1Medicinal Plants Research Center, University of Medical Sciences of Zabol, Zabol, Sistan and Baloochestan, Iran
2Department of chemistry, College of science, University of Tehran, Tehran, Iran
3Department of Pharmacology and Toxicology, University of Medical Sciences of Zabol, Zabol, Sistan and Baloochestan, Iran
4,5,6Department of Basic Sciences, Faculty of Veterinary Medicine, University of Zabol, Zabol, Sistan and Balochestan, Iran
7Department of Microbiology and Parasitology, University of Medical Sciences of Zabol, Zabol, Sistan and Baloochestan, Iran
Article Publishing History
Received: 10/10/2017
Accepted After Revision: 12/12/2017
Insulin resistance and overweight have been associated with major risk factors such as blood pressure (BP) for cardiovascular disease. In this study the effect of Nano-capsules of resveratrol (RV-NC) on BP control is evaluated. RV-NC nanoparticles were analyzed by SEM, Zeta sizer, Potentiometer and HPLC. The analysis resulted from RV-NC synthesis showed that the Nano capsules have characteristics such as size of 207 nm, zeta potential of -7.15 and loading efficiency of 99.54% ± 1.02. BP reduction was associated with reduction of weight and enhance of QUICKI index which represents insulin resistance. RV-NC were prepared by interfacial deposition and then its applicability was evaluated in metabolic syndrome induces mice. The effect of RV-NC was studied on fourteen mice. Induction of syndrome by high fat diet and high BP was observed. The collected data were analyzed by ANOVA and Turkey criteria were used to compare the distinction between the groups. Finally, the results indicated that RV-NC-treated mice have regulated in systolic and diastolic blood pressure (compare to the other group (p< 0.05). The effective formulation of nano-capsules for resveratrol delivery not only can be helpful in increasing the in vivo stability, but also in regulation of the patient’s blood pressure with at least cost of therapy.
Resveratrol, Nano-Capsulation, Cardiovascular Disease, Drug Delivery
Shahraki A, Bahadorikhalili S, Hashemzaei M, Hajinezhad M, Afsharimoghaddam A, Sarani F, Tajrobekar O. Resveratrol Nano-Capsule as an Efficient Tool for Blood Pressure Regulation: A Study on Metabolic Syndrome Induced Mice. Biosc.Biotech.Res.Comm. 2017;10(4).
Shahraki A, Bahadorikhalili S, Hashemzaei M, Hajinezhad M, Afsharimoghaddam A, Sarani F, Tajrobekar O. Resveratrol Nano-Capsule as an Efficient Tool for Blood Pressure Regulation: A Study on Metabolic Syndrome Induced Mice. Biosc.Biotech.Res.Comm. 2017;10(4). Available from: https://bit.ly/2WPTnWH
Introduction
Hypertension (BP: 140/90 mmHg), as one of the main symptoms of metabolic syndrome, can be caused by fatty and high-calorie diet associated with obesity and insulin resistance. This problem has been introduced as a serious warning sign in patients with heart disease. (Danaei et al, 2013; Jahandideh et al, 2016; Nonogaki et al, 2016). Global Research has been found that, approximately % 45 and 51% of deaths are resulted by coronary stroke and artery disease, respectively (Brook, 2013; Movahed et al, 2016). The clinical studies have shown that prescription of anti-hypertensive drugs for the hypertensive patients might cause a number of side effects. Therefore, medical researchers are interested in using natural sources instead of chemicals for the production antihypertensive drugs (Aluko et al, 2015; BC Guidelines, 2016).
Recently, plant polyphenols such as resveratrol have been successfully applied in improving the symptoms of insulin resistance and obesity in metabolic syndrome, and therefore it has opened a special place in global trade as a medicinal compound in the regulation of blood pressure in patients with heart problems, diabetes and other diseases (Raj et al, 2013; Liu et al, 2015; Movahed et al, 2016). However, the natural polyphenols suffer from a number of disadvantages such as low biological half-life, high volatility and rapid removal, which limits the in vivo applicability of these compounds (Cottart et al, 2015; Khaled et al, 2016)
Therefore, new studies have been conducted on the basis of nanotechnology to achieve effective formulation of pharmaceutical medicines (Smoliga, 2014; Penalva et al, 2015; Reis et al, 2016; Jadhave et al 2016; Shindikar et al, 2016). One methods are Nano capsule formation by coating the unstable medicinal compounds by biodegradable (Venturini et al ,2011; Frozza et al, 2013; Friedrich et al ,2015; Conte et al, 2016). Regarding the advantages of Nano capsules, the main goal of this study is to use an effective formulation of resveratrol in a stable Nano capsule to improve the fluctuations problems in blood pressure. The effect of the capsulated resveratrol is studied in mice with metabolic syndrome by fat diet
| Table 1: The physiochemical characteristics of Nano capsules containing RV-NC and B-NC |
| Formulation |
| B-NC R-NC |
| Size (nm) 205± 0.05 207±0.03 |
| PDI 0.12±0.09 0.12±0.04 |
| Zeta potential (mv) -6.21± 0.45 -7.15± 0.19 |
| PH 6.47± 0.02 6.22±0.04 |
| Encapsulation efficiency (%) – 99.54±1.02 |
| Table 2: Composition of High Fat Diet (HFD) and Low Fat Diet (LFD) | ||||
| HFD | LFD | |||
| Present of total kcal (kcal%) | Present of total mass (g%) | Present of total kcal (kcal%) | Present of total mass (g%) | |
| 25 | 25.84 | 30 | 28.1 | protein |
| 30 | 34.69 | 60 | 58.04 | carbohydrate |
| 45 | 42.5 | 10 | 13.15 | Fat |
| 4.26 | 3.02 | total (kcal/g) | ||
Material and Methods
Trans-resveratrol, PCL, Span 60 and Tween 80 obtained from Stigma Aldrich. Other chemicals and solvents were from analytical and pharmaceutical types. The Low Fat Diet (LFD) was prepared from Khorasan Seedling Company and to prepare High Fat Diet (HFD), fat-tail was used that had high levels of saturated fat. RV-NC was prepared by interfacial deposition method as described previously (Frozza et al, 2010). Briefly, to prepare the aqueous phase, polysorbate (0.0380 g) was dissolved in 53 ml of distilled water. The organic phase was prepared by vigorous stirring of RV, PCL, capric triglycerides, and sorbitanmonostearate in 27 ml of acetone at 40 0C. At the end, the organic phase was added to the aquatic phase and acetone was evaporated after 10 min and the suspension was concentrated under reduced pressure and filtered by 8 micrometer filter paper. Then, the non-loaded B-CN Nano capsules suspension was synthesized with the above method as the control formulation.
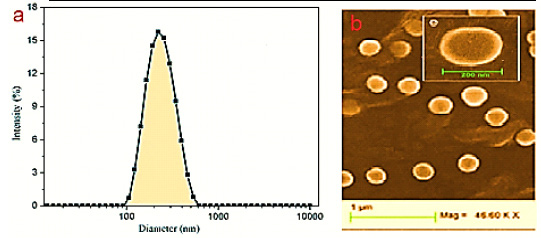 |
Figure 1: Size distribution graph for resveratrol – loaded Nano capsules (RV-NC) obtained by (a) DLS and (B) SEM photomicrographs. |
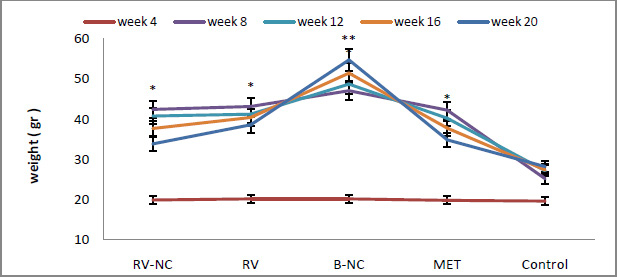 |
Figure 2: Weight changes in 5 different groups in 20 weeks. All data are expressed as mean ± standard deviation (n=8) and different letters show a significant difference at p < 0.05. |
To determine the size, zeta potential and polydispersity of the Nano capsules, zeta sizer and particle sizes (20101 SA, made in Japan) with laser light scattering method at 250C were used. Before the experiment, the sample was diluted with MilliQ water or 0.01 µM NaCl and filtered by MILLIPORE 0.45 µM. The measurement was repeated for each formulation in triple mode. To determine PH, AL-1703 and MUNCHEN an immersed electrode in suspension were used at room temperature. The concentration of the loaded active substance (RV) in suspension Nano capsules was determined by HPLC using CLC-C8 column and equipped with a UV detector and using water and acetonitrile as mobile phase with the flow rate of 1.2 ml/min and inhibition time of 3.45 min. The capsulation efficiency was calculated as below:
Here, resveratrol load and resveratrol in supernatant are active and free substance concentrations respectively. The free substance concentration was obtained by acetonitrile and the active substance extraction from suspension formulation was obtained by integrating ultrafiltration and centrifuge. 40 male mice (C57BL/6, 20-24 g, 4 weeks) were selected. The animals were kept in vitro under standard conduction such as free access to food and water in a room with controlled temperature (20-24 0C) and on a 12 h-light/dark cycle. The experimental protocol of this study was approved by Animal Ethical Committee of Zabol University of Medical Sciences. Before the experiment, all mice were acclimatized for an adaptation period a week and then, all groups except the control group (n=8, LFD), were kept under high fat diets (n=32, HFD) for 12 weeks. Measuring the parameters such as weight (each week), insulin levels and glucose were done by FG4000, Cayman kit by ELISA method, and ARKRAY, respectively.
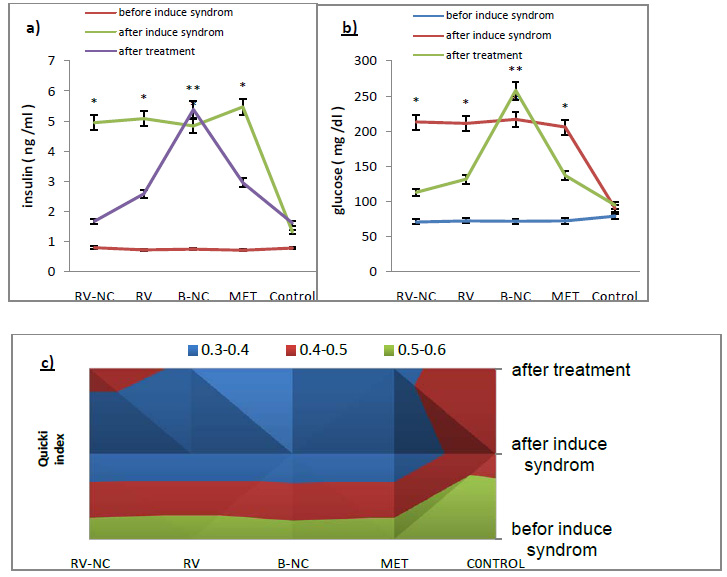
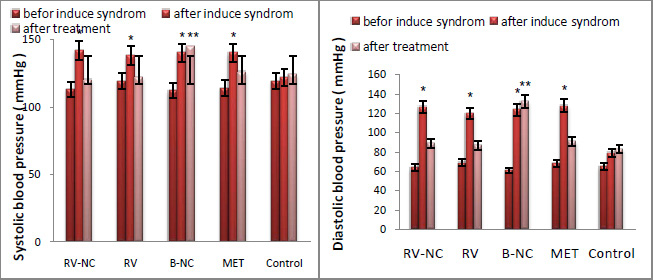
The non-invasive blood pressure (BP) system (URIT, Poland) with a tail-cuff sphygmomanometer was used to measure systolic and diastolic (approximate measurement) blood pressure. A clear plastic tube used to placed mice and tail hole pieces secured at either end. A nervous, stressed animal may have diminished circulation in the tail so the animals were placed in the holders at least 10 to 15 minutes prior to obtaining pressure measurements. At this step, mice which have consumed high fat diet randomly place in 4 groups which included; the groups treated with resveratrol Nano capsule (RSV-NC; 5 mg/kg/day), blank Nano capsule (B-NC; 5mg/kg/day), free resveratrol (RSV; 100 mg/kg/day), and metformin (MET; 250 mg / kg / day). The measured variables were done similarly at 3 steps: before and after induce syndrome and after treatment. The results are reported from AVONA as mean and standard deviation for at least 3 different experiments (Mean ± SD) and accordingly, significant difference between the groups can be observed (P<0.05). For between-group comparison, Tukey criteria are used as suitable criteria in making distinction between groups.
Results and Discussion
RSV-NC and B-NC were synthesized using biodegradable materials such as poly- caprolactone (PCL) with no need to additional steps with interfacial deposition method. The physiochemical characteristics of Nano capsules are mentioned in Table 1. The zeta potentials of RSV-NC and B-NC were obtained as -7.15 and 6.21, respectively. The negative values indicate the existence of polysorbate 80 in formulation that leads to their increased spatial resistance in water/particle surface. Also, the Nano capsules suspensions were analyzed by DLS and monomodal stability in size distribution and polydispersity index were observed to be lower than 0.3, which shows the narrow size. Particle size for RSV-NC and B-NC were about 200 nm and according to resulting of Friedrich et al, 2015 is an acceptable size. In addition, the pH for the formulations of both Nano capsules was larger than 6. HPLC method showed the capsulation efficiency of RSV-NC to be .54±1.02. These results are consistent with the new findings in this formulation.
Fat Diet
Ruminant fat was used to induce metabolic syndrome in mice. The HFD and LFD components are presented in Table 2. The diet in this study has 30% carbohydrate, 45% fat and 25% protein that is almost similar to the diets in various societies.
The weight means of HFD and LFD groups before and after induced metabolic syndrome were compared. According to the statistical results obtained from ANOVA, the significance level between the two groups is lower than 0.05. This states that there is a significant difference between the weights of HFD (n=32) and LFD (n=8), due to the higher level of saturated fat, that the HFD group have received (fig.2). Analyzing the results by Tukey test show that, HFD subgroup (RV-NC, B-NC, RV and MET, n = 8), had higher weights which was because of receiving high levels of saturated fat for 12 than control group that used standard diet. According to the findings in fig 2, the weight means of RV-NC, RV and MET groups show a significant reduction during 4 weeks of treatment and among these, group RV-NC showed a weight loss in a shorter time.
The QUICKI index was the main index for the insulin resistance measurement, which is directly obtained from glucose and insulin values. In this study, the results of fig3, show that there is a significant difference between HFD group compared to the control group (P<0.05). HFD groups have the highest value in glucose and insulin and therefore have the lowest QUICKI index. Results of testing between groups, Tukey test, show that glucose and insulin levels in 5 groups of RV-NC, B-NC, RV, MET and control are different. In this study, B-NC group has the highest levels of insulin and glucose and the lowest values QUICKI index, in contrast to the control group. In the RV-NC group, QUICKI index reach normal range, while the glucose and insulin values were normal. RV and MET groups have also the same result, relatively. Generally, it can be understood that RV-NC group operate better in increasing QUICKI index in shorter period of time. Since the blood pressure is one of the main symptoms of the metabolic syndrome, this parameter was investigated in mice fed fat diet for 12 weeks and 4-week treatment compare to control group. The results of changes in values systolic and diastolic blood pressure which Statistical analysis by ANOVA, in Figure 4, section A and B respectively, show that there was significant difference between HFD group and LFD groups in values of systolic and diastolic blood pressure (P<0.05). After separation the animals HFD into 5 subgroups (RV-NC, B-NC, RV, MET) and conducted treatment phase, Tukey method for compare between groups were used. The results show a significant reduction of systolic and diastolic blood pressure in RV-NC, RV and MET groups. B-NC group which has used Nano capsules without pharmaceutical active ingredient, have the highest amount in blood pressure, while not observed in the control group significant changes over time. It appears that changes in Systolic blood pressures are more obvious than diastolic. It is clear that the group RV-NC in the regulation of blood pressure in Comparisons between groups of RV-NC, RV, MET, is better. Discussion
This study assessed the potential effects of resveratrol –loaded Nano capsules suspension on insulin to resistance (IR) and systolic and diastolic blood pressure in metabolic syndrome induced in mice. The results show that there is a major association between the resistance to the effects of insulin on both glucose uptake and insulin-induced vasodilatation in obese hypertensive patients, which are in accordance with the previous findings (Ferrannini et al, 1987; Laakso et al, 1989; Natali et al, 1997; Lastra et al, 2010; Horita et al, 2011, Zhou et al, 2012).
The homeostasis model assessment-estimated insulin resistance (HOMA-IR) has been widely used for the estimation of IR in research (Matthews et al, 1985). It is calculated multiplying fasting plasma insulin (FPI) by fasting plasma glucose (FPG), then dividing by the constant 22.5, i.e. HOMA-IR = (FPI×FPG)/22.5 (Wallace et al, 2004). index that we used to determine IR is the quantitative IR check index (Quicki index) which that is a novel mathematical transformation of fasting blood glucose and insulin levels and useful index of IR in subjects with hypertension, obesity, type 2 diabetes, gestational diabetes, pregnancy, PCOS, premature adrenarche, hyperandrogenism, and nonalcoholic steatohepatitis (Katz et al, 2000; Hui et al, 2003).
Lifestyle factors such as excess body fat, excess dietary fat (total, Trans, and saturated fat), fake carbohydrates, smoking, stress and insufficient exercise are causes IR. This problem is a central part of a cluster of metabolic abnormalities called the metabolic syndrome. Candidate mechanisms whereby this metabolic syndrome might lead to hypertension include stimulation of sympatho-adrenergic activity, altered cellular electrolyte transport and composition, growth promoting effects, renal sodium retention and vascular hyper responsiveness (Lithell et al, 1998; Velliquette et al, 2003). In person with IR, the cells do not respond to insulin normally and glucose cannot easily enter the cells. As a result, the insulin level in blood will be high. Finally, the body will not be capable of building enough insulin to control blood glucose at normal level and diabetes, cardiovascular disorder and others occurs (Borkman et al, 1993; Vessby et al, 2001; Risérus et al, 2009; Sandeep et al, 2010). The diet used in this study consisted of proteins (25%), carbohydrates (30%) and saturated fat (45%) that is almost similar to the diets in most of the societies. The available diets for animal model almost contain 60% fat so that insulin resistance can be observed, but is not similar to the diets that are used by Individuals and cannot be generalized simply (Nishina et al, 1990; Surwit et al, 1995).
Several studies have been conducted on the edible containing the active ingredient in the prevention and treatment of insulin resistance and blood pressure regulation .The results show that edibles containing polyphenolic compounds (such as Red Grapes, Dark Chocolate and Blueberries) could be effective in this (Dauchet et al. 2005; Hu and Willett, 2002). Since Polyphenols such as resveratrol which that improve the risk factors of cardiovascular disorders (Poulsen et al, 2013), diabetes (Hausenblas et al, 2014) and pathologic conditions (Fernández et al,2011) is unstable in vivo and so use it expensive for patients (Joseph et al, 2006), Studies developed towards to produce new formulations to improve protecting and reaching acceptable level of bioavailability (Finley et al, 2010 and Francioso et al, 2014)
Nowadays, scientists could be using new technologies, especially nanoscience for drug delivery of active ingredients unstable to form of nanocapsules (Contri et al, 2016; Scognamiglio et al 2016 and Vivienne et al, 2016). Some of the advantages this method, Include the development of controlled-release system, maintaining the drug concentration in blood plasma for a long time, the possibility of developing drugs with very low dose and stability and efficacy impressive. In this study, we prepared protected form of resveratrol in the coating of biodegradable polymer PCL (poly-caprolacton) with a size of approximately 200 nm. However, the results of experiments in this plan highlight the successful performance of RV-NC compared to other groups to reduction of IR and regulation of blood pressure.
Conclusion
This is the first study on the effect of resveratrol loaded Nano capsule (RV-NC) on insulin resistance (IR) and blood pressure profiles in animal model metabolic syndrome. The results demonstrated that using high saturated fat in daily diet can cause IR and hypertension. On the other hand, RV-NC regulates blood pressure and reduces IR by reducing the amount of the fat in whole body. These findings suggest that further studies should be conducted on the effect of RV-NC on animal and human induced hypertension models, obesity, type 2 diabetes, gestational diabetes, pregnancy, PCOS, premature adrenarche, hyperandrogenism, and nonalcoholic steatohepatitis.
Acknowledgement
The findings of this study are obtained by the financial support of Medical University of Zabol. The authors express their gratitude to Dr. Zahra Sepehri, the Deputy of Research and Technology of the university.
References
- Aluko RE. (2015). Antihypertensive peptides from food proteins. Annu Rev Food Technol. 6:235-262.
- Borkman, M, Storlien, LH, Pan, DA et al, (1993). The relation between insulin sensitivity and the fatty acid composition of skeletal muscle phospholipids. NEJM. 328:238–244.
- Brook RD, Appel LJ, Rubenfire M, Ogedegbe G, Bisognano JD, and Elliott WJ. (2013). Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the American Heart Association. Hypertension. 61(6):1360–1383.
- Conte R, Calarco A, Napoletano A, Valentino A, Margarucci S, Di Cristo F, Di Salle A and Peluso G. (2016). Polyphenols Nanoencapsulation for Therapeutic Applications. Journal of Biomolecular Research & Therapeutics. 5:139.
- Contri RV, Fiel LA, Alnasif N, Pohlmann AR, Guterres SS, Schäfer-Korting M. (2016). Skin penetration and dermal tolerability of acrylic nanocapsules: Influence of the surface charge and a chitosan gel used as vehicle. International Journal of Pharmaceutics. 507: 12-20.
- Cottart CH, Nivet-antoine V, Laguillier-morizot C, Beaudeux JL. (2010). Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res. 54(1):7-16.
- Danaei G, Singh GM, Paciorek CJ, Lin JK, Cowan MJ, Finucane MM, Farzadfar F, Stevens GA, Riley LM, Lu Y, Rao M, Ezzati M. (2013). The global cardiovascular risk transition: associations of four metabolic risk factors with national income, urbanization, and Western diet in 1980 and 2008. Circulation. 127(14): 1493–1502.
- Dauchet L., Amouyel P., Dallongeville J. (2005). Fruit and vegetable consumption and risk of stroke: a meta-analysis of cohort studies. Neurology 65: 1193–1197.
- Fernandez, J., Curt, M. D., & Aguado, P. L. (2006). Industrial applications of Cynara cardunculus L. for energy and other uses. Ind. Crops Prod. 24: 222-229.
- Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S. (1987). Insulin resistance in essential hypertension. N J Med. 317(6):350-7.
- Finley JW, Kong AN, Hintze KJ, Jeffery EH, Ji LL and Lei XG. (2011). Antioxidants in Foods: State of the Science Important to the Food Industry. J. Agric. Food Chem. 59 (13): 6837–
6846. - Francioso A, Mastromarino P, Masci A, Mosca L. Chemistry, (2013). Stability and Bioavailability of Resveratrol. Medicinal chemistry. 10(3).
- Friedrich, R.B.; Kann, B.; Coradini, K.; Offerhaus, H. L.; Beck, R.C.; Windbergs, M. (2015). Skin penetration behavior of lipid-core nanocapsules for simultaneous delivery of resveratrol and curcumin. Eur. J. Pharm. Sci. 78: 204–213.
- Frozza RL, Bernardi A, Paese K and Salbego C. (2010). Characterization of trans-Resveratrol-Loaded Lipid-Core Nanocapsules and Tissue Distribution Studies in Rats. Journal of Biomedical Nanotechnology. 6(6):694-703.
- Frozza RL, Bernardi A, Hoppe JB, Meneghetti AB, Matté A, Battastini AMO, Pohlmann AR, Guterres SS and Salbego C. (2013). Neuroprotective Effects of Resveratrol Against Aâ Administration in Rats Are Improved by Lipid-Core Nanocapsules. Molecular Neurobiology. 47, (3 ): 1066–1080.
- Hausenblas, H.A.; Schoulda, J.A.; Smoliga, J.M. (2014). Resveratrol treatment as an adjunct to Pharmacological management in Type 2 diabetes mellitus-systematic review and meta-analysis. Mol. Nutr. Food Res. 59: 147-159.
- Horita S, Seki G, Yamada H, Suzuki M, Koike K, and Fujita T. (2011). Insulin Resistance, Obesity, Hypertension, and Renal Sodium Transport. Int J Hypertens. 391762.
- Hu FB, Willett WC. (2002). Optimal diets for prevention of coronary heart disease. Journal of the American Medical Association. 288(20):2569–2578.
- Hui JM, Sud A, Farreii GC. (2003). Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression. Gastroenterology. 125:1695–1704.
- Jadhav P, Bothiraja C and Pawar A. (2016). Resveratrol-piperine loaded mixed micelles: formulation, characterization, bioavailability, safety and in vitro anticancer activity. RSC Adv. 6: 112795-112805.
- Jahandideh F, Chakrabarti S, Majumder K, Li Q, Panahi S, Morton J, Davidge ST and Wu J. (2016). Egg white protein hydrolysate reduces blood pressure, improves vascular relaxation and modifies aortic angiotensin II receptors expression in spontaneously hypertensive rats. Journal of Functional Foods. 27: 667–673.
- Joseph A. Baur & David A. Sinclair. (2006). Therapeutic potential of resveratrol: the in vivo evidence. Nature Reviews Drug Discovery. 5: 493-506.
- Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. (2000). Quantitative insulin-sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 85:2402–2410.
- Khaled K, Abu-Amero, Altaf A, Kondkar and Kakarla V. Chalam. (2016). Resveratrol and Ophthalmic Diseases. Nutrients, 8(4), 200.
- Laakso M, Sarlund H, Salonen R, Suhonen M, Pyorala K. (1991). Asymptomatic atherosclerosis and insulin resistance. Arterioscler Thromb. 11: 1068–1076.
- Lastra G, Dhuper S, Johnson MS and Sowers JR. (2010). Salt, aldosterone, and insulin resistance: impact on the cardiovascular system. Nature Reviews Cardiology. 7: 577-584.
- Lithell H. (1998). Insulin resistance and diabetes in the context of treatment of hypertension Suppl 3. Blood Press. 28–31.
- Liu FC, Tsai H, Yu HP. (2015). Organ-Protective Effects of Red Wine Extract, Resveratrol, in Oxidative Stress-Mediated Reperfusion Injury. Oxidative Medicine and Cellular Longevity. 568634.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. (1985). Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28(7):412-419.
- Movahed A, Ostovar A, Iranpour D, Thandapilly SJ, Raj P, Louis XL, Smoliga JM and Thomas Netticadan. (2016). The efficacy of resveratrol in controlling hypertension: study protocol for a randomized, crossover, double-blinded, placebo-controlled trial. 17: 296-304.
- Natali A, Bonadonna R, Santoro D, Galvan AQ, Baldi S, Frascerra S, Palombo C, Ghione S, Ferrannini E.Insulin (1994). Resistance and Vasodilation in Essential Hypertension. Studies with Adenosine. J Clin Invest. 94 (4): 1570-1576. 1994.
- Nishina, P.M., Verstuyft, J., Paigen, B.Synthetic (1990). Low and High Fat Diets for the Study of Atherosclerosis in the Mouse.The Journal of Lipid Research. 31: 859-869.
- Nonogaki K, Yamazaki T, Murakami M, Satoh N, Hazama M, Takeda K, Tsujita N, Katoh S, Kubota N. (2016). Low-frequency and very low-intensity ultrasound decreases blood pressure in hypertensive subjects with type 2 diabetes. International Journal of Cardiology. 215:147-149.
- Penalva R., Esparza I., Larraneta E., González-Navarro C. J., Gamazo C., Irache J. M. (2015). Zein-Based nanoparticles improve the oral bioavailability of resveratrol and its anti-inflammatory effects in a mouse model of endotoxic shock. Journal of Agricultural and Food Chemistry. 63(23):5603–
5611. - Poulsen MM, Jørgensen JO, Jessen N, Richelsen B, Pedersen SB. (2013). Resveratrol in metabolic health: an overview of the current evidence and perspectives. Ann N Y Acad Sci. 1290:74–82.
- Raj P, Louis XL, Thandapilly SJ, Movahed A, Zieroth S, Netticadan T. (2013). Potential of Resveratrol in the Treatment of Heart Failure. Life Sci. 95 (2): 63-71.
- Reis S, Neves AR, Nunes C and Amenitsch H. (2016). Effects of resveratrol on the structure and fluidity of lipid bilayers: a membrane biophysical study. Soft Matter. 12: 2118-2126.
- Risérus U, Willett WC, Hu FB. (2009). Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 48(1):44-51.
- Sandeep S, Gokulakrishnan K, Velmurugan K, Deepa M, Mohan V. (2010). Visceral and subcutaneous abdominal fat in relation to insulin resistance and metabolic syndrome non-diabetic south Indians. Indian J Med Res. 131:629-635.
- Scognamiglio F, Travan A, Borgogna M, Donati I, Marsich E, Bosmans JM, Perge L, Foulc MP, Bouvy ND, Paoletti S. (2016). Enhanced bioadhesivity of dopamine-functionalized polysaccharidic membranes for general surgery applications. Acta Biomaterialia. 44: 232-242.
- Shindikar A, Singh A, Nobre M and Kirolikar S. (2016). Curcumin and Resveratrol as Promising Natural Remedies with Nanomedicine Approach for the Effective Treatment of Triple Negative Breast Cancer. Journal of Oncology. ID9750785.
- Smoliga JM, Blanchard O. (2014). Enhancing the Delivery of Resveratrol in Humans: If Low Bioavailability is the Problem, what is the Solution? Molecules. 19: 17154-17172.
- Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, et al. (1995). Differential effects of fat and sucrose on the development of obesity and diabetes in c57bl/6j and a/j mice. Metabolism. 44(5):645–651.
- Velliquette RA, Ernsberger P. (2003). The role of I (1)-imidazoline and alpha (2)-adrenergic receptors in the modulation of glucose metabolism in the spontaneously hypertensive obese rat model of metabolic syndrome X. J Pharmacol Exp Ther. 306: 646–657.
- Venturini CG, Jäger E, Oliveira CP, Pohlmann AR. (2011). Formulation of lipid core nanocapsules. Colloids and Surfaces A Physicochemical and Engineering Aspects. 375(1-3):200-
208. - Vessby B, Unsitipa M, Hermansen K et al (2001). Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in health men and women: the KANWU study. Diabetologia 44, 312–319.
- Vivienne H. Tam, Chris Sosa, Rui Liu, Nan Yao, Rodney D. Priestley. (2016). Nanomedicine as a non-invasive strategy for drug delivery across the blood brain barrier. International Journal of Pharmaceutics. 515: Pages 331-342.
- Wallace TM, Levy JC, Matthews DR. (2004). Use and Abuse of HOMA Modeling. Diabetes Care. 27 (6):1487-1495.
- Zhou MS, Wang A, and Yu H. (2014). Link between insulin resistance and hypertension: What is the evidence from evolutionary biology? Diabetol Metab Syndr. 6: 12.