Seri-biotech Research Laboratory, Central Silk Board, Kodathi, Carmelram Post, Bengaluru- 560 035, India.
Corresponding author email: ravikumarpillai@gmail.com
Article Publishing History
Received: 10/02/2020
Accepted After Revision: 27/03/2020
Silk sericin is a natural polymer with wide utility in biomedical applications. The use of native sericin protein has been well documented in cell culture and cryopreservation of various types of cells. However, the use of recombinant sericin is limited and currently there is no report on the effect of recombinant sericin fusion protein on cell culture. In this work, we report the use of recombinant sericin-cecropin B fusion in the proliferation and cryopreservation of primary human dermal fibroblast cells. Compared with cells cultured in the control with FBS, those cultured in sericin/sericin-cecropin B supplemented media (along with FBS) showed enhanced proliferation of cells. The culture medium containing sericin or its fusion also proved effective in cryopreservation of human dermal fibroblasts with reduced amount of FBS. However, low DMSO concentration did affect the viability of cells post-freezing. Both proliferation and cryoprotection properties were found enhanced in the recombinant sericin-cecropin B treated group than in recombinant sericin alone. Our results clearly show that the use of sericin-cecropin B could act as potential media supplement for the enhanced proliferation and improved cryopreservation of human dermal fibroblasts.
Silk sericin, Fusion protein, Proliferation, Cryopreservation.
Thomas D. S, Manoharan C, Rasalkar S, Mishra R. K, Gopalapillai R. Recombinant Sericin-Cecropin B Fusion Protein Aids in the Proliferation and Cryopreservation of Human Dermal Fibroblast Cells. Biosc.Biotech.Res.Comm. 2020;13(1).
Thomas D. S, Manoharan C, Rasalkar S, Mishra R. K, Gopalapillai R. Recombinant Sericin-Cecropin B Fusion Protein Aids in the Proliferation and Cryopreservation of Human Dermal Fibroblast Cells. Biosc.Biotech.Res.Comm. 2020;13(1). Available from: https://bit.ly/2ufsfDQ
Copyright © Thomas et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Sericin and fibroin are the two major proteins in the silk fibre produced by the domesticated silkworm, Bombyx mori. Sericin proteins are produced by the middle silk gland (MSG) and Ser1 is the most abundant sericin protein. It is characterized by a serine-rich peptide consisting of 38-amino acids repeats reported to play important roles in hydrophilicity, cryoprotection, and cell proliferation (Tsujimoto et al., 2001; Terada et al., 2005). Fibroin has been used in textile manufacturing and for several biomaterial applications, whereas sericin is treated as a waste material in the textile industry. However, sericin has shown interesting applications in cosmetics and pharmaceuticals (Kundu et al., 2008 Cao and Zhang, 2017
Zhang et al., 2019 ).
The use of cell and tissue culture is essential for various research and medical applications. Hence, the culture and storage of cells is of critical importance in a standard cell culture laboratory. Fetal bovine serum (FBS) is widely used in cell culture as it aids in cell proliferation, growth and storage. However, the serum is expensive and also causes concern regarding bovine spongiform encephalopathy (BSE). Sericin has been presented as a potential alternative to FBS, can promote proliferation of several types of cells and protect cells from freezing stress. Dermal fibroblasts are responsible for producing the extracellular matrix forming the connective tissue of the skin and play a crucial role in wound healing, tissue repair, and remodelling, (Cao and Zhang, 2017).
Proteins derived from genetic engineering are characterized by control over composition, sequence, molecular mass, and stereochemical purity. Most importantly, unlike native sericin, recombinant sericin is free from contamination. Recently we have recombinantly expressed partial repeat sequences of sericin 1 protein and its fusion with cecropin B in bacteria (Thomas et al., 2020 – paper communicated). The recombinant sericin/sericin-cecropin B has shown strong antibacterial activity against gram-negative and positive bacteria. In this paper, we describe the effect of recombinant sericin-cecropin B fusion protein in the proliferation and cryoprotection of primary human dermal fibroblasts. This is the first report on the use of recombinant sericin fusion protein in cell culture applications.
MATERIALS AND METHODS
Recombinant sericin and sericin-cecropin B proteins :Both sericin and its fusion with cecropin were expressed in Escherichia coli as described by us (Thomas et al, 2020-paper communicated). Briefly, 282 bp Ser1 sequence of Bombyx mori (Accession No. AB112019) coding for 94 amino acids containing one copy of 38-amino acid motif and two copies of decapeptide repeats alone or fused with an open reading frame of B. mori cecropin B (Accession No.NM_001102561) without its signal sequence were cloned in to pET-47b(+) and expressed in E. coli Rosetta (DE3) strain. Recombinant proteins were purified by immobilized metal affinity chromatography (IMAC). The following recombinant proteins were used for the assays: 1. sericin, 2. sericin-cecropin B fusion, and 3. proteins purified from bacteria harbouring empty vector as control.
Cells and culture conditions: Human primary adult dermal fibroblast cells (EZXpand Dermal Fibroblast, HiMedia) were cultured in HiFibroXL Fibroblast Expansion Medium, supplements and containing gentamycin-amphoterecin B (HiMedia). This modified culture medium contained reduced amount of FBS (2%, unlike the conventional 10%) as indicated by the manufacturer. Cells were cultured in an incubator at 37oC under 5% CO2 till they attained confluency. Each well (6-well culture plates) was seeded with 1 x 105 cells and each treatment had three replicates. Recombinant protein concentration was 25 or 150µg/ml and added in to medium in the respective groups. Cells were allowed to grow and observations on adherent cells under microscope followed by digestion with trypsin and counting of cells. The following groups were employed for the assays: 1. CM (Culture medium) alone, 2. CM + PB (Protein purified from bacteria harbouring empty vector), 3. CM + RS (Recombinant sericin), and 4. CM + RSC (Recombinant sericin-cecropin B).
Cryopreservation of cells: Cells were grown to confluency and resuspended at 1 x 105 cells/ml in the freezing medium containing culture medium as above, 23% FBS (MP Biomedicals) and 10% DMSO (Sigma). Different concentrations of FBS (11.5%) and DMSO (5%) were also tested. Recombinant protein concentration was 500 µg/ml or 2mg/ml and added in to medium in the respective groups. Cells after adding the freezing media were chilled on ice for 5 min and then kept at –80oC. After 24 h, cells were thawed, fresh culture medium was added and centrifugation done to remove the DMSO. Cells were then cultured on 6-well plates with 1 ml fresh culture media for a day and observations taken. Cells without the addition of recombinant proteins were also used as control.
Cell morphology, number and viability: Morphology of the cells was studied by visualizing the cells under inverted microscope (Leitz, Germany). The viable and non-viable cell densities were determined by the Trypan Blue (HiMedia) exclusion method using a Neubauer haemocytometer.
RESULTS AND DISCUSSION
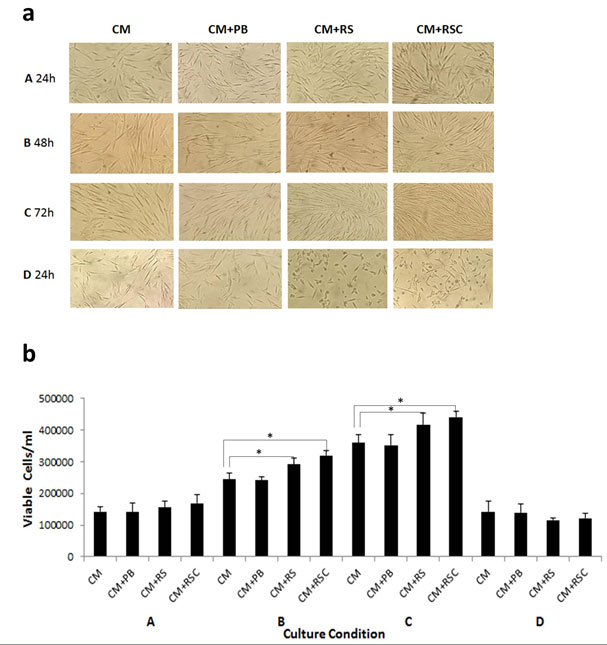
Cell proliferation: Results are presented in Figure 1 a & b. The cells grown in complete culture media (CM) were used as control. The attachment, growth and proliferation of cells were found in all groups and as same as that of control. The morphology of cells was typically spindle shaped and comparable to the cells grown in normal culture media. The CM+PB exhibited normal growth as it contained the complete culture media. Comparatively, the cells grown in recombinant sericin (CM+RS) and sericin-cecropin B fusion (CM+RSC) supplemented media showed marked increase in density of viable cells. Subsequently, the cell density gradually increased and attained confluence at 48-72 h. The highest proliferation of cells was observed in media supplemented with recombinant sericin-cecropin fusion protein (CM+RSC). Compare to CM, the number of cells in the CM+RSC and CM+RS increased by 1.22 and 1.16 times, respectively, on 72 h. However, higher concentration of sericin and sericin-cecropin fusion protein drastically inhibited the cell growth and proliferation, resulting in round shaped and reduced number of cells (Figure 1a& b-d). We could not alter the concentration of FBS as the culture media supplied by the manufacturer already contained FBS with other ingredients. However, reduced amount of FBS was used in cryopreservation studies (below).
Figure 1: Effect of recombinant sericin and sericin-cecropin fusion proteins on human dermal fibroblast cell proliferation. CM: culture medium, CM+PB: culture medium containing proteins purified from bacteria harbouring empty vector, CM+RS: culture medium containing recombinant sericin, CM+RSC: culture medium containing recombinant sericin-cecropin fusion protein. Recombinant protein concentration was 25 µg/ml in the respective groups except in D where it was 150 µg/ml. Observations were taken at 24, 48, and 72 h (A-C) and 24 h in D. a: Microscope observations showing cell attachment, morphology and proliferation, b: Estimation of viable cells. *Significant difference from control (P < 0.05) by Student’s t-test.
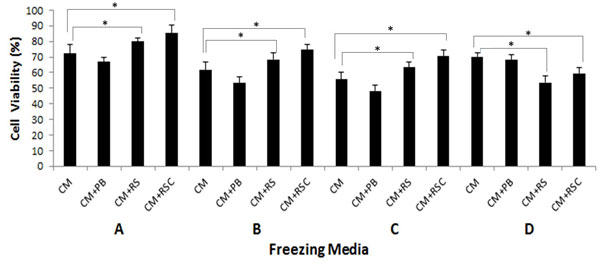
Cryopreservation: As shown in Figure 2, addition of recombinant sericin or its fusion with cecropin in the standard freezing media (CM containing 10% DMSO and 23% FBS) resulted in an increased number of viable cells recovered after freezing- thawing. Compared to all groups, the maximum cell viability after cryopreservation was achieved with CM+RSC followed by CM+RS. Reduced amount of FBS to 11.50% did not alter the viability of cells in the presence of CM+RS or CM+RSC when compared to cells treated with CM alone. However, FBS below 11.50% was found harmful (data not shown). The concentration of DMSO at 5% had deleterious effect on the survivability of cells although the effect was less pronounced in CM+RS and CM+RSC groups. Further, when the concentration of recombinant sericin and its fusion protein was increased to 2 mg/ml, significant decline in viability of cells was observed (Figure 2-D).
Figure 2: Human dermal fibroblast cell viability after 24 h of cryopreservation using varying concentrations of freezing media. Number of frozen cells was set as 100%. A: culture medium (CM), 10% DMSO and 23 % FBS; B: CM, 10% DMSO and 11.50% FBS; C: CM, 5 % DMSO and 25% FBS; D: CM, 10% DMSO and 25% FBS; Recombinant protein concentration was 500 µg/ml in A-C and 2 mg/ml in D. *Significant difference from control (P< 0.05) by Student’s t-test.
In our study, addition of recombinant sericin or sericin-cecropin fusion proteins along with FBS supplemented media can be used for enhanced growth of human dermal fibroblast cells. Effect of sericin on serum-free or serum-reduced media on cell culture is reported (review by Cao and Zhang, 2017). However, previous studies are limited by the use of native sericin except one report which shows recombinant sericin helps in the proliferation of cells (Terada et al., 2002). In addition, no report on the recombinant sericin fusion protein on cell culture studies yet.
The recombinant sericin in our constructs has repeat regions of Ser1 protein composed of 94 amino acid repeats containing one copy of 38-amino acid motif and two copies of decapeptide. The molecular mass of the recombinant sericin and sericin-cecropin is approximately 12 and 16 kDa, respectively. Low molecular weight sericin is most suitable for cell cultures (Terada et al. 2005; Cao and Zhang, 2017). The repeats in Ser1 protein is reported to have mitogenic and cryoprotective properties (Cao and Zhang, 2017). Sericin can promote proliferation of various types of cells including human fibroblast cells (Terada, 2002; 2005; Tsubouchi et al., 2005, Toyosawa et al., 2006; Aramwit et al, 2010; Liu et al, 2016).
At the same time, sericin also suppresses carcinogenesis and tumour promotion by reducing oxidative stress (Zhaorigetu et al, 2003). CHO and Hela cells cultured in the medium with 15 µg/ml sericin hydrolysate instead of FBS show survivability of both cells is similar or higher than that of FBS group (Zhang et al., 2019). The addition of recombinant sericin-cecropin further increased the proliferation of cells indicating that a combination of both sericin and cecropin yielded superior cultures than sericin alone. Similar to other polycationic peptides, cecropin B possesses broad antimicrobial spectrum, antioxidant and anti-endotoxin activity (Moore et al 1996). Further, Cecropin B and analogues have shown cytotoxic activity against several cancer cell lines but do not affect normal cells and shown proliferation of fibroblast cells (Reed et al., 1992; Chen et al., 1997).The concerted action of both sericin and cecropin resulted in the enhanced proliferation of cells in our study. The proliferation of cells promoted by sericin is shown to be concentration-dependent. Sericin concentration from 0.01 to 0.1% was simulative while 1% is harmful to the culture (Terada et al, 2002) and our results are comparable with this observation. Using synthetic gene constructs, varying number of 38-amino acid repeats of B. mori sericin 1 protein are expressed in bacteria (Tsujimoto et al., 2001; Huang et al., 2003). In the presence of 0.01% of a recombinant sericin consists of two to four repeats of the consensus sequence, stimulated the hybridoma proliferation as that of native sericin (Terada et al, 2002). To conclude, addition of sericin-cecropin fusion protein into FBS culture medium yields superior cell viability to FBS culture medium alone.
The cryopreservation of cells is crucial for the continuous source of functional cell lines. The conventional freezing media contains 10% DMSO and 25% FBS. There is significant difference in the viability of cells in the freezing media when supplemented with either recombinant sericin or sericin-cecropin B fusion protein. However, reduced FBS concentration to half results in lower cell viability, but the cell viability is still at par with control in sericin and sericin-cecropin treated groups. Reduction in DMSO affects cell viability with a slight change in fibroblast survivability in the presence of recombinant sericin-cecropin. Our results show that replacement of FBS may not be advisable but freezing media with reduced amount of FBS, 10% DMSO along with sericin-cecropin is better for the cryopreservation of human primary dermal fibroblast cells. A 10% DMSO concentration is optimal both for the freezing of primary hMSCs and SAOS-2 cell line, and that sericin cannot compensate for a 5% decrease of DMSO (Verdanova et al., 2014). Most studies to date have not shown sericin superior to FBS as a cryopreservative agent (Toyosawa et al, 2009; Miyamoto et al, 2012; Ohnishi et al., 2012).
However, freezing medium containing 1% sericin, 0.5% maltose, 0.3% proline, 0.3% glutamine and 10% DMSO in PBS is found better than medium with FBS using various cell lines (Sasaki et al., 2005). The serine-rich repetitive motif of 38 amino acid residues in the silk protein ser1 is found to act as a cryoprotectant of cells under freezing conditions and it functions as an antioxidant barrier against oxidative stress (Tsujimoto et al., 2001). The combined action of both sericin and cecropin B, which is also an effective antioxidant, gives extra protection to cells under freezing stress conditions.
Our results clearly show that recombinant sericin or sericin-cecropin fusion proteins have significant impact on proliferation and cryopreservation of primary human dermal fibroblast cells and can be used as cell culture supplements. Another potential application of the recombinant sericin-cecropin fusion protein is that it can be an attractive alternative to conventional antibiotics used in cell culture media.
ACKNOWLEDGEMENTS
DST, CM, and SR are thankful to Central Silk Board (CSB), Bengaluru, India for financial assistance in the form of Junior and Senior Research Fellowships. This work was supported by a grant from CSB to RG.
REFERENCES
Aramwit P, Kanokpanont S, Nakpheng T, Srichana T. (2010) The effect of sericin from various extraction methods on cell viability and collagen production. Int J Mol Sci, 11:2200–2211
Cao, T and Zhang, Y.(2017) The potential of silk sericin protein as a serum substitute or an additive in cell culture and cryopreservation. Amino acids, DOI 10.1007/s00726-017-2396-3
Chen H, Wang W, Smith D, Chan SC (1997) Effects of the anti-bacterial peptide cecropin B and its analogs, cecropins B-1 and B-2, on liposomes, bacteria, and cancer cells. Biochimica et Biophysica Acta, 1336:171-179
Huang, J., Valluzzi, R., Bini, E., Vernaglia, B., and Kaplan, D. L.(2003) Cloning, expression, and assembly of sericin-like protein. J Biol Chem, 278: 46117–46123
Kundu SC, Dash BC, Dash R and Kaplan DL.(2008) Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog Poly Sci,33: 998–1012
Liu LY, Wang JH, Duan SH, Chen L, Xiang H, Yang Dong, Wang W (2016) Systematic evaluation of sericin protein as a substitute for fetal bovine serum in cell culture. Sci Rep,6:31516 (2016).doi:10.1038/srep31516.
Miyamoto Y, Oishi K, Yukawa H, Noguchi H, Sasaki M, Iwata H and Hayashi S. (2012) Cryopreservation of human adipose tissue-derived stem/progenitor cells using the silk protein sericin. Cell Transplant, 21: 617–622
Moore AJ, Beazley WD, Bibby MC, Devine DA.(1996) Antimicrobial activity of cecropins. J AntimicrobChemother,37:1077–1089
Ohnishi K, Murakami M, Morikawa M, and Yamaguchi A. (2012) Effect of the silk protein sericin on cryopreserved rat islets. J Hepato Bil-Pan Sci, 19:354–360
Reed WA, White KL, Enright FM, Holck J, Jaynes JM, and Jeffers, GW. (1992) Enhanced in vitro growth of murine fibroblast cells and preimplantation embryos cultured in medium supplemented with an amphipathic peptide. Mol Rep Dev, 31:106–113
Sasaki M, Kato Y, Yamada H, Terada S. (2005) Development of a novel serum-free freezing medium for mammalian cells using the silk protein sericin. Biotechnol Appl Biochem, 42:183–188
Terada S, Nishimura T., Sasaki M., Yamada H and Miki M.(2002) Sericin, a protein derived from silkworms, accelerates the proliferation of several mammalian cell lines including a hybridoma. Cytotechnology, 40: 3–12
Terada, S., Sasaki, M., Yanagihara, K., and Yamada, H. (2005) Preparation of silk protein sericin as mitogenic factor for better mammalian cell culture, J BiosciBioeng,100: 667–671
Thomas DS, Manoharan C, Rasalkar, S, Mishra, RK and Gopalapillai, R. (2020) Recombinant expression of sericin-cecropin fusion protein and its functional activity (Communicated).
Toyosawa T, Terada S, Sasaki M, Yamada H, Kino-oka M. (2006) Observation of individual cell behaviors to analyzemitogenic effects of sericin. Animal Cell Technol: Basic &Appl Aspects, 3:155–161
Toyosawa T, Oumi Y, Ogawa A. ( 2009)Novel serum-free cryopreservation of mammalian cells using sericin. Animal Cell Technology, 15:41–45
Tsubouchi K, Igarashi Y, Takasu Y, Yamada H.(2005) Sericin enhances attachment of cultured human skin fibroblasts. BiosciBiotechnolBiochem, 69:403–405
Tsujimoto, K., Takagi, H., Takahashi, M, Yamada, H., and Nakamori, S. (2001) Cry protective effect of the serine-rich repetitive sequence in silk protein sericin, J Biochem,129: 979–986
Verdanova M, Pytlik R, and Kalbacova MH. (2014) Evaluation of sericin as a fetal bovine serum-replacing cryo protectant during freezing of human mesenchymal stromal cells and human osteoblast-L like Cells. Biopreserv Biobank, 12: 99-105
Zhang M, Cao T, Wei Z, and Zhang Y. ( 2019) Silk sericin hydrolysate is a potential candidate as a serum-substitute in the culture of Chinese hamster ovary and henrietta lacks cells. J Insect Sci, 19: 10 (2019).doi: 10.1093/jisesa/iey137.
Zhaorigetu S, Yanaka N, Sasaki M, Watanabe H, and Kato N. (2003) Sericin consumption suppresses development and progression of colon tumorigenesis in 1,2-dimethylhydrazine-treated rats. Oncol Rep, 10: 537–543