1Department of Crop Protection – Faculty of Agriculture – Nile Valley University Atbara, Sudan
2Department of Biology, College of Science, University of Hail, Hail, Saudi Arabia
3Department of botany and agricultural biotechnology – Faculty of
Agriculture, University of Khartoum Sudan
4Department of plant production and processing technology-Faculty of Agricultural
Production and Processing Technology, International University of Africa
5Department Health Information Technology -Medical Sterilization
Applied College- King Abdel Aziz University, Jedda Saudi Arabia
6Department of Biotechnology- National Research Center
7Department of Research and Training, Research and Training Station,
King Faisal University, Al-Ahsa , Saudi Arabia
Corresponding author email:rndabyrm@gmail.com
Article Publishing History
Received: 14/08/2023
Accepted After Revision: 25/12/2023
Most terrestrial plants respond to colonization by symbiotic mycorrhizal fungi, and these fungi have various benefits to their hosts under different stress conditions, especially phosphorus (P) limitation. A pot experiment was conducted to determine how tomato seedlings were affected by varying levels of mycorrhiza species under phosphorus deficiency conditions. A block experiment based on a completely randomized design was conducted in pots. Seeds of the tomato (Solanum lycopersicum L.) cultivar Beeli were inoculations with two species of arbuscular mycorrhizal (AM), namely; Glomus mosseae (MG), locally-isolated mycorrhizal spores (ML), Fusarium oxysporum f. sp. lycopersici (FOL1) pathogen, the myco-parasitic fungus (Trichoderma harzianum) (Th) and effective microorganisms (EM@TM) at two different time 6 and 13 weeks. Results showed that the above-ground dry matter of inoculated Tomato at both.
Arbuscular Mycorrhizal, Phosphorus Deficiency, Tomato Seedlings,
Fusarium Oxysporum F. Sp. Lycopersici
Bairum R.S, Sulieman A.M.E, Mahdi A. A, Mohamedelnour A, Sherfi S.A, Elamin H.B, Aloufi B, Almuzaini N, Salih Z.A. Role of Mycorrhiza Colonization in Phosphorus Deficiency in Tomato Seedlings Affected By Different Levels of Mycorrhiza Species. Biosc.Biotech.Res.Comm. 2023;16(4).
Bairum R.S, Sulieman A.M.E, Mahdi A. A, Mohamedelnour A, Sherfi S.A, Elamin H.B, Aloufi B, Almuzaini N, Salih Z.A. Role of Mycorrhiza Colonization in Phosphorus Deficiency in Tomato Seedlings Affected By Different Levels of Mycorrhiza Species. Biosc.Biotech.Res.Comm. 2023;16(4). Available from: <a href=”http://surl.li/pegft“>http://surl.li/pegft</a>
INTRODUCTION
Tomato (Solanum lycopersicum L.), with an annual production of 160 million tons, is one of the world’s leading vegetables used in raw and processed forms (almost 40 million tons of tomatoes annually).Tomatoes are subject to many pests and diseases from the time of emergence to harvest. Among these, diseases incited by Fusarium are responsible for significant reductions in tomato quality and yield every year. Because the impact of tomato diseases cannot be predicted from one year to the next, certain precautions must be taken yearly to ensure maximum fruit production with minimum Fusarium wilt occurrence.
Charoenporn et al. (2010) believe that fusarium wilt is one of the most severe tomato diseases worldwide. This disease is caused by Fusarium oxysporum f. sp. lycopersici (Sacc.), leading to severe economic losses Snyder and Hansen, (1940). It becomes one of the most prevalent and damaging diseases wherever tomatoes are grown intensively because the pathogen can persist indefinitely in infested soils (Agrios, 1997). Tomato is a critical horticultural crop that provides a wide range of necessary nutrients for human health (Imane, 2020).
Terrestrial fungi can adopt different life strategies to exploit nutrient sources. They grow as saprotrophs on simple or complex organic substrates, or they can establish a nutritional relationship with higher plants, either biotrophs or necrotrophs. Mycorrhizal associations are the most critical mutualistic biotrophic interactions. Over 80% of vascular flowering plants can enter symbiotic associations with arbuscular mycorrhizal (AM) fungi. The fungi that form these associations are members of the zygomycetes, and the current classification places them all into one order: Glomales. They strictly depend on their host plant to complete their life cycle, whereas other mycorrhizal fungi, such as ericoid fungi, can be grown in pure culture.
The AM association is a relatively nonspecific, highly compatible, long-lasting mutuality from which both partners derive benefit. The plant supplies the fungus with carbon, on which it is entirely dependent. The fungal contribution is more complex. Although it is clear that the fungi assist the plant with acquiring phosphate and other mineral nutrients from the soil, AM fungi also may influence the plant’s resistance to invading pathogens. In addition to its ecological significance, the association also may have applications in agriculture. This is particularly important because mycorrhizae link soil and plant and can improve plant nutrition efficiency and soil conservation.
The interaction begins when fungal hyphae arising from spores in the soil and adjacent colonized roots or hyphae contact the root surface. Here, they differentiate to form appressoria and penetrate the root. Appressoria formation is one of the first morphological signs of recognition between the plant and the fungus. Once inside the root, the fungus may grow inter- and intracellularly throughout the cortex, but AM fungi do not invade the vascular or the meristematic regions. The types of internal structures that develop depend on the plant/fungal combination and may include intracellular differentiated hyphae called arbuscules and intracellular coils.
Biotrophic fungi usually are thought to penetrate host tissues mechanically. It has been calculated that high pressure can be generated by appressoria of Magnaporte grisea (a nonmycorrhizal fungus) at the penetration point. This mechanical pressure allows the fungus to perforate the host wall by forming a penetration peg. Some wall components, such as melanin, play an essential role in increasing hydrostatic pressure since they trap solutes within the appressoria, causing water to be absorbed because of the increasing osmotic gradient.
There is an urgent need for environment-friendly management techniques, such as using arbuscular mycorrhizal fungi (AMF) to enhance crop productivity. AMF are commonly known as bio-fertilizers. Moreover, many believe that the inoculation of AMF provides tolerance to host plants against various stressful situations like heat, salinity, drought, metals, and extreme temperatures. AMF may assist host plants in the up-regulation of tolerance mechanisms and prevent the down-regulation of critical metabolic pathways. AMF, being natural root symbionts, provide essential plant inorganic nutrients to host plants, thereby improving growth and yield under unstressed and stressed regimes.
The role of AMF as a bio-fertilizer can strengthen plants’ adaptability to changing environments. Thus, further research on AMF-mediated crop quality and productivity promotion is needed. The present study provides comprehensive, up-to-date knowledge on AMF and its influence on host plants at various growth stages, their advantages and applications, and consequently, the importance of the relationships of different plant nutrients with AMF, (Naheeda et al.2019).
MATERIAL AND METHODS
Site description: The study site was the Demonstration Farm of the at the Faculty of Agriculture, University of Khartoum, at Shambat (15º 32´ N; 32º 32´E), Sudan.
Source of tomato cultivars: Tomato(Solanum lycopersicum L.)cultivar: Seeds of the cultivars were obtained from Shambat Research Station, Agricultural Research Corporation (ARC).
Isolation of the pathogen: F. oxysporium lycopersici was isolated from naturally diseased tomato plants exhibiting typical symptoms of wilt disease. Infected parts of the plants will be excised with a sterile scalpel and was surface sterilized with 30% (w/w) NaOCl for 2 min. Sterilized pieces was washed with sterile water and cut into small pieces (1cm length) and transferred on to antibiotic amended PDA plates.
Plateswas incubated at room temperature for 48 h and mycelial growth from the infected stem pieces will be transferred into new PDA plates. After incubation for 5 days, single spores were isolated and cultured on new PDA plates. The pathogen was identified based on the characteristics described by Booth (1977). Koch’s postulates were demonstrated for the pathogen and confirmed as the causal agent of wilt of the tomato plant.
Pathogen inocula: The pathogen inocula was produced on PDA plates. The plates was inocubated with an agar plug (5 mm in diameter) containing actively growing F. oxysoprium mycelium and incubated under fluorescence for 10 days at room temperature. Spores were washed from the plates with sterile distilled water and the concentration was adjusted to 106 spores mL-1 with a haemocytometer.
Disease assessment: Disease Severity (DS) and Disease Incidence (DI) of Fusarium wilt and Rhizoctonia were assessed 21 days after inoculation for each treatment.
Disease severity was estimated visually by assessing brown rot on the root and hypocotyls using a rating scale of 0-5
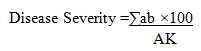
Where:
a = No. of diseased plants having the same degree of infection
b = Degree of infection
A = Total no. of examined plants
K = Highest degree of infection
Analysis of growth and yield parameters: Three plants of each treatment were harvested, three weeks after inoculation with the pathogens.
2-washed under running water to remove soil particles and evaluated for the following growth parameters:
- a) shoot fresh and dry weight (g). b) root fresh and dry weight (g)
- c) shoot and root length (cm).
- d) leaf area (cm2).
Dry weights were recorded after drying the samples at 800 C for 48 h. in a hot air oven until constant weight.
AM inocula: In this investigation, a mixture of formulated AM (Multi-VAM) spores kindly provided to units of Glomus mosseae in suspension form (1×106 unit L-1 in concentration). In addition to this Glomus mosseae inoculum, another treatment consisting of locally-isolated mycorrhizal spores was used. These spores were isolated from Shambat soil.
Planting and growth conditions: Pots were filled with disinfested soil at the rate of 2.5 kg pot-1; clay: sand (2:1, v/v). Five healthy seeds of tomato will be sown in each pot. Half of the pots will receive AM inocula as a suspension twice, in the tomato seed bed at the beginning and as a soil drench 14 days after the sowing at dilution of 5 ml L-1 water (El-Haddad et al., 2004). All plants were fertilized with phosphorus, all pots was kept outdoor under natural conditions and watered when necessary.
After four weeks of AM inoculation, five milliliters of spore’s suspension (F. oxysporium) were applied by pipette just below the collar region around the hypocotyls of each plant. Ten pots were treated only with plain water to serve as a control. Ten other pots were used as replicates for each treatment. Fifteen plants from each treatment were harvested after three weeks after inoculation with the pathogen for different analyses. The treatments to be applied in this study has been summarized as follows: Control (CNM), AM (CM), Pathogen (PNM) and Pathogen + AM (PM). The trials were conducted twice, and the experiments have been arranged in a completely randomized block design.
Concentration of phosphorus: The phosphorus content was determined according to Chapman and Pratt (1961). Shoots dried samples (2g) of tomato seedlings were ground. The samples were ashed in a furnace at 550C for 3 hr. Ten mL of 0.5N HCl were added to the ashed sample and heated gently on a hot plate. The ashed solution was quantitatively transferred to a 100 mL volumetric flask, made up to volume with deionized distilled water, and shaken. 2 ml from the ashed solution were taken in a conical flask (50), then 10 ml ammonium vanadates were added, and the volume was completed to 50 ml with distilled water. After 30 min, the absorbance at 470 nm was then determined by dosing a UV spectrophotometer (JENWAY 6305UV/vis).
Statistical analysis: Analysis of data variance was performed using Statistics Package 8.
Staining and estimation of mycorrhizal root colonization: Fixed roots were rinsed repeatedly in tap water; cut into small segments (0.5 to 1cm) and bleached once in a KOH (10%) solution for 45 min at 900 C, darker roots will be bathed in alkaline hydrogen peroxide for 20 min (Kormanik and McGraw, 1982). Thereafter, the roots were washed with tap water three times and stained with 0.05% trypan blue in lactophenol for 15 min at 900 C (Phillips and Hayman,1970). The excessive stain was washed with tap water. Fifty randomly selected stained root pieces were mounted on slides in lactoglycerol and examined microscopically for estimation of mycorrhizal root colonization according to the method of Trouvelot et al. (1986).
Experiment design: A block experiment based on a completely randomized design was carried out with three replications. The experimental setup consisted of three treatments: a control group with no inoculums and two groups inoculated with mycorrhizal fungus (Glomus mosseae) (MG) and locally isolated mycorrhizal spores (ML), respectively. These treatments were organized as the first and second variables.
The two species of AM fungi (MG) and (ML) used in this study were isolated from Shambat soil by using the “wet sieving and decanting” technique of Hayman (1982). Fusarium oxysporum f. sp. lycopersici (FOL1) pathogen was isolated from naturally diseased tomato plants exhibiting typical symptoms of wilt disease and identified based on the characteristics described by Booth (1977).
The Effective Microorganisms (EMTM) was obtained in solution form from Moroug Co. Khartoum North, an agent of EmroJapan.com.
The Myco-parasitic fungus (Trichoderma harzianum) (Th) Rifai was isolated from commercial BIOCONT from Organicsolutions-me.com by plating and preparing in PDA.
The tomato seeds were sterilized with 0.05% sodium hypo-chloride for 45 minutes before sowing. Seeds were germinated into sterilized soils (clay: sand) at a ratio of (2:1, v/v) in plastic pots (15×15 cm). Half of the pots received AM inoculum as a suspension twice: in the tomato seedbed at the beginning and as a soil drench 14 days after sowing, added dilution of 5 ml L-1 water of 6.12×103spores /mL (El-Haddad et al. 2004). Three weeks after sowing, seedlings were inoculated with the pathogen (FOL1) by injecting spores suspension near the roots at the rate of 5 mL (containing approx. 5.13×104spores /mL-1) per seedling.
Control plants were sown with nonmycorrhizal and no injection. Plants were grown under natural photoperiods, temperature, and relative humidity conditions, fertilized with phosphorus super phosphate P2O5 (5.23×10-4 g/pot), and watered every other day. At 21 days, the percentage of disease incidence (DI) and disease severity (DS) were recorded (Filion et al., 2003). At 6 and 13 weeks after transplanting, the parameters include leaf area (cm2), shoots and roots length (cm), and ground fresh and dry weights (g) of seedlings were measured after harvesting. The new weights (g) were measured before drying at 80°C for 28 h in a hot air oven until constant weight led to the dry matter weights (g).
The extent of colonization of tomato roots by arbuscular mycorrhizal (AM) fungi was assessed at two distinct time points (6 and 13 weeks) after harvest. Roots were rinsed repeatedly in tap water, cut into small segments (0.5 to 1cm), and bleached once in a KOH solution (10%) for 45 min at 900 C and stained in 0.05% lactic acid– glycerol–Trypan Blue (Phillips & Hayman, 1970). Darker roots were bathed in alkaline hydrogen peroxide for 20 minutes (Kormanik & McGraw, 1982). Fifty randomly selected stained root pieces were mounted on slides in lactoglycerol and examined microscopically for estimation of mycorrhizal root colonization following the method of Biermann and Linderman (1981).
RESULTS AND DISCUSSION
Tomato variety was experimentally infected with FOL1, with a disease incidence of 77.50%, significantly dropping 13 weeks from transplanting (Table 1). However, all inoculation treatments, including the local mycorrhiza (ML), dual mycorrhizal inoculation (MG), T. harzianum (Th), and the Effective Microorganisms (EMTM), were effective in reducing both disease incidence and severity at 6 and 13 weeks (Table 1). However, no clear superiority could be attributed to any of these treatments as their effects on disease amelioration showed apparent fluctuations between 6 and 13 weeks. It is noted that disease incidence and severity in the non-infected plants at 13 weeks were lower than in all infected plants, albeit vaccination with any of the above treatments.
Figure 1. F. oxysporium lycopersici inocula
Table 1: Disease Incidence (DI%) and Disease Severity (DS%) on Tomato
var. Beeli at 6th and 13th Weeks from Transplanting.
| Treatments | Six weeks DI (%) | Thirteen weeks
DI (%) DS (%) |
|
| Control | 57.50b | 7.05c | 12.80a |
| FOL1 | 77.50a | 53.25a | 40.78a |
| ML | 50.00b | 20.25bc | 12.78a |
| FOL1+ ML | 65.00ab | 23.00b | 22.50a |
| FOL1+ ML + MG | 50.00b | 29.00b | 34.78a |
| FOL1+ Th | 62.50ab | 25.50b | 18.05a |
| FOL1 + EM | 62.50ab | 18.25bc | 31.25a |
| ± SEM | 9.06 | 7.06 | 13.43 |
Mean separations were performed, and differences at P < 0.05
Table 2.a. shows the effects of the treatments on some above-ground growth parameters of Tomato var. Beeli after six weeks from transplanting. In the FOL1-infected plants, all treatments (ML, ML+MG, T. harzianum, or EMTM) resulted in significantly (p ≤ 0.05) higher leaf areas, which were even more significant than those of uninfected plants (controls and those receiving the local mycorrhiza). The highest benefit was due to the combination of mycorrhizae and EMTM. A substantial increase was recorded in the 3.47% -16.52% range in the leaf area. A noticeable decrease in the leaf area in the 25.00% – 4.00% range was also obtained.
As for shoot length, all inoculation treatments could reduce the effects due to FOL1, except for plants inoculated with T. harzianum, and were all better than control plants except in the case of T. harzianum and EMTM. (Table 2. a). Results of the investigations in which Tomato var. Beeli and FOL1 (alone on shoot length) showed a substantial increase in the 2.38% -7.14% range. A noticeable decrease in the shoot length range of 15.20% – 0.25% was also obtained. In varying degrees, the various inoculation treatments improved shoot fresh weight in the FOL1-infected plants. Still, these treatments did not yield new weights higher than control plants except for plants treated with the dual mycorrhizal inoculum or T. harzianum.
On the other hand, none of the inoculated treatments could produce shoot dry weights higher than those of the control plants. They could not improve the infected plants’ dry weight except those receiving the dual mycorrhizal inoculation or T. harzianum. A basic increase in shoot fresh weight was recorded in the 20.71% -46.44% range. Also, there was a noticeable decrease in the shoot new weight in the range of 28.10% – 14.79%, whereas in shoot dry weight, the reduction was 11.11%. A noticeable decrease in the shoot dry weight in the 41.66% – 19.44% range was also obtained (Table 2. a).
Table 2.a: The effects of the treatments on some above-ground growth parameters of
Tomato var. Beeli, at six weeks from Transplanting
| Treatments | Leaf area
(cm) |
Change
(%) |
Shoot length
(cm) |
Change
(%) |
Shoot fresh wt.(g) | Change
(%) |
Shoot dry wt. (g) | Change
(%) |
| Control | 94.83d | 0.00 | 27.30c | 0.00 | 3.38d | 0.00 | 0.36ab | 0.00 |
| FOL1 | 71.00g | -25.00 | 23.15f | -15.20 | 2.43f | -28.10 | 0.26abc | -27.77 |
| ML | 98.13c | +3 .47 | 27.95b | +2.38 | 2.68e | -20.71 | 0.24bc | -33.33 |
| FOL1+ ML | 76.13f | -19.71 | 25.08d | -8.13 | 4.08c | +20.71 | 0.21c | -41.66 |
| FOL1+ ML + MG | 110.50a | + 16.52 | 29.25a | +7.14 | 2.88e | -14.79 | 0.29abc | -19.44 |
| FOL1+ Th | 90.95e | -4.09 | 27.23c | -0.25 | 4.95a | +46.44 | 0.40a | +11.11 |
| FOL1+ EM | 100.25b | +5.71 | 24.20e | -11.35 | 4.56b | +34.91 | 0.21c | -41.66 |
| ±SEM | 0.58 | 0.15 | 0.10 | 0.07 |
Mean separations were performed, and differences at P < 0.05
At 13 weeks, all inoculated treatments produced leaf area greater than in the FOL1-inoculated treatment, and all, except for the combined mycorrhizal treatment, were more significant than the control treatment. A substantial increase in leaf area was recorded in the range of 9.81% -30.47%. A noticeable decrease in the leaf area in the 15.76 % – 9.55% range was also obtained (Table 2.b).
Shoot fresh weights in the FOL1-infected plants were also improved, to varying extents, by all inoculation treatments, which produced higher new weights than in the control plants except for plants receiving the dual mycorrhizal inoculation. Likewise, all inoculation treatments improved the infected plants’ shoot dry weight and shot length. Still, two of them (the two mycorrhizal inoculations) could not surpass control plants in these two parameters. A basic increase in shoot fresh weight was recorded in the 17.64 % -99.60% range. Also, a noticeable decrease in the shoot new weight in the range of 39.12% – 31.96%, whereas in shoot dry weight was recorded in the range of 10.08% -54.62%. A noticeable decrease in the shoot dry weight in the range of 56.30% – 13.44% was also obtained (Table 2.b).
Table 2.b: The effects of the treatments on some above-ground growth parameters of
Tomato var. Beeli, at 13 weeks from Transplanting
| Treatments | Leaf area
(cm) |
Change
(%) |
Shoot length
(cm) |
Change
(%) |
Shoot fresh wt.(g) | Change
(%) |
Shoot dry wt. (g) | Change
(%) |
| Control | 100.17e | 0.00 | 33.05d | 0.00 | 5.10e | 0.00 | 1.19d | 0.00 |
| FOL1 | 84.38g | -15.76 | 30.10g | -8.92 | 3.10g | -39.12 | 0.52g | -56.30 |
| ML | 110.00d | +9.81 | 32.03e | -3.08 | 6.00d | +17.64 | 1.03e | -13.44 |
| FOL1+ ML | 120.27b | +20.06 | 34.08c | +3.11 | 9.03b | +77.05 | 1.31c | +10.08 |
| FOL1+ ML + MG | 90.60f | -9.55 | 31.05f | -6.05 | 3.47f | -31.96 | 0.61f | -48.73 |
| FOL1+ Th | 115.45c | +15.25 | 35.00b | +5.90 | 8.46c | +66.88 | 1.58b | +32.77 |
| FOL1+ EM | 130.70a | +30.47 | 36.00a | +8.92 | 10.18a | +99.60 | 1.84a | +54.62 |
| ±SEM | 0.96 | 0.05 | 1.22 | 1.02 |
Mean separations were performed, and differences at P < 0.05
Figure. 2. Experiment design

At six weeks, the data in Table 3. showed that the root length was also more significant in the inoculated FOL1-infected plants than in the un-inoculated treatments, whether infected or not. The longest roots were recorded in infected plants inoculated with T. harzianum, followed by those treated with EMTM, whereby a substantial increase was recorded in the range of 26.31% -117.93%. A noticeable decrease in the root length of 16.56% was also obtained. Root fresh weight was also significantly (p ≤ 0.05) higher in the inoculated treatments than in the FOL1-infected plants, except for those receiving the mycorrhizal combination, than in the uninfected plants.
Similarly, all inoculation treatments resulted in higher root dry weight than in FOL1-infected plants, all of which, except those receiving ML or EMTM, were superior to the uninfected plants. A substantial increase in root fresh weight was recorded in the 28.12% – 46.87% range. There was also a noticeable decrease in the root new range of 34.37% – 6.25%, whereas root dry weight was recorded in the 2.12%- 63.82% range. A noticeable decrease in the root dry weight in the 59.57% – 20.22% range was also obtained (Table 3.a).
Table 3.a: The effects of the treatments on some root growth parameters of Tomato var. Beeli,
at six weeks from Transplanting
| Treatments | Root length (cm) | Change
(%) |
Root fresh wt. (g) | Change
(%) |
Root dry wt. (mg) | Change
(%) |
| Control | 5.13e | 0.00 | 0.32c | 0.00 | 9.4bc | 0.00 |
| FOL1 | 4.28f | -16.56 | 0.21e | -34.37 | 3.8d | -59.57 |
| ML | 5.28e | +29.23 | 0.41b | +28.12 | 7.5c | -20.22 |
| FOL1+ ML | 6.48d | +26.31 | 0.26d | -18.75 | 5.3d | -43.61 |
| FOL1+ ML + MG | 7.53c | +46.78 | 0.30cd | -6.25 | 9.7b | +3.19 |
| FOL1+ Th | 11.18a | +117.93 | 0.47a | +46.87 | 9.6bc | +2.12 |
| FOL1+ EM | 10.10b | 96.88 | 0.41b | +28.12 | 15.4a | +63.82 |
| ±SEM | 0.33 | 0.03 | 9.69 |
Mean separations were performed, and differences at P < 0.05
At 13 weeks, the root length of the infected plants was improved by all inoculation treatments, which produced roots longer than in control plants except for plants receiving the mycorrhizal injections (Table 3.b). Likewise, all treatments had higher root fresh weights, which were more elevated than the FOL1-infected plants. Still, only three (those co-inoculated with the local mycorrhiza, with T. harzianum, and those inoculated with the local mycorrhiza without FOL1 infection) were better than control plants.
Similarly, all produced higher root dry weights than the FOL1-infected plants, but only two (those receiving the local mycorrhiza and those inoculated with T. harzianum) were better than control plants. A substantial increase in root fresh weight was recorded in the 9.63 % -48.19% range. The degree of colonization of tomato roots by arbuscular mycorrhizal (AM) fungi was evaluated at two specific time intervals (6 and 13 weeks) following harvest. A noticeable decrease in the root dry weight in the range of (77.44% – 25.00%) was also obtained (Table 3.b).
Table 3.b: The effects of the treatments on some root growth parameters
of Tomato var. Beeli, at 13 weeks from Transplantin
| Treatments | Root length (cm) | Change
(%) |
Root fresh wt.(g) | Change
(%) |
Root dry wt.(mg) | Change
(%) |
| Control | 8.88ab | 0.00 | 0.83d | 0.00 | 0.31c | 0.00 |
| FOL1 | 5.17d | -41.77 | 0.42g | -49.39 | 0.07f | -77.41 |
| ML | 9.08ab | +2.25 | 0.91c | +9.63 | 0.13e | -58.06 |
| FOL1+ ML | 8.05bc | -9.34 | 1.23a | +48.19 | 0.41b | +32.25 |
| FOL1+ ML + MG | 7.13c | -19.70 | 0.50f | -39.75 | 0.09f | -70.96 |
| FOL1+ Th | 9.85a | +10.92 | 1.01b | +21.68 | 0.45a | +45.16 |
| FOL1+ EM | 9.08ab | +2.25 | 0.64e | -22.89 | 0.23d | -25.80 |
| ±SEM | 0.58 | 0.02 | 9.43 |
Mean separations were performed, and differences at P < 0.05
Table (4) shows root colonization by the mycorrhizal fungi in Tomato var. Beeli at six weeks from transplanting. Very low colonization occurred in FOL1-infected plants that did not receive vaccination and in control plants. Highest root colonization was recorded in FOL1-infected plants co-inoculated with EMTM, followed by plants co-inoculated with the local mycorrhiza or T. harzianum, and then uninfected plants inoculated with the local mycorrhiza. A basic increase in root colonization was recorded in the range of (120.00% -265.00 %).
Table 4: Mycorrhizal Colonization percentage of Tomato var. Beeli affected by different
levels of mycorrhiza species in 6th Week from Transplanting
| Treatments | Root colonization (%) |
| Control | 20.0 |
| FOL1 | 44.0 |
| ML | 59.0 |
| FOL1 + ML | 62.0 |
| FOL1 + ML + MG | 60.0 |
| FOL1+ Th | 62.0 |
| FOL1+ EM | 73.0 |
Mean separations were performed, and differences at P < 0.05
Table 5 shows that plant phosphorus content (ppm) of FOL1-pathogenic plants was much higher than in un-pathogenic (control) plants at 6 and 13 weeks, and the values recorded at 13 weeks were much lower than those recorded at six weeks. However, this content was significantly improved by injection of the pathogenized plants with either the local mycorrhiza or both mycorrhizae at six weeks and the local mycorrhiza at 13 weeks. In the un-pathogenic plants, although the phosphorus content was much lower than in FOL1-pathogenic plants, vaccination with the local mycorrhiza significantly improved the phosphorus content at 6 and 13 weeks.
Table 5: Phosphorus Content (ppm) of Tomato var. Beeli is affected by different levels
of mycorrhiza species at the 6th and 13th Weeks from Transplanting
| Treatments | Six weeks (ppm) | Thirteen weeks (ppm) |
| Control | 0.94cd | 0.45c |
| FOL1 | 1.33cd | 0.71bc |
| ML | 2.28b | 0.91ab |
| FOL1 + ML | 3.65a | 1.17a |
| FOL1 + ML + MG | 1.70bc | 0.54c |
| FOL1 + Th | 1.00cd | 0.66bc |
| FOL1+ EM | 0.74d | 0.44c |
| ±SEM | 0.39 | 0.16 |
Mean separations were performed, and differences at P < 0.05
Fungi are involved in a wide range of intimate symbiotic associations with other organisms, and it would be no exaggeration to say that they have shaped the history of life on land. In several cases, fungi and their partners have become so intimately dependent on one another that they have lost the ability to live separately. In many cases, it is possible to cultivate fungi in laboratory media. Still, they are, in effect, ecologically obligate symbionts or parasites because they seldom grow as free-living organisms in nature (Deacon, 2006).
Tomatoes are susceptible to a wide variety of fungal pathogens and other diseases. The most important are those caused by pathogenic fungi (Apodaca-Sánchez et al., 2002; Carrillo-Facio et al., 2003). Tomato’s most crucial fungal disease is Fusarium wilt, caused by Fusarium oxysporum Schlechtend, f. sp. lycopersici (Sacc.) W.C.Snyder and H.N. Hans (FOL) can reduce yield by up to 60% and affect fruit quality (Agrios, 2004). Fusarium wilt of Tomato is a hypoplastic disease that causes reduced development and is similar in most respects to vascular fusarioses of various other plants (Fierro-Coronado et al., 2013).
In the present study, the plants invariably exhibited symptoms of vascular wilt, formation of yellow patches, and wilting after 21 days of pathogenization. Plant growth parameters were, to a large extent, negatively affected, and the degrees of disease incidence and severity were great. Nevertheless, injection of these Fusarium-pathogenic plants with the two arbuscular mycorrhizal fungi (Glomus mosseae or the locally isolated mycorrhiza, or their combination) improved Tomato plant growth.
It succeeded in alleviating the harmful effects of vaccination with the Fusarium isolates alone, suggesting that these fungi can be an effective and environmentally sustainable biological treatment to counter these ill effects and increase Tomato plants’ growth. These AM fungi could be inoculated during transplantation or in tomato nurseries (Utkhede, 2006).
Considered a contribution to protect Tomato plants (Solanum lycopersicum L.) against wilt disease, this study highlighted physiological and biochemical aspects of Tomato AMF mycorrhization, which could enhance resistance through the improvement of growth (nutrient supply) and the activation of the defense mechanisms of host plants against of F. o. f. sp. lycopersici, the causal agent of wilt disease. Nutrients such as N, P, K, Mg, and Ca are required by tomato crops at the right time and in the correct quantity for sound production and yield (Olaniyi JO and Ajibola AT, 2008).
The various microorganisms present in the rhizosphere arbuscular mycorrhizal (AM) fungi are of great value in promoting the growth and yield of plants (Siddiqui & Mahmood, 1998). Colonization by AM fungi has been studied to increase the absorption of minerals, particularly immobile nutrients, from the soil by the host.
The present study revealed that inoculation with AM fungi singly or in a combination enhanced plant growth in all the varieties of Tomato plants tested. This considerable increase in growth and dry weight could be attributed to the increase in intake of nutrients such as phosphorus, nitrogen, potassium, and other micronutrients by the co-inoculated AM fungi. Previous studies in different vegetable crops (Artursson et al., 2006) have reported significantly increased shoot and root dry weights when inoculated with AM fungi.
CONCLUSION
The present study has demonstrated the beneficial role that can be played by some biocontrol agents (the two mycorrhizal fungi Glomus mosseae and the local mycorrhizal isolate, the fungus T. harzianum and the commercial microbial blend EMTM) in increasing plant resistance against the infection with FOL. Different physical and physiological mechanisms have been shown to play a role in plant protection by these treatments, namely, improved plant nutrition and growth.
REFERENCES
Agrios, G. N.(2004).Plant Pathology.4th ed. Academic Press.NewYork, USA.pp 635.
Apodaca-Sánchez, M.A.; Zavaleta- Mejía, E.;García-Espinoza, R.; Osada-Kawosoe,S.;Valenzuela-Ureta,J.G. (2002).Frecuencia de campos infestados con Fusarum oxysporum f. sp. Radicis lycopersici en Sinaloa, México y su control. Mexico Journal of Phytopathology20: 1-7.
Artursson, V.; Finlay, R.D. and Jansson, J.K.(2006).Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environmental Microbiology 8, 1–10.
Carrillo-Facio, J.A.; Montoya-Rodrí – guez, T.J.; García-Estrada, R.S.; Cruz-Ortega, J.E.; Márquez-Ze –quera, I. and Sañudo-Barajas, A.J.(2003).Razas de Fusarium oxysporum f. sp. Lycopersici Sny-der y Hansen, en tomate ( Lyco persicon esculentum Mill.) en el Valle de Culiacán, Sinaloa, México.Mexico Journal of Phytopathology,21: 123-127.
Charoenporn, C.; Kanokmedhakul, S.; Lin, F.C., Poeaim, S. and Soytong, K.(2010).Evaluation of bio-agent formulations to control Fusarium wilt of tomato.African Journal of Biotechnology,9(36):5836-5844. http://www.academicjournals.org/AJB.
Deacon, J.W. (2006). Fungal biology.4th ed. Malden, Massachusetts: Blackwell Publishing.
El-Haddad, S. A.; Abd El-Megid, M.S. and Shalaby O.Y.(2004). Controlling onion white rot using Egyptian formulated endo-mycorrhiza (Multi-VAM)—annual of Agriculture Science, 49:733-745.
El-Khallal, S.M. (2007). Induction and modulation of resistance in tomato plants against Fusarium wilt disease by bioagent fungi (arbuscular mycorrhiza) and hormonal elicitors (jasmonic acid and salicylic acid): 2-changes in the antioxidant enzymes, phenolic compounds, and pathogen related-proteins. Australian Journal of Basic Applied Sciences, 1(4): 717-32.
Fierro-Coronado, R. A.; Castro-Moreno, M. G.; Ruelas-Ayala, R. D.; Apodaca-Sánchez, M. A. and Ignacio Eduardo Maldonado-Mendoza, I. M. (2013). Induced protection by Rhizophagus intraradices against Fusarium wilt of Tomato. Interciencia, 38 (1): 48- 53.
Fierro-Coronado, R. A.; Castro-Moreno, M. G.; Ruelas-Ayala, R. D.; Apodaca-Sánchez, M. A. and Ignacio Eduardo Maldonado-Mendoza, I. M. (2013). Induced protection by Rhizophagus intraradices against Fusarium wilt of Tomato. Interciencia, 38 (1): 48- 53.
Imane Haddidi 1, Nguyen Hong Duc 1 , Szende Tonk 2 , Eszter Rápó 1,2 and KatalinPosta 1,3,* (2020). Defense Enzymes in Mycorrhizal Tomato Plants Exposed to Combined Drought and Heat Stresses. Agronomy
Imane Haddidi 1, Nguyen Hong Duc 1 , Szende Tonk 2 , Eszter Rápó 1,2 and KatalinPosta 1,3,* (2020). Defense Enzymes in Mycorrhizal Tomato Plants Exposed to Combined Drought and Heat Stresses. Agronomy
Naheeda Begum 1, Cheng Qin 1, Muhammad Abass Ahanger 1, Sajjad Raza 2, Muhammad Ishfaq Khan 3, Muhammad Ashraf 4, Nadeem Ahmed 1,5 and Lixin Zhang 1(2019): Role of Arbuscular Mycorrhizal fungi in plant growth regulation :implication in abiotic stress tolerance. Frontiers in plant sciences Applied soil Ecology 12 4 335 349
Olaniyi JO, Ajibola AT (2008).Effect of organic and inorganic fertilizers application on the growth, fruit yield and quality of Tomato. Journal of Applied Biosciences, 8: 236-242.
Siddiqui, Z.A.; Mahmood, I. and Hayat S. (1998).Biocontrol of Heterodera cajani and Fusarium udum on pigeonpea using Glomus mosseae, Paeciliomyces lilacinus and Pseudomonas fluorescens. Thailand Journal of Agricultural Sciences, 31: 310-321.
Utkhede, R. (2006). Increased growth and yield of hydroponically grown greenhouse tomato plants inoculated with arbuscular mycorrhizal fungi and Fusarium oxysporum f. sp. Radicis-lycopersici. Biocontrol, 59: 393-400.


