1Department of Mycology and Plant Ppathology, Institute of Agricultural
Science, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
2Department of Botany, Institute of Science, Banaras Hindu University,
Varanasi, Uttar Pradesh, India
3Department of Genetic and Plant Breeding, Institute of Agricultural Science,
Banaras Hindu University, Varanasi, Uttar Pradesh, India.
Corresponding author email: rakeshsingh@bhu.ac.in
Article Publishing History
Received: 15/10/2021
Accepted After Revision: 13/12/2021
There are significant losses which have occurred in crops due to the infestation of plant parasitic nematode which are known as hidden enemy due to their presence in rhizosphere and their infection site on the roots. Synthetic nematicidal control is an effective strategy to combat this biotic stress but an inappropriate and deficient application of chemical pesticides have an adverse effect on soil micro-flora and fauna. Due to the environmental and regulatory pressure, use of potential biocontrol agents is the new approach for nematode management by the farming community. For this study, four potential rhizobacteria from different habitats BHU1, BHU2, BHU3 and BHU4 were assessed for their antagonistic activities against Meloidogyne incognita infecting tomato plant. These were characterized on the basis of their morphological and biochemical activities. In vitro screening of bacterial isolates was conducted in a 25-microwell plate by addition of second stage juvenile (J2) of M. incognita with nematode application.
Among four bacteria,, three potential antagonistic bacteria were able to kill nematode within 24 hours. Mortality percentage of J2 M. incognita observed in sterile distilled water selected bacterial isolates ranged from 23.33 to 100% in 3h to 24h periods. Moreover, all bacterial isolates except BHU2 isolate were found positive for production of extracellular enzymes like catalase, oxidase, chitinase, amylase and gelatinase which favour effective biopesticide activity of bacteria. Further selected isolates of bacteria associated with tomato have shown a great potential as biocontrol agents against root-knot nematode in tomato during pot experiment. Based on the fact stated above, the current research focused on plant growth promoting rhizobacteria based nematodes biocontrol strategies with direct and indirect mechanism of PGPR for nematode management.
Biocontrol, Knot-Root, Lycopersicon esculentum, Meloidogyne incognita, PGPR.
Gupta S. K, Singh R. K, Patel A. K, Banjare U. Role of Growth-Promoting Bacteria as Biocontrol Agent Against Root Knot Nematode of Tomato. Biosc.Biotech.Res.Comm. 2021;14(4).
Gupta S. K, Singh R. K, Patel A. K, Banjare U. Role of Growth-Promoting Bacteria as Biocontrol Agent
Against Root Knot Nematode of Tomato. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/30mVmEY“>https://bit.ly/30mVmEY</a>
Copyright © Gupta et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Rhizosphere is one of the most crucial zones where plant and microbes interact symbiotically. In this aspect, plants mainly benefit from microbial consortium for acquiring nutrients as a vital mineral and in return providing habitat to micro-organism. Root-knot nematodes (RKNs) are mainly involved in causing damage to vegetable crop and estimated loss upto 21.3% to agricultural products annually. M. incognita is highly destructive plant parasitic nematode with rapid multiplication in seasonal vegetable crop likes tomato, brinjal, okra, spinach etc (Razavi et al. 2017; Kumar et al. 2020).
incognita penetrate epidermis of root and produced several hydrolytic enzymes from stylet that degrade cell wall of plant root in order to enter through plant and not only caused diseases but also restricted water and minerals uptake of plants, due to hindering biochemical, physiological and morphological status of plants. Farmers recognized insect pests and other constraints as production problems but overlooked plant parasitic nematodes due to its indirect effect on the plants. Nematode diseases are difficult to control when it enter in the root system hence pre adaptive measures are more successful to control them rather than post adaptive management practices. Plant parasitic nematodes not only cause damage individually but form disease-complexes with other microorganism and increased the crop losses (Etesami and Adl 2020).
In rhizospheres several beneficial micro-organisms viz bacteria fungi actinomycetes etc are found which control the nematode population naturally. In natural rhizospheric zone many plant growth promoting bacteria having very good growth promotion activities and potential biocontrol ability have been found. Which have direct mechanism for plant growth with mineral solubilization, phytohormone production while indirect mechanism with production of hydrogen cyanide, siderophore, antibiotic production and also have induced systemic resistance activities. Rhizobacteria are reported to produce diverse range of bioactive chemical which promote growth of plant and provide protection against many phytopathogen including M. incognita (Khanna et al. 2019; Liu et al. 2019; Etesami and Adl 2020).
Tomato is one of the most susceptible vegetables against root knots, nematodes mostly causing 30 to 40 % yield losses in tropical regions, and also it has been also found that M. incognita is responsible in deteriorating the quality of fruit and yield of tomatoes, (El-Shawadfy 2021). Among the various microorganisms, plant growth promoting rhizobacteria have attracted considerable attention for their usefulness in biofertilizer and biological control ability.
Hence the current research work was focused on their biofertilizer ability as well as on the eco-friendly management of M. incognita by plant growth promoting traits for enhancing plant defense mechanism of tomato seedlings, raised under nematode infested soil as previously reported by El-Ashry et al. (2020) and Zao et al. (2021).
MATERIAL AND METHODS
Rhizospheric soil samples were collected from agricultural field of rice, tomato, oat and barley growing at four districts viz Varanasi, Mirzapur, Chunar and Jaunpur of Uttar Pradesh in India. Collected soil samples were kept in sterilized plastic bags at 40C. Serial dilution technique was used for screening of bacterial colonies on King’s B medium. Serial diluted plates were incubated at 30°C for 48-72 hours (h) and bacterial colonies were isolated, maintained as pure cultures on King’s B media. For the morphological and biochemical characterization of growth promoting bacteria, isolated bacteria were identified firstly by macroscopic observation i.e., gram reaction, colony morphology, pigmentation, mobility and cell shape followed by several biochemical and enzymatic test viz Catalase test, Oxidase, Chitinase, Amylase and Gelatinase (Hayward 1960; Blazevic and Ederer 1975; Collins 1980; Fadden 1980; Somasegaran and Hoben 1994; Kaur et al. 2012).
Qualitative assay of phosphate solubilizing activity of bacteria was measured by Pikovaskaya on Pikovaskaya’s Agar Medium and clear transparent zone around the bacterial colony confirmed phosphate solubilization activity within 24 to 72 h (Pikovaskaya 1948).Indole acetic acid (IAA) Production was detected as described by Bric et al. 1991. Appearance of pink colour in broth by adding of Salkowaski reagent indicated IAA production. For the quantification of IAA, absorbance was taken at 540 nm by using UV/visible spectrophotometer (Bric et al. 1991).
HCN production was evaluated on Whatman filter paper No.1 soaked with picric acid (0.05%) solution in 2% sodium carbonate and placed in the test tube sealed with parafilm and incubated. A colour change of the filter paper from deep yellow to reddish-brown colour at 30°C for 48-72 h indicated HCN production and quantified (Baker et al. 1987). Siderophore production of bacterial isolates was detected by observing orange halo zone around the bacterial colony on chrome azurol S agar media after 72 h of growth. Quantification of siderophore was calculated in µg/ml (Schwyn and Neilands 1987).
For the preparation and screening of bacterial strains against M. incognita, nematode inoculum was prepared from large numbers of egg masses by hand-picked using a sterilized forceps from heavily infected tomato roots. These egg masses were washed in distilled water and placed in 10-cm diameter 15 mesh sieves. The hatched juveniles were collected and incubated in a micro-well plate containing 3 ml sterile distilled water for at 250C. For the screening of bacterial isolates having ability to kill M. incognita in vitro, four bacterial isolates were screened for their nematode killing ability against M. incognita in microwell plate (25 wells). Heavy cell suspension for each bacterial isolates was prepared (1×106cfu/ml) in sterile distilled water using a UV/Visual spectrophotometer. Each well contained 100 (J2) of M. incognita in 1ml of sterile distilled water with 10µl/ml of bacterial suspension. Mortality of M. incognita was evaluated at 3, 6, 12 and 24 h time interval.
For the pot experiment, all the four promising bacteria were inoculated on tomato seeds (variety Kashi Abhiman) by seed biopriming. Seeds were soaked with bacteria containing 109 CFU/ml for overnight. Bioprimed seeds were sown in pots containing I kg sterilized soil followed by 2000J2 population of M. incognita in each pot. Subsequently single plants per pot was maintained at 60% water holding capacity and plants were uprooted after 21 days. Seed germination and vigour index were observed by towel method. Leaf area was calculated by using following formula (Yashida et al. 1972; International Seed Testing Association 1993). The experiment was performed in completely randomized block design. Statistical significance between the treatments was compared by least significant difference test at P < 0.05 probability level on different growth promoting parameters using SPSS16.0 software.
RESULTS AND DISCUSSION
Four potential bacteria viz BHU1, BHU2, BHU and BHU4 were isolated from different rhizospheric locations (Table1) and their morphological characteristics of rhizobacterial isolates BHU1, BHU2, BHU3 and BHU4 showed variation in colony, shape, colour, pigmentation, colony surface and margin.
Table 1. Isolation of bacteria from rhizospheric soil from different locations
| S.No. | strains | Location | Rhizospheric soil associated with Crops |
| 1 | BHU1 | Varanasi (25.3176 N, 82.9739 E) | Rice (Oryza sativa) |
| 2 | BHU2 | Mirzapur (25.32N, 83.33E) | Tomato (Lycopersicon esculentum) |
| 3 | BHU3 | Chunar (25.1037 N, 82.8721 E) | Oat (Avena sativa) |
| 4 | BHU4 | Jaunpur(24.240N, 82.70E) | Barley (Hordeum vulgare) |
Bacterial strain was identified on the basis of morphological appearance of bacterial colony and their biochemical performance which is mention in table 1. BHU1 showed green pigmentation while BHU3 isolates showed yellow pigmentation on YEMA agar media with rough and shiny surface (Table2).
Table 2. Morphological characterization of selected isolate
| Ss.No. | Bacteria | Strain | Gram stain | Colony
Shape |
Colony Colour | Pigmentation | Colony Surface | Colony Margin |
| 1 | Pseudomonas fluorescens | BHU1 | -ve | Round | Yellow | Green | Smooth | Entire |
| 2 | Enterobacter spp | BHU2 | -ve | Biconcave | White | No pigmentation | Rough | Entire |
| 3 | Bacillus spp | BHU3 | -ve | Circular | Creamy white | Yellow | Rough | Undulate |
| 4 | Burkholderia spp | BHU4 | -ve | Circular | Yellow | No pigmentation | Rough | Entire |
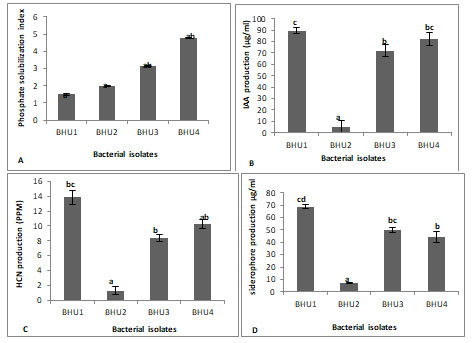
Bacterial strain shows various type of biochemical characterization like phosphate solubilization, indole acetic acid, HCN and siderophore production (Table 3). Selected bacteria showed phosphate solubilization activities on Pikovaskaya’s media by forming clear transparent zone around the bacterial colony. Among four isolates highest phosphorus was solubilized by BHU4 strain (Figure1&2A). Phosphorus is a major nutrient required for plant growth and development but plants are unable to utilize phosphate because 95-99% phosphate found in the soil in insoluble form. Making phosphorous in available form for plants is an important trait for PGPR selection (Shahzad et al. 2008).
Some PGPR have been reported to produce citrate, lactate, succinate activity that dissolve the mineral phosphates as a result of anion exchange or chelation of Fe and Al ions associated with phosphate. This leads to an increase in the soluble P in the rhizosphere resulting its more uptake by the plants. IAA is important phytohormones which stimulatory effect on plant growth under biotic stress condition. Among four bacteria, three bacteria produced IAA in tryptophan yeast broth. Highest IAA production was recorded by BHU1 (89μg/ml) followed by BHU4 (82 μg/ml) and minimum IAA production by BHU2 (5 μg/ml) production (Figure 1&2B) (Shahzad et al. 2008).
HCN producing bacteria (BHU1, BHU3 and BHU4) showed antagonistic activities against phytopathogen three bacteria produced HCN with highest HCN production 13ppm by BHU1 (Figure 2C). HCN production is a type of volatile organic compounds (VOCs) toxic for phytopathogenic nematodes and provides protection from various biotic stresses. As the plants exposed to nematode infection grow weak in their defense system hence shielding them by HCN producing bacteria which can be helpful in overcoming the chances of pathogenic infection. Siderophore production was confirmed by the change of CAS broth colour to brown colour (Fig. 1C).
Three bacteria (BHU1, BHU3 and BHU4) were observed producing siderophore and highest siderophore production was observed by BHU1 (68μg/ml) (Figure 2D). Siderophores are low molecular weight iron chelating compound which restrict the growth of phytopathogen, indirectly by depriving fungal pathogens and plant parasitic nematodes from iron uptake and supported plant growth by iron supply resulting in increased dry biomass of plant and rootlet elongation (Cheng et al. 2017; Sahebani and Gholamrezaee 2021).
Figure 1: Biochemical characterization of PGPR Phosphate solubilization
(A), IAA production (B), Siderophore (C).
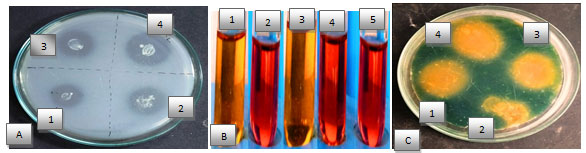
Figure 2: Biochemical characterization of growth promoting bacteria (A) P-solubilization, (B) IAA production, (C) HCN production and (D) Siderophore production. Significant of variance (p < 0.05) has been analyzed by Duncan Multiple Range Test.
Extracellular enzyme activities are important parameter for selection of growth promoting bacteria and also favour strong evidence for improve defense mechanism. Selected bacteria were showing positive catalase activity, maximum catalase production was observed by BHU1 and BHU4. The oxidase test used to identify bacteria that produce cytochrome C oxidase, an enzyme of the bacterial electron transport chain. The reagent N, N-dimethyl-p-phenylenediamine is a dark-blue to maroon color when oxidized and become colorless when reduced. BHU4 was observed strongly positive for oxidase test. Chitinase enzyme serves various functions such as antagonistic activity against chitin-containing pests and in nutrient cycling. BHU4 strain produced chitin followed by BHU2. Gelatin hydrolysis test is used to detect the ability of an organism to produce gelatinase which is a type of proteolytic enzyme that liquefy gelatin hydrolysis of gelatin indicates the presence of gelatinase (Subedi et al. 2020).
Maximum gelatin was produced by BHU4 bacteria followed by BHU2 (Table3). Three bacterial isolates BHU1, BHU3 and BHU4 had greater efficiency to produced lytic enzyme like catalase, oxidase, chitinase amylase and gelatinase. Calalase and oxidase enzyme reduce the oxidative damage while α-amylase enzyme in the aleurone layer helping in hydrolyzing the endosperm starch into sugars, which provide the energy for vigour index parameter. Other important mechanisms include production of extracellular lytic enzymes, such as CHI, β-1,3-GLU and protease, which lyse chitin and glucan (present in the cell wall of fungi) and protein in the nematode cuticle (Lee et al. 2015; Gupta et al. 2017; Subedi et al. 2020; Sahebani and Gholamrezaee 2021).
Table 3. Extracellular enzyme production by selected bacteria
| S. no | strains | Catalase | Oxidase | Chitinase | Amylase | Gelatinase |
| 1 | BHU1 | +++ | + | + | + | + |
| 2 | BHU2 | + | – | ++ | – | – |
| 3 | BHU3 | ++ | ++ | + | ++ | ++ |
| 4 | BHU4 | +++ | +++ | +++ | +++ | +++ |
(-) no production, (+) weak, (++) moderate and (+++) strong
When these bacteria were examine for their antagonistic properties against M. incognita three bacteria BHU1, BHU3 and BHU4 kill nematode but BHU2 did not show ability to kill it (table 4 and 5). Bacteria BHU1 and BHU3 kill M. incognita 100% and 93% respectively after 12 hours. The bacterial mechanism of infection caused the lysis of nematode M. incognita particularly on the oesophageal region (BHU1) and intact body (BHU3) which is mention in table 4 and 5.
In many studies it has been found that growth promoting microbes produced various types of secondary metabolites and volatile organic compound (VOC) at infected region. Antagonistic bacteria tend to prohibit the invasion of RKNs into the roots and maintain the balance of nutrients and mineral status in plants moreover, growth promoting bacteria also control the gall formation and maintain the vascular integrity in order to boost the physiology and biochemistry of the plant. Consequently, the most vital mechanism to prevent gall formation through PGPR’s by production of nematicidal compound like hydrogen sulfide, ammonia, butyric acid and degradation of nematode attractants of root exudates. Furthermore, they bind to the root surface and produced hydrolytic enzyme that can kill the nematode (Safni et al. 2018; Sahebani and Gholamrezaee 2021).
Table 4. Mechanism of antagonistic bacterial infection inside the body of M. incognita
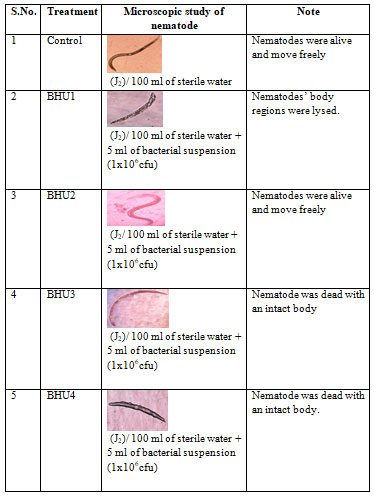
Table 5. Mortality percentage of M. incognita hours after inoculation
| S.No. | Treatments | Mortality percentage of M. incognita hours after inoculation | |||
| 3 h | 6 h | 12 h | 24 h | ||
| 1 | Control | 0±0a | 0±0a | 0±0a | 0±0a |
| 2 | BHU1 | 33.33±3.9b | 66.66±4.5c | 100±0d | 100±0b |
| 3 | BHU2 | 0±0a | 0±0a | 0±0a | 0±0a |
| 4 | BHU3 | 23.33±2.8b | 46.66±3.4b | 93.3±6.9cd | 100±0b |
| 5 | BHU4 | 23.33±2.8ab | 56.66±4.8bc | 76.66±6.4b | 100±0b |
Values are mean of three replicates SE. Significant of variance (p < 0.05) is analyzed by Duncan Multiple Range Test
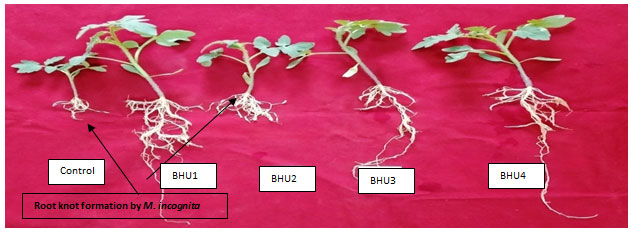
Biopriming tomato seed with bacteria showed higher root length, shoot length, vigour index and reduced gall/ knot formation after 21 days of seed germination. In the current study, it has been found that M. incognita cause root knot disease in tomato seedling resulted in declined root length, shoot length, fresh weight and dry weight, leaf area, leaf number of tomato seedlings. This reduction is due to the formation of root knot in the root leads to the reduction in the growth promotion due to disruption of root system of tomato plant and inhibition of nutrient uptake from the root system (Safni et al. 2018; Sahebani and Gholamrezaee 2021).
Biopriming tomato seed with bacteria showed higher root length, shoot length, vigour index and reduced gall/ knot formation after 21 days of seed germination. Plants inoculated with BHU1, BHU2, BHU3 and BHU4 strain exhibited significant increase in root and shoot length compared to the un-inoculated control. Maximum root length and shoot length of tomato plant was observed by BHU1 strain followed by BHU4 bioprimed bacteria as compare to control plant i.e. 475% root length increment and enhanced shoot length by 6.74cm. Highest fresh root weight as compare to untreated plant infested with M. incognita and shoot weight was enhanced upto 2.36g by BHU1strain over the control plant (Figure 3). Similarly seed germination and viour index reduced in tomato crop under M. incognita infection which is a type of biotic stress (Khanna et al. 2019; Sahebani and Gholamrezaee 2021).
Growth promoting attributes like nutrient mobilization and hormone production maintained even under stressed condition by BHU1, helped in enhancing the root growth, nutrient and water uptake and augmenting the dry weight. Highest root and shoot dry weight were observed by BHU1 and BHU3 strain as compare to control plant. High phosphate solubilizing activity of BHU1 more than 4.8cm zone of clearance must have favored the plant growth directly and depicted as enhancement of fresh and dry root weight and shoot weight respectively and the leaf area had significantly increased and the treated plant with BHU1 and BHU4 was observed to impart more leaf area with 124% increment as compare to control plant. Biopriming of seed with selected bacteria significantly enhanced percentage of seed germination ranged from 57-100%. Maximum vigour index 3880 as compare to untreated plant was observed in BHU1 treated seed which is a very important parameter for promotion of plant growth (Table 6). While almost similar type of result was also observed in tomato by bacterial induction of resistance in tomato against root-knot nematode M. javanica with biocontrol agents (Zhai et al. 2018; Dey and Raghuwanshi 2020; Mazrou et al. 2020).
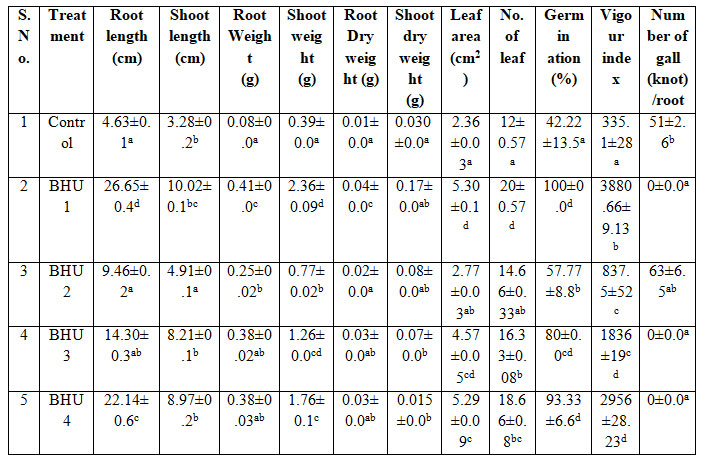
Values are mean of three replicates SE. Significant of variance (p < 0.05) is analyzed by Duncan Multiple Range Test
Figure 3: Effect of selected isolates in tomato plants under nematode infection.
CONCLUSION
The finding of the present study suggests that BHU1 bacterial strain have a very good potential to augment plant growth activity and potential biological control managing root knot nematodes in tomato and in feature leads to incorporate compatible other microorganism enhancing their positive attributes to replace chemical application with promising biocontrol agent. While studying the rhizospheric microorganism and their interaction with plant under biotic stresses there are chances to recognized some microorganism which can enhance directly or indirectly defense immunity in the plant. The performance of individual bacterial strain will always lead to screen potential microorganism for harnessing them to mitigate losses occurred due to biotic stresses.
ACKNOWLEDGEMENTS
This study was financially supported by Banaras Hindu University, Varanasi, Uttar Pradesh, India.
Conflict of Interests: Authors have no conflict of interests to disclose.
REFERENCES
Baker, A.W., and Schippers, B. (1987). Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas SPP-mediated plant growth-stimulation. Soil Biol Biochem, 19(4), 451–457.
Blazevic, D.J., and Ederer, G.M. (1975). Principles of biochemical tests in diagnostic microbiology, Wiley and Company, NewYork,13-45.
Bric, J.M., Bostock, R.M., and Silverstone, S.E. (1991). Rapid in-situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane. Appl Environ Microbiol, 57(2):535-8.
Cheng, W., Yang, J., and Nie, Q. (2017). Volatile organic compounds from Paenibacillus polymyxa KM2501-1 control Meloidogyne incognita by multiple strategies. Sci Rep7: 16213.
Collins, C.H., Patricia, M., Lyne, J., and Grange, M. (1995). Collins and Liyne’s Microbiological Methods, Butterworth-Heinemann, UK, (7), pp.117.
Dey, R., and Raghuwanshi, R. (2020). Comprehensive assessment of growth parameters for screening endophytic bacterial strains in Solanum lycopesicum (Tomato). Heliyon, 6. e05325.
El-Ashry, R.M., Abdelhadi, A.I., Ramadan, M. (2020). Enhancing application efficiency of Pseudomonas SPP. and Serratia marcescens isolates against Meloidogyne incognita in Tomato Plants. Acad. J. Biolog. Sci, 12(2): 127-145 (2020).
El-Shawadfy, M., Mousa, I., and Magdy, E. (2021). Plant growth promoting rhizobacteria (PGPR) as ecofriendly alternatives for management root-knot nematodes, Meloidogyne spp. on tomato plants. IJSRSD, 4:(2), 2537-0715.
Etesami, H., and Adl, S.M. (2020). Plant growth-promoting rhizobacteria (PGPR) and their action mechanisms in availability of nutrients to plants. Phyto-Microbiome in Stress Regulation, pp.147-203.https://doi.org/10.1007/978-981-15-2576-6_9.
Fadden, M.C. (1980). Biochemical tests for identification of medical bacteria. Williams and Wilkins, Baltimore. USA, 51-4.
Gupta, S.K., Prasad, J.K., and Raghuwanshi, R. (2017). Characterizing rhizospheric plant growth promoting bacteria for their effects on oat Avena sativa. Int J Pharma Bio Sci, 8(4):(B) 142-151.
Hayward, A.C. (1960). A method for characterizing Pseudomonas solanacearum. Nature, 186:405. doi: 10.1038/186405a0.
International Seed Testing Association (1993). International rule for seed testing. Seed Sci Technol, 21:25-30.
Kaur, K., Duttajirao, V., Shrivastava, V., et al. (2012). Isolation and Characterization of Chitosan-Producing Bacteria from Beaches of Chennai, India. Enzyme Research, 421683, 6. doi:10.1155/2012/421683.
Khanna, K., Jamwal, V.L., and Kohli, S.K. (2019). Role of plant growth promoting Bacteria (PGPRs) as biocontrol agents of Meloidogyne incognita through improved plant defense of Lycopersicon esculentum. Plant Soil, 436, 325–345.
Kumar, V., Khan, M.R., and Walia, R.K. (2020). Crop Loss estimations due to plant-parasitic nematodes in Major Crops in India. Natl. Acad. Sci. Lett. 43, 409–412.
Lee, Y.S., Nguyen, X.H., and Naing, K.W. (2015). Role of lytic Enzymes secreted by Lysobacter capsici YS1215 in the control of root-knot nematode of tomato plants. Indian J Microbiol, 55, 74–80.
Mazrou, Y.S.A., Makhlouf, A.H., Hassan, M.M., et al. (2020). Microbial induction of resistance in tomato against root-knot nematode Meloidogyne javanica with biocontrol agents. Journal of Environmental Biology, 41:1054-1060.
Pikovskaya, R.I. (1948). Mobilization of phosphorous in soil in connection with vital activity of some microbial species. Mikrobiologiya, 17:362–370.
Razavi, B.S., Hoang, D.T.T., Blagodatskaya, E., et al. (2017). Mapping the footprint of nematodes in the rhizosphere: cluster root formation and spatial distribution of enzyme activities. Soil Biol Biochem, 115:213–220.
Safni, I., Lubis, L.K., Tantawi, A.R., et al. (2018). Isolation and characterization of rhizobacteria for biological control of root-knot nematodes in Indonesia. J ISSAAS, 24;1: 67-81.
Sahebani, N., and Gholamrezaee, N. (2021). The biocontrol potential of Pseudomonas fluorescens CHA0 against root knot nematode Meloidogyne javanica is dependent on the plant species. Biological control, 152, 104445.
Schwyn, B., and Neilands, J.B. (1987). Universal chemical assay for the detection and determination of siderophore. Anal Biochem, 160, 47–56.
Shahzad, M.S., Khalid, A., Arshad, M., et al. (2008). Integrated use of plant growth promoting bacteria and P-enriched compost for improving growth, yield and nodulation of chickpea. Pak J Bot, 40, 1735–1744.
Somasegaran, P., and Hoben, H.J. (1994). Handbook for Rhizobia. Methods in Legume–Rhizobium Technology. Heidelberg, NY: Springer, 10.1007/978-1-4613-8375-8.
Subedi, P., Gattoni, K., Liu, W., et al. (2020). Current utility of plant growth-promoting rhizobacteria as biological control agents towards plant-parasitic nematodes. Plants, 9: 1167.
Yoshida, S., (1972). Physiological aspects of grain yield. Ann Rev Plant Physiol, 2, 437-464.
Zhai, Y., Shao, Z., Cai, M., et al. (2018). Zhang J. Multiple Modes of Nematode Control by Volatiles of Pseudomonas putida 1A00316 from Antarctic Soil against Meloidogyne incognita. Frontiers in Microbiology, 9.
Zhao, J., Wang, S., Zhu, X., et al. (2021). Isolation and characterization of nodules endophytic bacteria Pseudomonas protegens Sneb1997 and Serratia plymuthica Sneb2001 for the biological control of root-knot nematode. Applied Soil Ecology, 164, 103924.


