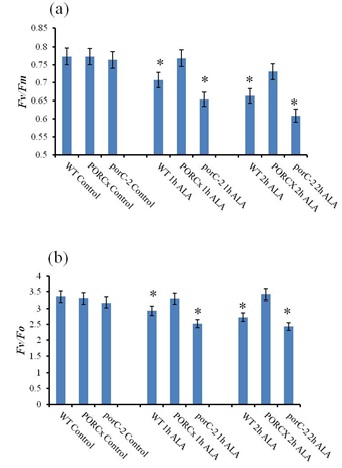 |
Figure 2: Pchlide derived singlet oxygen decreases chlorophyll fluorescence yield |
To understand the effect of accumulated singlet oxygen due to ALA treatment on the quantum yield of PS II, the chlorophyll a fluorescence parameters were analyzed in ALA or distilled water treated dark incubated WT, PORCx and porC-2 mutant plants after light exposure. (a) the ratio of variable to maximum fluorescence (Fv/Fm) represents the maximum photochemical efficiency of PS II (b ) Fv/Fo denotes the relative activity of water splitting complex on the donor side of PS II. Each data point is the average of 5 replicates and error bars represent ±SE. Asterisks indicate significant differences determined by t test (*P<0.05).
ALA-induced oxidative stress decreased the quantum yield of PS II photochemistry and electron transport rates: The impact of 1O2-induced oxidative stress on the overall photochemical quantum yield of PS II (ø PSII) was determined at 530 μmol photons m-2 s-1 light intensity using the equation described in materials and methods. In ALA-treated dark incubated porC-2 plants, the effective quantum yield of PS II decreased by 50.3% within an hour of light exposure that later declines to 72.6 % after 2 h of light treatment (Fig.3a). In the WT plants, the effective quantum yield decreases from 35% and 63% due to 1h and 2h of light treatment respectively. However, in the PORC overexpressors, the decline in ø PSII was 17 % after 2 h of light exposure. The decrease in the electron transport rate (ETR) of PS II measured at 530 μmol photons m-2 s-1 light intensity was determined in control, and ALA-treated plants. ALA sprayed porC-2, and WT plants, exposed to light 75 μmol photons m-2 s-1 for 2 h had 50% and 35 % reduction in ETR respectively (Fig. 3b). Under identical conditions, the ETR of PORCx plants was declined by 17.41% after 2 h of light exposure.
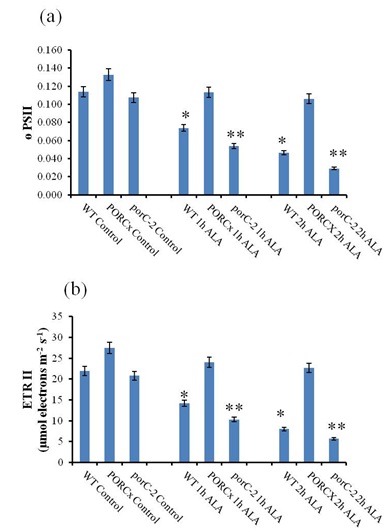 |
Figure 3: Exogenous application of ALA decreases quantum yield and electron transport rates of porC-2 mutant |
Pulse amplitude modulated chlorophyll a fluorescence parameters were analyzed from ALA or distilled water treated samples. (a) Quantum yield of photosystem II (b) Electron transport rate of photosystem II was calculated using Imaging Win Software. Each data point is the average of 5 replicates and error bars represent ±SE. Asterisks indicate significant differences determined by t test (*P<0.05, **P<0.01). Activation of heat dissipation machinery in PORCx in response to 102-induced oxidative stress: NPQ is an indicator of activation of heat dissipation machinery in response to stress. Increased NPQ in PORC overexpressor indicates the activation of gradient-dependent heat dissipation machinery (Fig.4a). Whereas, in porC-2 and WT, the decrease in NPQ indicate the absence of activation of the protective mechanism. In the control conditions, the Y(NO), that denotes quantum yield of energy dissipation in PS II, were similar in all the three types of plants (Fig.4b). After ALA treatment, the increase in Y(NO) in porC-2 and WT indicates that both the processes of photochemical energy conversion and protective regulatory mechanisms are unable to cope up even with the low light (75 μmol photons m-2 s-1). Under identical conditions in PORCx plants, the decrease in the Y(NO) indicates that due to excess of PORC enzyme, the Y(NO) remained unaffected after 2 h of light exposure.
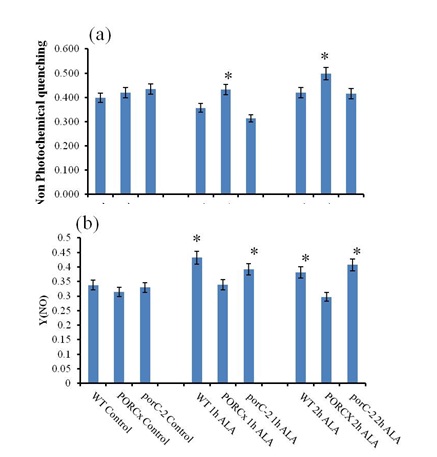 |
Figure 4: Activation of heat dissipation machinery in PORCx in response to ALA-induced oxidative stress |
Pulse amplitude modulated Chlorophyll a fluorescence parameters in ALA and distilled water treated samples were determined by Imaging PAM. (a) Non photochemical quenching (NPQ) (b) Quantum yield of non regulated energy dissipation in PS II. Each data point is the average of 5 replicates and error bars represent ±SE. Asterisks indicate significant differences determined by t test (*P<0.05). In a plant cell, 1O2 is produced as a byproduct of cellular metabolic function. The limited amount of 1O2 can be easily quenched by carotenoids (Ramel et al., 2012). However, when plants are exposed to photooxidative conditions, the balance between 1O2 productions and quenching gets perturbed, resulting in an elevation in 1O2 level. The accumulation of 1O2 not only oxidizes several biomolecules but severely impact the metabolic processes (Tripathy and Pattanayak, 2010). In plants, chloroplasts are the major source of 1O2 generation as the tetrapyrroles that act as photosensitizers are exclusively located in the organelle (Chakraborty and Tripathy, 1992; Triantaphylides et al., 2008; Ambastha et al., 2017).
ALA is the precursor of tetrapyrroles such as chlorophyll, haem, and phycobilins. In the dark, the accumulation of Pchlide modulates ALA synthesis by a feedback mechanism. Regulation of ALA synthesis is essential to prevent the accumulation of photodynamic chlorophyll metabolic intermediates. This enables plants to evade photooxidative stress (Rebeiz et al., 1988; Tripathy and Chakraborty, 1991; Chakraborty and Tripathy, 1992). Unlike other isoforms of POR, which are prominently present in the etiolated seedlings, PORC expression is present throughout the leaf development and it increases in response to high light. In PORCx plants, the rapid conversion of Pchlide to Chlide takes place due to maximum availability of PORC enzyme (Pattanayak and Tripathy, 2002, Pattanayak and Tripathy, 2011). Chlide produced as a result of phototransformation is immediately converted into chlorophyll that associates itself to the chlorophyll binding proteins present in the thylakoid membranes and transfers the absorbed energy to the reaction centers to drive photosynthetic reactions.
To understand the effect of Pchlide derived 1O2 on the photosynthetic machinery of plants, their chlorophyll fluorescence parameters were studied by IMAGING-PAM (Kandoi et al., 2016). The energy absorbed by Chl can be utilized through either one of the three processes. Most primarily, the energy absorbed by the Chl molecule is directed to initiate the photochemical reactions. Secondly, the absorbed energy is dissipated in the form of heat, and third, the excited Chl molecules return to the ground state by fluorescence. These are three competing processes. Analysis of modulated chlorophyll fluorescence in response to different light pulses provide valuable information regarding the photosystem II activity in a quick and non-invasive manner. The ratio of variable to maximal fluorescence (Fv/Fm) indicates the health status of dark-adapted plants. Under control conditions, a dark-adapted, healthy and non-stressed plant emits maximum fluorescence (Fm) after illuminated with a short pulse of saturating light Therefore the ratio of variable to maximum fluorescence was closer to 0.78 in non-stressed leaves, which is an effective parameter to study the maximal quantum efficiency of PS II (Björkman and Demmig, 1987). Similarly, under stress, a decline in the light-adapted maximal fluorescence (Fʹm) indicates the release of the absorbed energy as heat due to activation of nonphotochemical quenching (NPQ). Likewise, other parameters of chlorophyll a fluorescence can be interpreted to obtain information regarding the quantum yield and PSII-dependent electron transport rate.
In this study, we have observed that plants overexpressing PORC enzyme were capable of tolerating ALA-induced oxidative stress. The porC-2 mutant has T-DNA inserted in the 4th exon of PORC gene (Masuda et al., 2003). The unavailability of PORC enzyme reduces efficient phototransformation of Pchlide to Chlide. The nontransformed Pchlide upon receiving light gets excited like a Chl molecule and returns to the ground state by generating singlet oxygen via type-II photosensitization process of Pchlide (Tripathy and Chakraborty, 1991; Chakraborty and Tripathy, 1992). Further, spraying porC-2 plants with ALA increases the accumulation of Pchlide and Pchlide derived 1O2. Therefore, maximum damage to the photosynthetic machinery was observed in the porC-2 mutant.Enhanced level of 1O2 can reduce photosynthetic efficiency by oxidizing structural and functional proteins associated with the PS II. During electron transport across the PS II, D1/D2 heterodimer constitutes the core of reaction center. D1 protein participates in the charge separation and electron transfer events during the electron transport. Production of 1O2 in the plastidic membrane due to the accumulation of membrane-bound Chl biosynthetic intermediates (Pchlide) can easily target D1 protein. When the oxidative damage caused by 1O2 increase beyond the D1 repair machinery, impairment in the reduction of P680 directly influences the electron transport rate and quantum yield of PS II (Edelman and Mattoo, 2008).
The increased production of 1O2 in porC-2 mutant bleached substantial amounts of Chls leaving a few green patches in the leaves. The fluorescence was emitted only from a few green patches that were not severely bleached. The WT plants using its endogenous POR could photo transform some of the Pchlide to Chlide, resulting in lower Pchlide accumulation than porC-2 mutant. Therefore, WT had lesser bleaching of leaves due to reduced generation of 1O2 than porC-2 mutants, and consequently, the fluorescence was emitted from a higher number of green leaves. The fluorescence imaging clearly reveals that the PORCx plants were protected from photooxidative damage having fluorescence emission from a large number of leaves due to the minimal generation of 1O2 in light.
The Fv/Fm ratio, a measure of the quantum efficiency of PSII, in dark-adapted leaves, declined in WT and porC-2 plants. The PORCx plants always had higher Fv/Fm ratio than that of the WT and porC-2 plants. In the same vein, the Fv/Fo that measures the oxygen-evolving activity in the oxidizing side of PSII implies the activity of water splitting complex, which is very sensitive to redox changes, was lower in light exposed porC-2 plants. It was due to 1O2-induced impairment of oxygen-evolving complex associated with PSII. Similarly, the quantum yield of PSII and the estimated PSII-dependent ETR were higher in PORCx plants due to reduced oxidative damage to the oxygen-evolving complex. Conversely, the heat dissipation of absorbed photons measured as NPQ and quantum yield of non regulated energy dissipation Y (NO) (Genty, Briantais and Baker, 1989) was higher porC-2 mutants exposed to light. Present results demonstrate the important role of protochlorophyllide oxidoreductase C in the protection of plants from 1O2-induced oxidative damage in light.
Acknowledgement
This work was supported by BSR Faculty Fellowship from the University Grants Commission to BCT.
Conflict of Interest
The authors declare that they have no conflict of interest
References
Ambastha V Sopory SK Tiwari BS et al (2017) Photo-modulation of programmed cell death in rice leaves triggered by salinity Apoptosis Springer US 22(1) pp 41–56 doi: 10.1007/s10495-016-1305-7
Armstrong GA Runge S Frick G et al (1995) Identification of NADPH:Protochlorophyllide Oxidoreductases A and B: A Branched Pathway for Light-Dependent Chlorophyll Biosynthesis in Arabidopsis thaliana Plant Physiology 108(4) pp 1505–1517 doi: 10.1104/pp.108.4.1505
Baker NR (2008) Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo Annual Review of Plant Biology 59 (1) pp 89–113 doi: 10.1146/annurev.arplant.59.032607.092759
Baroli I Niyogi KK (2000) Molecular genetics of xanthophyll-dependent photoprotection in green algae and plants Philosophical Transactions of the Royal Society B: Biological Sciences 355(1402) pp 1385–1394. doi: 10.1098/rstb.2000.0700
Björkman O and Demmig B (1987) Photon yield of O 2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins Planta 170(4) pp. 489–504 doi: 10.1007/BF00402983.
Chakraborty N and Tripathy B C (1992) Involvement of Singlet Oxygen in 5-Aminolevulinic Acid-Induced Photodynamic Damage of Cucumber (Cucumis sativus) Chloroplasts Plant Physiology 98(1) pp 7–11 doi: 10.1104/pp.98.1.7
Dat J Vandenabeele S Vranova´ E et al (2000) Dual action of the active oxygen species during plant stress responses Cellular and Molecular Life Sciences 57(5) pp 779–795 doi: 10.1176/ajp.88.1.103
D’Alessandro S and Havaux M (2019) Sensing β‐carotene oxidation in photosystem II to master plant stress tolerance New Phytologist doi: 10.1111/nph.15924
Edelman M and Mattoo AK (2008) D1-protein dynamics in photosystem II: The lingering enigma, Photosynthesis Research 98(1–3) pp 609–620 doi: 10.1007/s11120-008-9342-x
Foote CS (1991) Definition of Type I and Type I1 Photochemistry and Photobiology 54(5) p 659 doi: 10.1111/j.1751-1097.1991.tb02071.x
Foyer CH and Shigeoka S (2011) Understanding Oxidative Stress and Antioxidant Functions to Enhance Photosynthesis Plant Physiology 155(1) pp 93–100 doi: 10.1104/pp.110.166181
Fujii S Kobayashi K Nagata N et al (2017) Monogalactosyldiacylglycerol Facilitates Synthesis of Photoactive Protochlorophyllide in Etioplasts Plant Physiology 174(4) pp 2183–2198 doi: 10.1104/pp.17.00304
Genty B Briantais J and Baker N (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence, Biochemica et Biophysica Acta 990(1) pp 87–92 doi: 10.1016/S0304-4165(89)80016-9
Kandoi D Mohanty S Govindjee et al. (2016) Towards efficient photosynthesis : overexpression of Zea mays phosphoenolpyruvate carboxylase in Arabidopsis thaliana Photosynthesis Research Springer Netherlands doi: 10.1007/s11120-016-0224-3
Krieger-Liszkay A Fufezan C and Trebst A (2008) Singlet oxygen production in photosystem II and related protection mechanism Photosynthesis Research 98(1–3) pp 551–564 doi: 10.1007/s11120-008-9349-3
Kwon C T Kim S H Song G et al (2017) Two NADPH: Protochlorophyllide Oxidoreductase (POR) Isoforms Play Distinct Roles in Environmental Adaptation in Rice Rice 10(1) pp 1–14 doi: 10.1186/s12284-016-0141-2
Laloi C and Havaux M (2015) Key players of singlet oxygen-induced cell death in plants Frontiers in plant science 6 pp 1–9 doi: 10.3389/fpls.2015.00039
Masuda T Fusada N Oosawa N et al (2003) Functional Analysis of Isoforms of NADPH:Protochlorophyllide Oxidoreductase (POR) PORB and PORC in Arabidopsis thaliana Plant and Cell Physiology 44(10) pp 963–974 doi: 10.1093/pcp/pcg128
Mohapatra A and Tripathy BC (2007) Differential distribution of chlorophyll biosynthetic intermediates in stroma Molecular genetics of xanthophyll-dependent photoprotection in green algae and plants Philosophical Transactions of the Royal Society envelope and thylakoid membranes in Beta vulgaris Photosynthesis Research 94(2–3) pp 401–410 doi: 10.1007/s11120-007-9209-6
Montoya S C N Comini L R Sarmiento M et al (2005) Natural anthraquinones probed as Type I and Type II photosensitizers: singlet oxygen and superoxide anion production Journal of Photochemistry and Photobiology B: Biology 78(1) pp 77–83 doi: https://doi.org/10.1016/j.jphotobiol.2004.09.009
Nishiyama Y Yamamoto H Allakhverdiev S I et al (2001) Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery 20(20) pp 1–8
Oosawa N Masuda T Awai K. et al (2000) Identification and light-induced expression of a novel gene of NADPH-protochlorophyllide oxidoreductase isoform in Arabidopsis thaliana FEBS Letters 474(2–3) pp 133–136. doi: 10.1016/S0014-5793(00)01568-4
Pattanayak GK and Tripathy BC (2002) Catalytic function of a novel protein protochlorophyllide oxidoreductase C of Arabidopsis thaliana Biochemical and Biophysical Research Communications 291(4) pp 921–924 doi: 10.1006/bbrc.2002.6543
Pattanayak GK and Tripathy BC (2011) Overexpression of Protochlorophyllide Oxidoreductase C Regulates Oxidative Stress in Arabidopsis PLoS one 6(10) doi: 10.1371/journal.pone.0026532
Ramel F Birtic S Cuine S et al (2012) Chemical quenching of singlet oxygen by carotenoids in plants Plant physiology 158(3) pp 1267–78 doi: 10.1104/pp.111.182394
Ramel F Ksas B Akkari E et al (2013) Light-induced acclimation of the Arabidopsis chlorina1 mutant to singlet oxygen The Plant cell 25(4) pp 1445–1462 doi: 10.1105/tpc.113.109827
Rebeiz C A Zouhoor A M Mayasich J M et al (1988) Photodynamic herbicides Recent developments and molecular basis of selectivity Critical Reviews in Plant Sciences 6(4) pp 385–436 doi: 10.1080/07352688809382256
Reinbothe S Reinbothe C Lebedev N et al (1996) POR A POR B Two light-dependent Protochlorophyllide-Reducing Enzymes of Angiosperm Chlorophyll Biosynthesis The Plant Cell 8 pp 763–769
Su Q Armstrong G and Apel K (2001) POR C of Arabidopsis thaliana : a third light- and NADPH-dependent protochlorophyllide oxidoreductase that is differentially regulated by light Plant Molecular Biology 47(2001) pp 805–813
Triantaphylides C Krischke M Hoeberichts F A et al (2008) Singlet Oxygen Is the Major Reactive Oxygen Species Involved in Photooxidative Damage to Plants Plant Physiology 148(2) pp 960–968 doi: 10.1104/pp.108.12569
Tripathy B C and Chakraborty N (1991) 5-Aminolevulinic Acid Induced Photodynamic Damage of the Photosynthetic Electron Transport Chain of Cucumber (Cucumis sativus ) Cotyledons Plant Physiology 96(3) pp 761–767 doi: 10.1104/pp.96.3.761
Tripathy BC Mohapatra A and Gupta I (2007) Impairment of the photosynthetic apparatus by oxidative stress induced by photosensitization reaction of protoporphyrin IX Biochimica et Biophysica Acta – Bioenergetics 1767(6) pp 860–868 doi: 10.1016/j.bbabio.2007.03.008
Tripathy BC and Oelmüller R (2012) Reactive oxygen species generation and signaling in plants Plant Signaling and Behavior 7(12) pp 1621–1633 doi: 10.4161/psb.22455
Tripathy BC and Pattanayak Gk (2010) Singlet Oxygen-Induced Oxidative Stress in Plants In Rebeiz CA et al (eds) The Chloroplast: Basics and Applications Dordrecht: Springer Netherlands pp 397–412 doi: 10.1007/978-90-481-8531-3_25
Vedalankar P and Tripathy BC (2019) Evolution of light-independent protochlorophyllide oxidoreductase Protoplasma pp 293–312
Yang B Tang J Yu Z et al (2019) Light Stress Responses and Prospects for Engineering Light Stress Tolerance in Crop Plants Journal of Plant Growth Regulation Springer US 0(0) p. 0. doi: 10.1007/s00344-019-09951-8


