Department of Oral and Maxillofacial Surgery, College of Dentistry, King Saud University, Riyadh, Saudi Arabia
Corresponding author email: oalghamdi@ksu.edu.sa
Article Publishing History
Received: 15/01/2020
Accepted After Revision: 10/03/2020
To evaluate the efficacy of enamel matrix derivative (EMD) with bioactive glass (BG) in the management of periodontal osseous defects. The addressed focused question was “Does the use of EMD with BG, improve its efficacy in the management of periodontal defects in comparison to EMD alone?” Databases were searched up to December 2019 using different combinations of MESH words. Six randomized clinical trials were included. One study showed significantly better periodontal outcomes for BG as an adjunct to EMD as compared to EMD alone. However, in two studies, improvement in the periodontal parameters for BG application as an adjunct to EMD and EMD alone were comparable. One clinical trial indicated significant improvement in clinical periodontal measures with the use of adjunctive EMD to BG compared with BG alone. However, one study showed equal outcomes between adjunctive EMD and BG alone. One study showed significant clinical improvement for BG compared with EMD. In conclusion, it remains unclear whether the efficacy of EMD in the management of periodontal osseous defects is improved when it is used in combination with BG as compared to when EMD is used alone given that the number of selected studies was relatively low and reported parameters were inconsistent.
Enamel Matrix Derivative; Bioactive Glass; Periodontal Osseous Defects; Systematic Review
Alghamdi O. Role of Novel Biomaterial Bioactive Glass with Enamel Matrix Derivative in Regeneration: A Systematic Review. Biosc.Biotech.Res.Comm. 2020;13(1).
Alghamdi O. Role of Novel Biomaterial Bioactive Glass with Enamel Matrix Derivative in Regeneration: A Systematic Review. Biosc.Biotech.Res.Comm. 2020;13(1). Available from: https://bit.ly/2S36Kye
Copyright © Alghamdi, This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Periodontitis is an inflammatory condition of periodontal tissues caused by complex oral biofilms and is characterized by irreversible periodontal tissue damage, which if not treated, may lead to tooth loss (Tonetti et al., 2018; Hajishengallis, 2015). This disease affects almost 50-90% of the global population and is considered one of the most common oral diseases and is the sixth most prevalent disease in the world (Preshaw et al., 2012). The putative microorganisms that includes Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, and Treponema denticola are responsible in the development of chronic periodontitis and activate innate, inflammatory, and adaptive immune responses (Van der Velden, 2017). As a result, this disease creates a local proinflammatory state and secretes a plethora of cytokine production which is manifested by dysregulated immune responses and results in periodontal tissue destruction (Akram et al., 2016).
Treatment of periodontitis aims to repair, regenerate and maintain the periodontal tissues. Multiple management strategies for periodontal disease have been utilized, including scaling and root planning (SRP) (Smiley et al., 2015), administration of local and systemic antibiotics (Keestra et al., 2015), photodynamic therapy (Akram et al., 2017), probiotic therapy (Ikram et al., 2018), metformin therapy (Akram et al., 2018), surgical intervention (Akram et al., 2019) and guided tissue regeneration (GTR) (Akizuki et al., 2005). Guided tissue regeneration (GTR), which is placement of barrier membranes and bone fillers or grafts in the periodontium (Bottino et al., 2012), aims to achieve the regeneration of lost periodontal tissues. Bioactive glass (BG), in particular, has been used as a contemporary alloplastic bone substitute to restore periodontal defects. It binds to natural bone and stimulates the regeneration of periodontal tissues in the implantation site (Hench, 2006). It induces the formation of a hydroxy carbonate apatite (HCA) layer, causing migration of osteoblasts to defect area, protein adsorption, incorporation of collagen fibrils, and attachment of stem cells and, therefore, regeneration of bone (Mondal et al., 2018).
Use of BG filler to restore periodontal defects requires much simpler surgical techniques as compared to using GTR membranes (Mengel et al., 2006; Yukna et al., 2001) and have demonstrated better bone regeneration when compared to surgical interventions alone (Zhang et al., 2016). In addition, when used with autogenous bone grafts, BG fillers have shown results comparable to autogenous grafts combined with hydroxyapatite (Galindo‐Moreno et al., 2008). Lately, enamel matrix derivative (EMD) has been used as an adjunct to surgical periodontics for the regeneration of lost periodontal bone (Miron et al., 2016). EMD comprises amelogenin and other proteins extracted from porcine fetal teeth and has shown to stimulate the regeneration of periodontal ligament (PDL) cells (Amin et al., 2016). In addition, it facilitates the proliferation and attachment of PDL cells such as fibroblasts, by stimulating the production and release of cyclic adenosine monophosphate levels, transforming growth factor-β, and interleukin-6 (Kawase et al., 2000; Lyngstadaas et al., 2001; Schwartz et al., 2000; der Pauw et al., 2000). It is hypothesized that the use of EMD in combination with conventional freeze-dried allografts could produce a synergistic effect in periodontal regeneration procedures. In a study by Sculean et al. (2005a), patients with intrabony defects treated with EMD as an adjunct to BG demonstrated substantial improvement in periodontal measures compared with BG alone. However, Sculean et al., (2002) in a clinical trial comparing the effect of EMD combined with BG and EMD alone in the management of periodontal osseous defects, concluded that all patients showed comparable clinical outcomes at follow up regardless of the materials used. Hence, there appears to be a debate and contradictory results in terms of the purpose of EMD with and without BG in the treatment of periodontal defects and therefore, a systemic review is deemed necessary. This review aims to systematically evaluate the efficacy of EMD in combination with BG in the management of periodontal osseous defects.
MATERIALS AND METHODS
Systematic review question and protocol: This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009). The focused systematic review question was “Does the use of EMD, when used with BG, improve its efficacy in the management of periodontal defects in comparison to EMD or BG used alone?”
Eligibility criteria: To conduct the systematic review, following criteria were considered: (a) Randomized controlled clinical trials (RCTs) in humans (b) Trials evaluating efficacy of EMD and BG in the treatment of intrabony defect. (c) Studies reporting pocket depth (PD), clinical attachment loss (CAL) as primary outcomes and gingival recession (REC), plaque index (PI), gingival index (GI) or bleeding on probing (BOP) as secondary outcomes and (d) English language articles only. The studies were excluded if they had in vitro or experimental design, letters to the editor, review papers and unpublished articles.
Search: The author searched the PUBMED, EMBASE, and CENTRAL databases up to December 2019 for appropriate articles addressing the focused question. A structured approach to literature searching was used to identify the relevant papers that directly compare the efficacy of EMD with or without BG in subjects with the presence of at least one intra-bony defect. Following that, reference lists of original studies were hand-searched to identify any articles that could have been missed during the initial search. Hand searching of the following journals was performed: Journal of Clinical Periodontology, Journal of Periodontology, and Journal of Periodontal Research. Different combinations of MeSH (Medical Subject Headings) terms were considered: enamel matrix derivative; enamel matrix protein; bioactive glass; bioglass; ceramics; intrabony defect; intraosseous defect.
Screening methods and data abstraction: Titles and abstracts of articles that satisfied the selection protocol were screened and checked for agreement. Thereafter, the full-text screening was done. The information from the accepted studies was tabulated according to the (1) study design, (2) demographic characteristics of study participants, (3) study groups, (4) intrabony defect, (5) assessed periodontal parameters, (6) subjects follow up, and (7) main outcome. The kappa value for the intra-assessor agreement was 0.92.
Quality of the studies: The methodological quality of the included studies according to a grading system was developed using the Jadad scale (Jadad et al., 1996).
RESULTS AND DISCUSSION
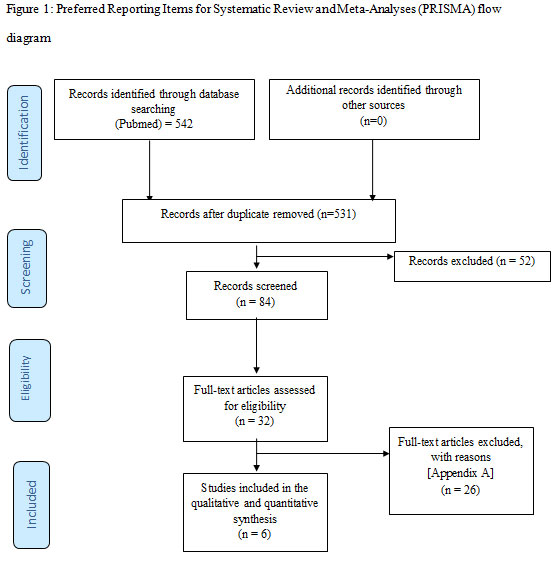
Study selection: A total of 542 studies were initially identified. Twenty-six studies which did not fulfill the eligibility criteria after full-text screening were excluded (Appendix A). In total, 6 studies (Kuru et al., 2006; Leknes et al., 2009; Sculean et al., 2002; Sculean et al., 2007; Sculean et al., 2005a; Sculean et al., 2005b) were included and processed for data extraction. All studies (Kuru et al., 2006; Leknes et al., 2009; Sculean et al., 2002; Sculean et al., 2007; Sculean et al., 2005a; Sculean et al., 2005b) were performed at either universities or health care centers. Figure 1 shows the PRISMA study identification flow chart.
Figure 1: Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) flow diagram
General characteristics of the selected articles: All 6 studies (Kuru et al., 2006; Leknes et al., 2009; Sculean et al., 2002; Sculean et al., 2007; Sculean et al., 2005a; Sculean et al., 2005b) included in the present systematic review were RCTs. The total number of patients in these clinical trials ranged between 6 and 30 individuals (Kuru et al., 2006; Leknes et al., 2009; Sculean et al., 2002; Sculean et al., 2007; Sculean et al., 2005a; Sculean et al., 2005b). Only two studies (Leknes et al., 2009; Sculean et al., 2007) reported the mean age of study participants, which was 46.1 and 52.5 (age range 38 to 74). Five clinical studies (Leknes et al., 2009; Sculean et al., 2002; Sculean et al., 2007; Sculean et al., 2005a; Sculean et al., 2005b) reported the number of female participants, which ranged from 6 to 16 individuals. Five studies (Sculean et al., 2002; Sculean et al., 2007; Sculean, et al., 2005a; Sculean et al., 2005b) used EMD and BG in the test group while one study (Leknes et al., 2009) used BG alone in the test group. In the control group, four studies (Kuru et al., 2006; Leknes et al., 2009; Sculean et al., 2007; Sculean et al., 2005b) used EMD while two studies (Sculean et al., 2002; Sculean, et al., 2005a) used BG (Table 1). In all the studies (Kuru et al., 2006; Leknes et al., 2009; Sculean et al., 2002; Sculean et al., 2007; Sculean, et al., 2005a; Sculean, et al., 2005b) the follow-up period ranged from 24 – 192 weeks. In one study (Sculean et al., 2007), 16.66% of patients dropped out of the trial. Smokers were in included in studies by Sculean et al and Leknes et al respectively (Leknes et al., 2009; Sculean et al., 2002). All the enrolled participants had a complication-free healing period with no side-effects related to BG and EMD.
Table 1: General characteristics of the selected studies.
| Investigator, year | Study design | Sample size (Female %) | Mean age range
(in years) |
Study groups | Follow-up
(weeks) |
Main outcome | |
| Test
(n) |
Control
(n) |
||||||
| Sculean et al.
2002 |
RCT | 28 (53.5) | NA | EMD+BG
(14) |
BG
(14) |
48 | Equal improvements in clinical parameters for both groups at follow-up |
| Kuru et al.
2006 |
RCT | 23 (NA) | NA | EMD+BG
(13) |
EMD
(10) |
32 | Clinical parameters were significantly better for test group as compared to control at follow-up |
| Sculean et al.
2005a |
RCT | 30 (53.3) | NA | EMD+BG
(15) |
EMD
(15) |
48 | Improvements in clinical parameters for both groups were comparable at follow-up |
| Sculean et al.
2005b |
RCT* | 6 (100) | NA | EMD+BG | BG | 24 | Clinical parameters were significantly better for test group as compared to control at follow-up |
| Leknes et al. 2009 | RCT* | 13 (61.5) | 52.5
(41 – 74) |
BG
(13) |
EMD
(13) |
48 | Clinical parameters were significantly better for test group as compared to control at follow-up |
| Sculean et al.
2007 |
RCT | 25 (56) | 46
(38 – 55) |
EMD+BG
(12) |
EMD
(13) |
192 | Improvements in clinical parameters for both groups were comparable at follow-up |
RCT; randomized clinical trial, *Split-mouth technique, EMD; enamel matrix derivative, BG; bioactive glass, PD; pocket depth
NA; not available
Clinical periodontal parameters of included studies: All clinical studies (Kuru et al., 2006; Leknes et al., 2009; Sculean et al., 2002; Sculean et al., 2007; Sculean, et al., 2005a; Sculean et al., 2005a; Sculean et al., 2005b) described clinical periodontal measures (Table 2). In four studies (Kuru et al., 2006; Sculean et al., 2002; Sculean et al., 2007; Sculean, et al., 2005a), the mean PI ranged from 0.18 to 0.9 at follow up. In four studies (Kuru et al., 2006; Sculean et al., 2002; Sculean et al., 2007; Sculean, et al., 2005a) gingival index was reported, which was 0.17 to 0.9 at follow-up. Three studies (Sculean et al., 2002; Sculean et al., 2007; Sculean, et al.,2005a) reported BOP in percentage which ranged from 22% to 43%. In 5 studies, the mean PD ranged from 1.0 mm to 5.73 mm at follow-up. All six studies (Kuru et al., 2006; Leknes et al., 2009; Sculean et al., 2002; Sculean et al., 2007; Sculean, et al., 2005a; Sculean et al., 2005b) reported CAL and five studies (Kuru et al., 2006; Leknes et al., 2009; Sculean et al., 2002; Sculean et al., 2007; Sculean et al., 2005a) reported REC which ranged from 6.3 mm to 13.7 mm and 2.4 mm to 6.5 mm respectively at follow-up. Relative bone loss was reported by only one study (Kuru et al., 2006).
Table 2: Clinical periodontal parameters of the included studies.
| Authors | PD (mm) | CAL (mm) | REC (mm) | PI | GI | BOP (%) | RBL (mm) |
| Sculean et al.
2002 |
EMD+BG
Baseline: 8.07±1.14 Follow up: 3.92±0.73 BG Baseline: 8.07±1.32 Follow up: 3.85±0.66 |
EMD+BG
Baseline: 9.64±1.59 Follow up: 6.42±1.08 BG Baseline: 9.78±1.71 Follow up: 6.71±1.89 |
EMD+BG
Baseline: 1.50±1.16 Follow up: 2.50±1.08 BG Baseline: 1.64±0.74 Follow up: 2.92±1.85 |
EMD+BG
Baseline: 0.9±0.5 Follow up: 0.6±0.4 BG Baseline: 0.8±0.7 Follow up: 0.7±0.4 |
EMD+BG
Baseline: 1.8±0.9 Follow up: 0.8±0.7 BG Baseline: 2.1±1.9 Follow up: 0.9±0.6 |
EMD+BG:
Baseline: 60 Follow up: 40 BG: Baseline: 58 Follow up: 43 |
NA |
| Kuru et al.
2006 |
EMD+BG
Baseline: 9.77±1.01 Follow up: 5.73±0.80 EMD Baseline: 9.47±0.81 Follow up: 5.03±0.89 |
NA | NA | EMD+BG
Baseline: 0.30±0.05 Follow up: 0.19±0.05 EMD Baseline: 0.29±0.06 Follow up: 0.18±0.05 |
EMD+BG
Baseline: 0.30±0.06 Follow up: 0.17±0.05 EMD Baseline: 0.27±0.04 Follow up: 0.19±0.02 |
NA | EMD+BG:
Baseline: 6.24±0.78 Follow up: 2.76±0.69 EMD: Baseline: 6.38±0.62 Follow up: 2.15±0.42 |
| Sculean et al.
2005a |
EMD+BG
Baseline: 8.5±1.1 Follow up: 4.4±1.2 EMD Baseline: 8.5±1.5 Follow up: 4.0±1.6 |
EMD+BG
Baseline: 10.4±1.5 Follow up: 7.1±1.5 EMD Baseline: 10.2±2.1 Follow up: 6.3±2.2 |
EMD+BG
Baseline: 1.9±1.1 Follow up: 2.8±0.9 EMD Baseline: 1.5±1.4 Follow up: 2.4±1.6 |
EMD+BG
Baseline: 0.5±0.3 Follow up: 0.4±0.4 EMD Baseline: 0.4±0.2 Follow up: 0.4±0.3 |
EMD+BG
Baseline: 1.2±0.4 Follow up: 0.5±0.4 EMD Baseline: 1.1±0.3 Follow up: 0.4±0.4 |
EMD+BG:
Baseline: 52 Follow up: 28 EMD: Baseline: 50 Follow up: 22 |
NA |
| Sculean et al.
2005b |
NA | EMD+BG§
Baseline: 11-13 Follow up: 7-8 BG¶ Baseline: 9-14 Follow up: 7-11 |
NA | NA | NA | NA | NA |
| Leknes et al. 2009 | EMD:
Buccal: Baseline: 1.1±0.5 Follow up: 1.1±0.4
Lingual: Baseline: 3.8±2.8 Follow up: 2.1±1.3
Proximal: Baseline: 6.5±1.3 Follow up: 4.0±2.2 BG: Buccal: Baseline: 1.1±0.5 Follow up: 1.0±0.6
Lingual: Baseline: 3.3±1.8 Follow up: 2.5±1.5
Proximal: Baseline: 6.9±1.6 Follow up: 4.3±1.6 |
EMD:
Buccal: Baseline: 11.2±2.5 Follow up: 12.2±2.9
Lingual: Baseline: 12.0±3.2 Follow up: 11.9±2.7
Proximal: Baseline: 14.2±2.4 Follow up: 13.6±2.7 BG: Buccal: Baseline: 11.4±2.8 Follow up: 11.6±3.1
Lingual: Baseline: 12.6±2.8 Follow up: 11.7±2.2
Proximal: Baseline: 14.9±3.0 Follow up: 13.7±2.9 |
EMD:
Buccal: Baseline: 3.5±1.0 Follow up: 5.3±2.2
Lingual: Baseline: 3.8±1.6 Follow up: 5.0±1.6
Proximal: Baseline: 4.2±1.3 Follow up: 6.5±1.8 BG: Buccal: Baseline: 4.4±2.1 Follow up: 5.0±1.8
Lingual: Baseline: 4.5±1.6 Follow up: 5.5±1.6
Proximal: Baseline: 5.5±1.6 Follow up: 6.2±1.3 |
NA | NA | NA | NA |
| Sculean et al.
2007 |
EMD+BG
Baseline: 8.6±1.0 Follow up: 4.1±1.0 EMD Baseline: 8.6±0.9 Follow up: 3.9±0.6 |
EMD+BG
Baseline: 10.3±1.6 Follow up: 6.7±1.2 EMD Baseline: 10.4±1.6 Follow up: 6.7±1.1 |
EMD+BG
Baseline: 1.7±1.0 Follow up: 2.6±0.9 EMD Baseline: 1.8±1.2 Follow up: 2.8±1.0 |
EMD+BG
Baseline: 0.8±0.4 Follow up: 0.9±0.4 EMD Baseline: 0.7±0.5 Follow up: 0.7±0.5 |
EMD+BG
Baseline: 1.7±0.5 Follow up: 0.6±0.4 EMD Baseline: 1.8±0.8 Follow up: 0.8±0.6 |
EMD+BG:
Baseline: 53 Follow up: 34 EMD: Baseline: 49 Follow up: 36 |
NA |
EMD; enamel matrix derivative, BG; bioactive glass, PD; pocket depth, CAL; clinical attachment loss, REC; recession, PI; plaque index, GI; gingival index, RBL; relative bone loss, BOP; bleeding on probing; NA; not available.
treatment on tooth #19, #30, #31
¶ treatment on tooth #19, #4, #3
The main outcome of the studies: All studies (Kuru et al., 2006; Leknes et al., 2009; Sculean et al., 2002; Sculean et al., 2007; Sculean, et al., 2005b; Sculean, et al., 2005b) that reported periodontal indices demonstrated that EMD was successful in intrabony periodontal osseous defects at follow-up. Among these clinical studies (Kuru et al., 2006; Leknes et al., 2009; Sculean et al., 2002; Sculean et al., 2007; Sculean, et al., 2005a; Sculean, et al., 2005b), one study (Kuru et al., 2006) showed significantly better periodontal outcomes for BG as an adjunct to EMD as compared to EMD alone. However, two studies by Sculean et al., (2007; 2005a) demonstrated equal improvement for BG as an adjunct to EMD and EMD alone. Sculean et al. (2005b), showed significantly better clinical measures for EMD as an adjunct to BG as compared to BG alone. However, one study (Sculean et al., 2002) showed equal improvement in periodontal indices for adjunctive EMD and BG alone. Leknes et al. (2009) reported significant improvement in clinical periodontal parameters with BG compared with EMD in intrabony osseous defects.
Quality of the clinical studies: All clinical studies in this systematic review were RCTs. Four studies used coin toss method for randomization (Kuru et al., 2006; Leknes et al., 2009; Sculean et al., 2007; Sculean et al., 2005a). Five studies (Kuru et al., 2006; Leknes et al., 2009; Sculean et al., 2002; Sculean et al., 2007; Sculean et al., 2005a a) reported the power analysis. The quality of one study was regarded as high (Leknes et al., 2009) and hence this study received a score of 5. Three studies were graded as moderate receiving a score of 3 (Kuru et al., 2006; Sculean et al., 2007; Sculean, et al., 2005a), whereas two studies were graded as poor receiving a score of 1 (Sculean et al., 2002; Sculean et al., 2005b). A summary of the quality scoring of the studies is presented in Table 3.
Table 3: Assessing the quality of included RCTs using the Jadad scale.
| Reference | Randomization | Blinding | An account of all patients | Total score | ||
| Sculean et al.
2002 |
0 | 0 | 0 | 0 | +1 | 1 |
| Kuru et al.
2006 |
+1 | +1 | 0 | 0 | +1 | 3 |
| Sculean et al.
2005a |
+1 | +1 | 0 | 0 | +1 | 3 |
| Sculean et al.
2005b |
0 | 0 | 0 | 0 | +1 | 1 |
| Leknes et al. 2009 | +1 | +1 | +1 | +1 | +1 | 5 |
| Sculean et al.
2007 |
+1 | +1 | 0 | 0 | +1 | 3 |
The present review was based on the hypothesis of whether EMD when used with BG, improves with efficacy in the management of periodontal osseous defects in comparison to EMD alone. All studies (Kuru et al., 2006; Leknes et al., 2009; Sculean et al., 2002; Sculean et al., 2007; Sculean et al., 2005a; Sculean, 2005b) included in the present systematic review showed that EMD was effective in the treatment of intrabony periodontal osseous defects at follow-up.Guided tissue regeneration (GTR) and grafting have been used extensively since the last few decades to restore periodontal defects (Bottino et al., 2009; Bottino et al., 2012). It has been observed in multiple RCTs that GTR is more effective than open-flap debridement in treating periodontal defects (Jepsen et al., 2002; Murphy & Gunsolley, 2003). EMD, a GTR material that is primarily composed of porcine amelogenin, is more effective in restoring clinical attachment levels and radiographic bone when compared to flap procedures (Amin et al., 2016). In addition to the clinical trials (Esposito et al.,2009), in vitro as well as in vivo studies have shown that EMD stimulates the proliferation of pre-osteoblastic cells (Boyan et al., 2000; Schwartz et al., 2000). Although a systematic review of using EMD against other types of GTR materials and periodontal treatments found it to be clinically effective, it did not include any studies which used EMD in combination with BG.
Studies included in this review assessed the clinical effectiveness of using EMD with BG and compared that with the use of BG or EMD alone. Comparable clinical periodontal parameters were observed among the studies using EMD with BG and those using BG or EMD alone (Kuru et al., 2006; Sculean et al., 2002; Sculean et al., 2007; Sculean, et al., 2005a; Sculean et al., 2005b). However, it is appropriate to mention that there was inconsistency observed among the studies with regards to study groups included and parameters measured. A major shortcoming of these studies is the limited follow-up period of the treated patients. Only one study (Sculean et al., 2005a) followed-up patients for up to 192 weeks while the remaining studies followed up patients for a period of only 12 to 48 weeks (Kuru et al., 2006; Leknes et al., 2009; Sculean et al., 2002; Sculean et al., 2007; Sculean 2005b). In addition, smoking harms periodontal health (Preber & BergstrÖM, 1986) and quitting smoking can improve the outcomes of periodontal treatment (Patel et al., 2012). Given this, in only 2 studies included in this review, smokers were included (Leknes et al., 2009; Sculean et al., 2002). Therefore, further studies with follow up of longer duration and strict inclusion and exclusion criteria are needed to assess the long-term efficacy of using EMD with BG in the management of periodontal osseous defects.
Only half of the studies (Sculean et al., 2002; Sculean et al., 2007; Sculean et al., 2005a) assessed in the present review reported all major clinical periodontal parameters (PD, CAL, REC, PI, GI, and BOP) and only one study (Kuru et al., 2006) assessed RBL. A comparison of only clinical parameters in the absence of RBL does not allow accurate prediction of periodontal outcomes (Haffajee et al., 1983). In addition, none of the studies assessed the subgingival microbial flora of the treated periodontal defects which is essential for an accurate surrogate assessment of periodontal recovery. Also, studies aimed at comparing the effectiveness of EMD+BG and BG or EMD through the use of biomarkers present in oral fluids are recommended (Taba., et al 2005) to accurately assess the efficacy of these GTR materials.
The study by Sculean et al. (2005b) also assessed the use of EMD+BG histologically and observed that healing around EMD+BG showed more mineralized tissue, PDL, and cementum. Conversely, when BG was used alone, more epithelial cellular growth was seen, suggesting that EMD+BG is more effective than BG. This lack of bone of formation around BG has been observed previously as well. Nevins et al observed that using BG to fill periodontal defects (Nevins et al., 2000) resulted in cellular growth that was characterized by the formation of junctional epithelium and limited formation of mineralized tissue and clinical attachment. The findings suggest that using BG alone decreases the likelihood of the formation of mineralized tissue. However, these histological observations are in contrast to the clinical findings shown in this systematic review i.e. comparable clinical outcomes for the use of EMD with and without BG in the management of periodontal osseous defects. Therefore, further randomized controlled trials aiming to assess the clinical and histological outcomes of the use of EMD with and without BG in the management of periodontal osseous defects are recommended.
CONCLUSION
It remains unclear whether the efficacy of EMD in the management of periodontal osseous defects is improved when it is used in combination with BG as compared to when EMD is used alone given that the number of selected studies was relatively low and reported parameters were inconsistent.
Conflict of interest statement: No conflict of interest
REFERENCES
Akizuki T, Oda S, Komaki M, Tsuchioka H, Kawakatsu N, Kikuchi A, Yamato M, Okano T, Ishikawa I. (2005) Application of periodontal ligament cell sheet for periodontal regeneration: A pilot study in beagle dogs. J. Periodontal Res. Vol 40: Pages 245-251.
Akram, Z., Abduljabbar, T., Hassan, A., Ibrahim, M., Javed, F., & Vohra, F. (2016) Cytokine profile in chronic periodontitis patients with and without obesity: a systematic review and meta-analysis. Dis. Markers Vol. 2016: Pages 4801418.
Akram, Z., Hyder, T., Al-Hamoudi, N., Binshabaib, M. S., Alharthi, S. S., & Hanif, A. (2017) Efficacy of photodynamic therapy versus antibiotics as an adjunct to scaling and root planing in the treatment of periodontitis: a systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. Vol. 19: Pages 86-92.
Akram, Z., Vohra, F., & Javed, F. (2018). Locally delivered metformin as adjunct to scaling and root planing in the treatment of periodontal defects: A systematic review and meta‐analysis. J. Periodontal Res. Vol. 53 No 6: Pages 941-949.
Akram, Z., Shafqat, S. S., Niaz, M. O., Raza, A., & Naseem, M. (2019) Clinical efficacy of photodynamic therapy and laser irradiation as an adjunct to open flap debridement in the treatment of chronic periodontitis: A systematic review and meta‐analysis. Photodermatol. Photoimmunol. Photomed.
Amin, H. D., Olsen, I., Knowles, J., Dard, M., & Donos, N. (2016). Interaction of enamel matrix proteins with human periodontal ligament cells. Clin. Oral Investig. Vol 20 No 2: Pages 339-347.
Bottino, M. C., Jose, M. V., Thomas, V., Dean, D. R., & Janowski, G. M. (2009). Freeze-dried acellular dermal matrix graft: Effects of rehydration on physical, chemical, and mechanical properties. Dent. Mater. Vol 25: Pages 1109-1115.
Bottino, M. C., Thomas, V., Schmidt, G., Vohra, Y. K., Chu, T.-M. G., Kowolik, M. J., & Janowski, G. M. (2012). Recent advances in the development of gtr/gbr membranes for periodontal regeneration-a materials perspective. Dent. Mater. Vol 28: Pages 703-721.
Boyan, B. D., Weesner T. C. , Lohmann C. H., Andreacchio D., Carnes DL, Dean DD, Cochran DL & Schwartz Z. (2000). Porcine fetal enamel matrix derivative enhances bone formation induced by demineralized freeze dried bone allograft in vivo. J. Periodontol. Vol 71: Pages 1278-1286.
Chitsazi, M.-T., Shirmohammadi, A., Faramarzie, M., Pourabbas, R., & Rostamzadeh, A. N. (2011). A clinical comparison of nano-crystalline hydroxyapatite (ostim) and autogenous bone graft in the treatment of periodontal intrabony defects. Med Oral Patol Oral Cir Bucal. Vol 16: Pages 448-453.
Eppley, B. L., Pietrzak, W. S., & Blanton, M. W. (2005). Allograft and alloplastic bone substitutes: A review of science and technology for the craniomaxillofacial surgeon. J. Craniofac. Surg. Vol 16: Pages 981-989.
Esposito, M., Grusovin, M. G., Papanikolaou, N., Coulthard, P., & Worthington, H. V. (2009) Enamel matrix derivative (Emdogain®) for periodontal tissue regeneration in intrabony defects. Cochrane Database of Systematic Reviews, Vol. 4.
Galindo‐Moreno, P., Ávila, G., Fernández‐Barbero, J. E., Mesa, F., O’Valle‐Ravassa, F., & Wang, H. L. (2008). Clinical and histologic comparison of two different composite grafts for sinus augmentation: A pilot clinical trial. Clin Oral Implants Res. Vol 19: Pages 755-759.
Hajishengallis, G. (2015) Periodontitis: from microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. Vol 15 No 1: Pages 30-44.
Hench, L. L. (2006). The story of bioglass®. J Mater Sci Mater Med. Vol 17: Pages 967-978.
Ikram, S., Hassan, N., Raffat, M. A., Mirza, S., & Akram, Z. (2018) Systematic review and meta‐analysis of double‐blind, placebo‐controlled, randomized clinical trials using probiotics in chronic periodontitis. J. Investig. Clin. Dent. Vol 9 No 3: Pages e12338.
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J. M., Gavaghan, D. J., & McQuay, H. J. (1996) Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin. Trials Vol 17: Pages 1-12.
Jepsen, S., Eberhard, J., Herrera, D., & Needleman, I. (2002) A systematic review of guided tissue regeneration for periodontal furcation defects. What is the effect of guided tissue regeneration compared with surgical debridement in the treatment of furcation defects? J. Clin. Periodontol. Vol 29: Pages 103-116.
Kawase, T., Okuda, K., Yoshie, H., & Burns, D. M. (2000) Cytostatic action of enamel matrix derivative (emdogain®) on human oral squamous cell carcinoma‐derived scc25 epithelial cells. J. Periodontal Res. Vol 35: Pages 291-300.
Keestra, J. A. J., Grosjean, I., Coucke, W., Quirynen, M., & Teughels, W. (2015) Non‐surgical periodontal therapy with systemic antibiotics in patients with untreated chronic periodontitis: A systematic review and meta‐analysis. J. Periodontal Res. Vol 50: Pages 294-314.
Kuru, B., Yılmaz, S., Argın, K., & Noyan, Ü. (2006) Enamel matrix derivative alone or in combination with a bioactive glass in wide intrabony defects. Clin. Oral Investig. Vol 10: Pages 227-234.
Leknes, K. N., Andersen, K.-M., Bøe, O. E., Skavland, R. J., & Albandar, J. M. (2009) Enamel matrix derivative versus bioactive ceramic filler in the treatment of intrabony defects: 12-month results. J. Periodontol. Vol 80: Pages 219-227.
Lyngstadaas, S. P., Lundberg, E., Ekdahl, H., Andersson, C., & Gestrelius, S. (2001) Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J. Clin. Periodontol. Vol 28: Pages 181-188.
Mengel, R., Schreiber, D., & Flores-de-Jacoby, L. (2006) Bioabsorbable membrane and bioactive glass in the treatment of intrabony defects in patients with generalized aggressive periodontitis: Results of a 5-year clinical and radiological study. J. Periodontol. Vol 77: Pages 1781-1787.
Miron, R. J., Chandad, F., Buser, D., Sculean, A., Cochran, D. L., & Zhang, Y. (2016) Effect of enamel matrix derivative liquid on osteoblast and periodontal ligament cell proliferation and differentiation. J. Periodontol. Vol 87 No 1: Pages 91-99.
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009) Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. Ann. Intern. Med. Vol 151: Pages 264-269.
Mondal, S., Hoang, G., Manivasagan, P., Moorthy, M. S., Nguyen, T. P., Phan, T. T. V., … & Oh, J. (2018) Nano-hydroxyapatite bioactive glass composite scaffold with enhanced mechanical and biological performance for tissue engineering application. Ceramics Int. Vol 44 No 13: Pages 15735-15746.
Murphy, K. G., & Gunsolley, J. C. (2003) Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects. A systematic review. Ann. Periodontol. Vol 8: Pages 266-302.
Nevins, M. L., Camelo, M., Nevins, M., King, C. J., Oringer, R. J., Schenk, R. K., & Fiorellini, J. P. (2000) Human histologic evaluation of bioactive ceramic in the treatment of periodontal osseous defects. Int. J. Periodontics Restorat. Dent. Vol 20: Pages 458-467.
Patel, R. A., Wilson, R. F., & Palmer, R. M. (2012) The effect of smoking on periodontal bone regeneration: A systematic review and meta-analysis. J Periodontol. 83: Pages 143-155.
Preber, H., & BergstrÖM, J. A. N. (1986) Cigarette smoking in patients referred for periodontal treatment. Eur. J. Oral Sci. Vol 94: Pages 102-108.
Preshaw, P. M., Alba, A. L., Herrera, D., Jepsen, S., Konstantinidis, A., Makrilakis, K., & Taylor, R. (2012) Periodontitis and diabetes: A two-way relationship. Diabetol. Vol 55: Pages 21-31.
Reynolds, M. A., Aichelmann-Reidy, M. E., & Branch-Mays, G. L. (2010) Regeneration of periodontal tissue: Bone replacement grafts. Dent. Clin. North Am. Vol 54: Pages 55-71.
Schwartz, Z., Carnes Jr, D.L., Pulliam, R., Lohmann, C.H., Sylvia, V.L., Liu, Y., Dean, D.D., Cochran, D.L. & Boyan, B.D. (2000) Porcine fetal enamel matrix derivative stimulates proliferation but not differentiation of pre-osteoblastic 2t9 cells, inhibits proliferation and stimulates differentiation of osteoblast-like mg63 cells, and increases proliferation and differentiation of normal human osteoblast NHOst cells. J. Periodontol. Vol 71: Pages 1287-1296.
Sculean, A., Barbé, G., Chiantella, G. C., Arweiler, N. B., Berakdar, M., & Brecx, M. (2002) Clinical evaluation of an enamel matrix protein derivative combined with a bioactive glass for the treatment of intrabony periodontal defects in humans. J. Periodontol. Vol 73: Pages 401-408.
Sculean, A., Pietruska, M., Arweiler, N. B., Auschill, T. M., & Nemcovsky, C. (2007) Four‐year results of a prospective‐controlled clinical study evaluating healing of intra‐bony defects following treatment with an enamel matrix protein derivative alone or combined with a bioactive glass. J. Clin. Periodontol. Vol 34: Pages 507-513.
Sculean, A., Pietruska, M., Schwarz, F., Willershausen, B., Arweiler, N. B., & Auschill, T. M. (2005a) Healing of human intrabony defects following regenerative periodontal therapy with an enamel matrix protein derivative alone or combined with a bioactive glass. J. Clin. Periodontol. Vol 32: Pages 111-117.
Sculean, A., Windisch, P., Keglevich, T., & Gera, I. (2005b) Clinical and histologic evaluation of an enamel matrix protein derivative combined with a bioactive glass for the treatment of intrabony periodontal defects in humans. Int. J. Periodontics Restorat. Dent. Vol 25: Pages 139-147.
Smiley, C. J., Tracy, S. L., Abt, E., Michalowicz, B. S., John, M. T., Gunsolley, J., . . . Forrest, J. L. (2015) Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J. Am. Dent. Assoc. Vol 146: Pages 508-524.
Taba, M., Kinney, J., Kim, A. S., & Giannobile, W. V. (2005) Diagnostic biomarkers for oral and periodontal diseases. Dent. Clin. North Am. Vol 49: Pages 551-571.
Tonetti, M. S., Greenwell, H., & Kornman, K. S. (2018) Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. Vol 45: Pages: S149-S161.
Van der Pauw, M. T., Van den Bos, T., Everts, V., & Beertsen, W. (2000) Enamel matrix-derived protein stimulates attachment of periodontal ligament fibroblasts and enhances alkaline phosphatase activity and transforming growth factor β1 release of periodontal ligament and gingival fibroblasts. J. Periodontol. Vol 71: Pages 31-43.
Van der Velden, U. (2017) What exactly distinguishes aggressive from chronic periodontitis: is it mainly a difference in the degree of bacterial invasiveness?. Periodontol. 2000 Vol. 75 No 1: Pages 24-44.
Yukna, R. A., Evans, G. H., Aichelmann-Reidy, M. B., & Mayer, E. T. (2001) Clinical comparison of bioactive glass bone replacement graft material and expanded polytetrafluoroethylene barrier membrane in treating human mandibular molar class ii furcations. J. Periodontol. Vol 72: Pages 125-133.
Zhang, X., Zeng, D., Li, N., Wen, J., Jiang, X., Liu, C., & Li, Y. (2016) Functionalized mesoporous bioactive glass scaffolds for enhanced bone tissue regeneration. Sci. Reports Vol 6: Pages 19361.