Department of Biochemistry, Shri Shivaji College, Akola, India
Article Publishing History
Received: 20/01/2016
Accepted After Revision: 05/04/2016
Hansunella polymorpha is extensively used as production strain for expressing recombinant proteins including recombinant Hepatitis B surface antigens (HBsAg). HBsAg is expressed in H. polymorpha and remains bounded to the membrane. Yeast cells are having very tough cell wall because of this; there is very little effect of chemicals on yeast cells for disruption. Some enzymes act on the yeast cells to break open the content, but their application is not feasible for industrial purposes. We established the cell lysis by using the mechanical disintegrater which gives more than 85% of cell lysis in one shot. This cell lysate was subjected to different types of chaotropic agents in different concentrations to selectively leach out the membrane bound HBsAg. Each chaotropic agent showed different levels of effect during extraction. The highest protein quantity was recorded with sodium chloride and least was with urea. Further, the quality of HBsAg extracted using urea was superior in comparison with other chaotropic agents. It was also found that the enrichment of HBsAg was found to be more in potassium thiocynate. In another experiment, the cells were exposed to the non-ionic detergents (triton X-100) before subjecting to the cell lysis. We found that there is strong link between the treatments of cells with non ionic detergent and leaching of the membrane bound protein. Very less protein could be extracted from the cells which are not exposed to non-ionic detergent. This was confirmed by running several experiments keeping the entire cell lysis parameters constant, only cells were either exposed or non exposed.
Hbsag, H.Polymorpha, Yeast Cells, Chaotropic Non Ionic Detergents
Ali A, Khan Z. H, Sahib M. K, Durrani S, Shaligram U, Raju E. B, Durrani A. Purification of Recombinant Subunit Vaccine by Hbsag Cell Lysis Using Various Enrichments of Chaotropic and Non-Ionic Detergents. Biosc.Biotech.Res.Comm. 2016;9(2).
Ali A, Khan Z. H, Sahib M. K, Durrani S, Shaligram U, Raju E. B, Durrani A. Purification of Recombinant Subunit Vaccine by Hbsag Cell Lysis Using Various Enrichments of Chaotropic and Non-Ionic Detergents. Coli, Enterohemorragic E. Coli And Shigella. Biosc.Biotech.Res.Comm. 2016;9(2). Available from: https://bit.ly/2nTlmoS
Introduction
During the early development of vaccine hepatitis B surface antigen,protein were purified from infected human plasma and used as a vaccine to prevent hepatitis B virus infection. It was having several complications and threat of transferring other viral agents to the recipient and it too difficult to have mass vaccination because of limited supply of vaccine. To overcome these problems the HBsAg genes have been cloned and expressed in E. coli, yeast cells and mammalian cells to reduce the dependency on plasma. The synthesis of HBsAg recovered as 22 nm particles in yeast cells (Hsieh et al. 1995), which is similar to the HBsAg isolated from the human plasma (Wijnendaele et al.1987; Wijnendaele
et al. 1989, Pointek et al., 2000; Gerd Gellissen 2012).
HBsAg is a lipoprotein, the Escherichia coli expressed HBsAg was devoid off lipid portion so it was not useful for vaccination. To overcome this issue the gene for HBsAg was cloned into Yeast, it expressed the correct molecule but the protein was remained bound to the membrane. Yeast is surrounded by very thick and tough cell wall and its not easy to break open the way it is performed for bacteria and mammalian cells. Several methods have been tried to break open the cell wall, (Zhang et al., 1999; Wijnendaele et al., 1989 Pointek et al., 2000; Gerd Gellissen, 2012).
Traditional extraction techniques often involve harsh and extreme conditions like high pressure could potentially of damaging the desired products or limit yields. Enzymatic digestion breaks open the cell wall but its application is limited to the lab and its not feasible for industrial perspectives, (Knorr et al., 1979; Wijnendaele et al., 1989). Furthermore, a second step is often required to break the spheroplasts generated from the commonly used digestive enzymes (zymolase, glucalase and/or lyticase). Sonication has been a widely used, successful method for cell disruption (Feliu et al. 1998) due to its speed, ease of operation, and cell lysis ability of various types, but until now it has only been relevant to single-sample techniques, (Bardiya 2006, Pointek et al., 2000; Gerd Gellissen, 2012)
The most popular mechanical methods used for yeast cell lysis at a lab scale is to agitate the glass beads of 0.5mm diameter and cell suspension in a bead disintegrator or simply on vortex mixer for 3-10min in a cold room or cold condition (Craig et al., 1987; Craig et al., 1991). But due to the scale-up limitation of the method and low recovery with high impurity profile its use is limited to lab. This was replaced with mechanical disintrating devise, Dynomill for the disruption of yeast cells. It is widely accepted because of easy operating and easy to scale up to handle larger volumes.
After disruption of the cells the next important objective was to extract the protein of interest from the cell lysate suspension containing high percentage of impurities.Chaotropic agents and non-ionic detergents are most commonly used for the extracting membrane proteins.(Wijnendaele et al.,1989, Hsieh et al., 1995). Chaotropic salts react with hydrophobic part of the membrane proteins and solubilized it in the aqueous phase. Such salts include compounds which contain the thiocynate ions, halide ions such as iodide and bromide and hypo halides ions such as perchlorate as cation such as lithium, calcium and barium (Hurni et al., 1993; Craig et al., 1987). Non-ionic detergents have a hydrophilic head group that is uncharged and are preferred for their ability to break lipid-lipid and lipid-protein interactions. They have limited ability to break protein-protein interactions and are often referred to as non-denaturing detergents and are used to isolate biologically active membrane proteins.
The high speed centrifugation is found to be the most effective method to remove the cell debris from the homogenate suspension, apart from this many other methods like use of protein aggregating agents example PEG-600, saturation with ammonium sulphate and non-ionic detergent polyoxyethylene (9, 5)-p-t-octyl-phenol (Triton® X-100) found effective methods to aggregate and separate the cell debris (Howard 1986; Gerd Gellissen Google book ) Under the light of above literature, the experiments were designed to standardize the yeast cell lysis and extraction of membrane bound HBsAg using chaotropic agents and non-ionic detergents.
Materials And Methods
Materials: All the raw materials used for fermentation and ER grade and the chemicals used in the purification were extra pure grade. These chemicals are from Merck Phosphate buffer: Phosphate buffer was prepared by adding 8.52 g Disodium hydrogen phosphate.12 H2O (Merck), 0.164 g Sodium dihydrogen phosphate. H2O (Merck) and 0.742 gm. EDTA into 90ml distilled water. Adjusted the final pH = 7.7±0.3 and final volume made up to 100 ml by adding distilled water.Cell lysis buffer: This was prepared by adding the triton X-100 (%) and PMSF (10mM) to the phosphate buffer. PNE Buffer: This was prepared by adding the sodium chloride (100mM) and EDTA (0.75%) to the phosphate buffer. Triton X-100 buffer: This was prepared by dissolving the 0.1%, 0.5% and 1.0% of Triton X-100 in PNE buffer, to get 0.1%, 0.5% and 1.0% triton X-100 buffer(s). Tween-20 : This was prepared by dissolving 0.1%, 0.5% and 1.0% of Tween 20 in PNE buffer, to get 0.1%, 0.5% and 1.0% tween 20 buffer(s). Glass beads: (0.5mm, Willy A.Bachhofen AG ) Methods:
Fermentation: During the fermentations, H. polymorpha cells grows on synthetic glycerol based medium. Once, the cell density reached to the required optical density (OD) 320-250 OD 600nm, cells were prepared for indication by slowly withdrawing the glycerol from the feeding medium. Once glycerol level reduced to minimum level, three methanol shots were given at equal intervals and then stop the fermentation. In this process methanol acts as inducing agent for expressing the HBsAg gene.
Cell clarification/ separation: After the completion of induction process, the cells were subjected to the filtration using the tangential flow filtration (TFF) 300kd NMWC. These cells further were washed three times with phosphate buffer to remove the fermented broth and lower the conductivity. At the end, the retentate is collected and diluted to required OD. This is starting material for cell breakage experiments. Alternatively for small scale preparations, 1000ml fermented broth (50-60gm/L cell dry weight) was subjected for centrifugation (6800 rcf for 10 min). Cell pellet was collected and washed twice with 1000ml of phosphate buffer by re-suspending the cell pellet and centrifuged at 6800 rcf for 10 min.
Cell disruptions: Two different types of methods were used for disrupting the yeast cells. 1. Cell disruption using the glass beads and vortex machine, and 2. Automated mechanical disintegrator (Dynomill KDL). For both the experiments, the starting material used was cell pellet suspension. Cell disruption by glass beads and overtaxing: The cell suspension was taken in a 50ml centrifuge tube and 7.5ml of glass beads were (0.5mm, Willy A.Bachhofen AG) added to the cell suspension. The suspension was subjected for vortex for different timings and cell lysis was confirmed by microscopic observation.
Cell disruption Dyno Mill KDL: The cell pellet re-suspended into 1000ml cell lysis buffer (1.4% phenylmethylsulphonylfluoride (PMSF) and made the uniform cell suspension for cell lysis. This quantity was equally distributed into pe-labeled five containers. Each container was labeled with their feeding flow rate by which it was fed to the Dynomill for cell lyisis. (80-120ml/minutes). Cell lysis was carried out in a glass bead homogenizer (Dyno Mill KDL) with 600ml glass chamber and 0.5mm glass beads (Willy A. Bachhofen AG ). To establish cell lysis process, the different feeding flow rates were tested (80, 90, 100,110, 120 ml/minutes) as labeled. We wanted to avoid the 100% cell lysis because it will contribute the unwanted proteins in cell lysate, so our target was to have 70%-75% cell lysis without damaging the protein of interest. The percent cell lysis was confirmed by microscopic observation of the cell homogenate and calculated the percentage lysis in approximate figure.
Extraction of HBsAg from Cell lysate: using Chaotropic agents: After establishing the required percentage of cell lysis, that is 70-80% lysis further extraction experiments were designed. Arranged 6 polypropylene tubes, and transferred 25ml cell lysate to each tube as shown in the table 1. Each polypropylene tube was labeled with extracting agents plus other details. One tube kept as negative control devoid off chaotropic agent. To each polypropylene tube (1-5), Chaotropic agent was added to get the final concentration 2000mM. All the tubes were incubated at 8 ºC on the rocker plateform for 16hr in cold room. After incubation period is over, 10ml from each tube was centrifuged at 6800rcf for 10min in a table top centrifuge. Supernatants were collected and HBsAg was estimated by Auszyme® EIA kit method (Abbott laboratories). The total protein contents in all samples were estimated using the Folin Lowry method and purity profile was checked by SDS-PAGE (Lammeli method ref.).
| Table 1: Chaotropic agents used for HBS Ag extraction | |||||
| Sr.# | Tube # | Chaotropic agent | Source | Concentration | Incubation time |
| 1 | 1 | Blank | – | – | 16hrs |
| 2 | 2 | Potassium thiocyanate | Merck | 2000mM | 16 hrs |
| 3 | 3 | Guanidine hydrochloride | Sigma | 2000mM | 16 hrs |
| 4 | 4 | Lithium chloride | Merck | 2000mM | 16 hrs |
| 5 | 5 | Potassium Iodide | Merck | 2000mM | 16 hrs |
| 6 | 6 | Urea | Merck | 2000mM | 16 hrs |
| 7 | 7 | Sodium chloride + PEG (5%) | Merck | 1000mM | 16hrs |
Extraction By Using Triton-X-100 And Tween-20
In an another extraction experiment, the above cell lysate (70% broken cells) was treated with two non-ionic detergents (Triton X-100 and tween-20) at three different concentrations to see the effect on the selective leaching of HBsAg from the lysed cells. The experiment is designed as mentioned in the table 2. Tube 1. The cell lysate was kept as such for incubation with out treating further, as a negative control for checking the HBsAg in the whole lysate
Tube 2. First the content of this tube was subjected for centrifugation, collected the pellet and supernatant separately and HBsAg was estimated in both (supernatant and in pellet).
| Table 2: Effect of Triton X-100 on HBsAg extraction | |||||
| Sr.# | Tube # | Contents | Triton X-100 | Tween-20 | Remarks |
| 1 | 1 | Whole cell lysate | No | No | Negative control |
| 2 | 2 | Supernatant from cell lysate | No | No | Negative control |
| 3 | 3 | Cell lysate | 0.1% | No | Effect of Triton X-100 |
| 4 | 4 | Cell lystae | No | 0.1% | Effect of Tween -20 |
| 5 | 5 | Cell pellet | 0.1% | No | Effect of Triton X-100 |
| 6 | 6 | Cell pellet | no | 0.1% | Effect of Tween -20 |
| 7 | 7 | Cell pellet | 0.5% | No | Effect of Triton X-100 |
| 8 | 8 | Cell pellet | No | 0.5% | Effect of Tween -20 |
| 9 | 9 | Cell pellet | 1.0% | No | Effect of Triton X-100 |
| 10 | 10 | Cell pellet | No | 1.0% | Effect of Tween -20 |
Tube 3. Taken whole cell lysate and added 0.1% Triton X-100 and kept for extraction.
| Table 3: Percentage of cell disruption | ||||
| S.No | Flow rate (ml/min) | Number of passes | Dry cell weight | Cell disruption (%) |
| 01 | 80 | Single | 55 +/-5mg/L | 90 to 95 |
| 02 | 90 | Single | 55 +/-5mg/L | 85 to 90 |
| 03 | 100 | Single | 55 +/-5mg/L | 70 to 80 |
| 04 | 110 | Single | 55 +/-5mg/L | 55 to 60 |
| 05 | 120 | Single. | 55 +/-5mg/L | 50 |
Tube 4 Taken whole cell lysate and added 0.1% Tween-20 and kept for extraction.
| Table 4: Effect of chaotropic agents on HBsAg extraction. | |||||
| Chaotropic agents. | Lysate volume | Pellet volume after centrifugation (ml) | Protein (mg/ml) | HBsAg (mg/ml) | HBsAg/ Protein (ratio) |
| Cell Lysate | 25 | whole | 9.40 | 0.072 | 0.0076 |
| Control supernatant | 25 | 1.8 | 7.39 | 0.072 | 0.0098 |
| Potassium thiocyanate | 25 | 1.5 | 7.51 | 0.050 | 0.00199 |
| Guanidine hydrochloride | 25 | 1.5 | 5.24 | 0.065 | 0.01124 |
| Lithium chloride | 25 | 1.3 | 5.8 | 0.072 | 0.01124 |
| Potassium Iodide | 25 | 1.8 | 7.4 | 0.0115 | 0.00200 |
| Urea | 25 | 1.8 | 8 | 0.073 | 0.00910 |
| PEG and Sodium chloride | 25 | 3.0 | 4.97 | 0.071 | 0.01142 |
Tube 5, 6, 7, 8, 9, 10. Taken equal volume of cell lysate in six tubes, all six tubes were subjected to centrifugation, collected the supernatant in one container and pellets were kept separately for treating them with triton X-100 and Tween-20. Tube 5, 7, 9. In these tubes, Triton X-100 was added to 0.1%, 0.5% and 1.0% respectively and kept for extraction.Tube 6, 8, 10. In these tubes, Tween-20 was added to 0.1%, 0.5% and 1.0% respectively and kept for extraction.
| Table 5: Effect of non-ionic detergent on HBsAg extraction | ||||||
| Sample ID | HBsAg (ng/ml) | Protein (mg/ml) | Volume (ml) | Total HBsAg (ng/ml) | Total protein (mg/ml) | HBsAg/ Protein (ratio) |
| 1 | 421.2 | 7.65 | 1.5 | 632 | 11.30 | 55.06 |
| 2 | 166.9 | 1.37 | 1.5 | 250 | 20.05 | 121.82 |
| 3 | 535.8 | 7.20 | 1.5 | 804 | 10.80 | 74.42 |
| 4 | 371.0 | 7.50 | 1.5 | 557 | 11.25 | 49.42 |
| 5 | 206.8 | 0.90 | 1.5 | 310 | 1.35 | 229.27 |
| 6 | 171.0 | 1.32 | 1.5 | 257 | 1.98 | 1129.27 |
| 7 | 306.0 | 0.40 | 1.5 | 459 | 0.60 | 765.00 |
| 8 | 97.2 | 0.44 | 1.5 | 146 | 0.66 | 220.89 |
| 9 | 1208.0 | 0.78 | 1.5 | 1812 | 1.17 | 1548.89 |
| 10 | 366.0 | 0.63 | 1.5 | 549 | 0.95 | 577.29 |
| 11 | 1423.0 | 0.69 | 1.5 | 2135 | 1.04 | 2062.32 |
| 12 | 346.0 | 0.59 | 1.5 | 519 | 0.87 | 594.50 |
| 1= Cell lysate , 2 = Supernatant from cell lysate , 3= Supernatant with 0.1% triton –X100, 4 = Supernatant with 0.1% Tween-20, 5 = supernatant of sample 3 , 6 = supernatant of sample 4, 7= pellet plus 0.1% triton-X100, 8= pellet plus 0.1% Tween-20, 9= pellet plus 0.5% triton-X100, 10= pellet plus 0.5% Tween-20, 11= pellet plus 1.0% triton-X100, 12= pellet plus 1.0% Tween-20. | ||||||
These all above tubes were subjected for overtaxing and incubated in cold room for 16hrs for extraction with continuous shaking on rocker platform. The centrifugation was performed (either before or after the incubation period) at 6800g for 10 minutes to separate the supernatant and collect the pellet. The volume of cell lysate in each tube was 1.5 ml.
Results And Discussion
H.polymorpah cell disruption by Vortex: The cell lysis was performed by subjecting the yeast cells to mechanical agitation in the presence of glass beads (0.55mm) but it gave 40-45% cell lysis eve after exposing several times to overtaxing force. H.polymorpah cell disruption by Dyno mill: The same lysate was passed through the mechanical cell designator at different flow rates and following % of cell lysis was recorded. The required cell lysis that is 70-80% was found to be at 100ml/%.
Extraction of HBsAg from homogenate by chaotropic agents: For extracting the HBsAg, 70-80% lysed cells were treated with different types of chaotropic agents and following results were found as per table no. 4
Extraction of HBsAg from cell lysate by non-ionic detergents (Tween-20 and Triton X-100): Cell lysate was treated with different concentrations of nonionic detergents to selectively remove the membrane bound HBsAg. The following results were recorded.
Cell disruption: Before establishing the cell lysis using dynomill, several experiments were conducted at small scale by playing with various parameters. The most important parameters were: the size of the glass beads (0.55, 75, 1.0mm), volume of glass beads, dry cell weight, flow rate of cell suspension feeding to the dynomill chamber and agitator speed. A comparative cell lysis study was also performed by treating the cells with tween 20 and without treatment. The data of initial experiments are not shown here which were considered pre-establishment work. We got few clues from the initial experiments, like glass beads (0.55-0.75mm)is suitable, dry cell weight (40-50mg/L) was working perfectly, and flow rates (80-120ml/minutes) give the promising results. Based on this information, other experiments were planed by fixing: the volume of glass beads 600ml, size of glass beads 0.55mm, dry cell weight (40-50mg/L) and agitator speed (2000rpm) were kept constant and passed the cell suspension with different velocity in the Dynomil Chamber.
The purpose of cell lysis, is to have limited broken cells with maximum number of damage cells/damaged cell wall so that the chaotropic agents can act on them to selectively remove the membrane bound HBsAg. And at the same time addition of un-necessary cell components can be minimized, which definitely helps in product purification.
While establishing the process, fixed all the above mentioned parameters and the flow rate of the cell suspension was changed with time and samples were collected and analyzed microscopically for the percent cell lysis. It was found that: 1. As the flow rate of feeding cell suspension increased from 80-120rpm, the percentage of cell lysis started decreasing because cells were not getting enough residence in the lysis chamber which is must for breakage. 2. While, at the low flow rate the cell lysis was very high and lots of broken cells found in the microscopic field. In this case the cells are staying for longer time in the lysis chamber and over exposed to the moving glass beads and because of this they were broken into pieces. 3. At the high flow rate, the cell lysis is less and there will be no effect of the chaotropic agents as the 50% of the cells are not lysate and it will definitely have a negative effect onto the final yield. 4. In low flow rates as the cells are damaged too much, this excessive lysis is contributing to increased impurity profile and this posed trouble during product purification.
From the on line sample analysis, it was found that the feeding flow rate (100rpm) at dry cell density (40-45mg/L), agitator speed (2000rpm), glass bead volume (600ml), glass bead diameter (0.5mm) gives optimum cell lysis with less number of broken cells, maximum number of damaged and very less number of cell debries. These all parameters were fixed for getting the consistent cell lysis (70-80%). Further, we used the same lysate for leaching experiments. The same types of results also reported (Pointek et al., 2000; Gerd Gellissen google book, 2012)
After establishing the cell lysis step, cell lysate was treated with different types of chaotropic agents. HBsAg was estimated in supernatant with Auszyme kit® and total quantity of extracted protein was estimated by using Folin lowery method (ref). All samples were also loaded onto the SDS-PAGE to see purity profile of the HBsAg (Fig 1). To our surprise, extraction of HBsAg was found more or less same in all the chaotropic treated samples (table 4). Except, extraction efficiency compare to the others was found to be less in potassium iodide and potassium thiocynate (0.011mg/ml and 0.050mg/ml respectively). The same findings were also reported by (Pointek et al., 2000; William et al., 1987; Friedman et al., 1987; Jih-han et al.1995; DeRizzo et al. 1972; Michel De Wilde et al. 2001). The purity profile on the SDS-PAGE was also confirmed by intensity of bands after staining the gel and if their intensity was not varying much from sample to sample (results are shown in figure 2) the same type results have also been confirmed by Craig et al. (1987).
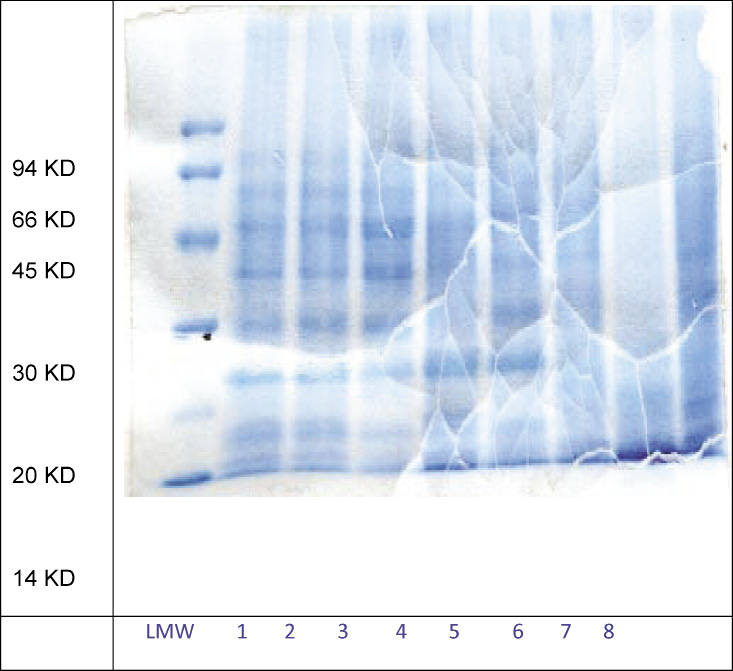 |
Figure 1: SDS-PAGE of HBsAg extracted from cell lysate using different chaotropic agents |
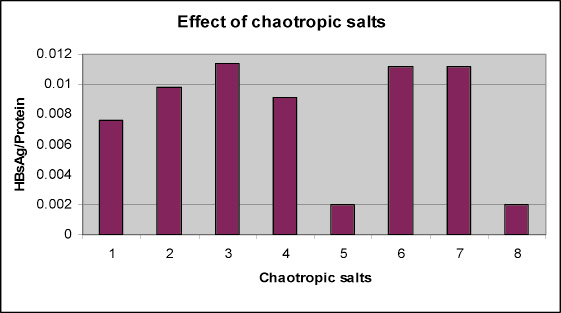 |
Graph 1: Graphical presentation of effect of chaotropic agents on HBsAg extraction |
HBsAg purity profile looks better in the sample treated with 5% PEG and 1000mM sodium chloride. This can be found in table 4. The PEG600 with Sodium chloride was found to be the most effective with 4.97mg/ml of HCP and 0.01142% purity ratio (HBsAg/HCP) as compare to the 7.39mg/ml of HCP and 0.0098% purity ratio (HBsAg/HCP) of the control sample, Guanidine hydrochloride and Lithium chloride shows similar profile with respect of HCP concentration (5.24mg/ml and 5.8mg/ml respectively) and % purity (HBsAg/HCP =0.01124%).
This result is supported by the earlier work of many researchers, (Craig et al. 1987; Jih-han et al. 1995; Vnek et al. 1976 and Wijnendaele et al. 1989). The performance of urea turned out to be very poor with respect to HCP concentration (8mg/ml), even though the concentration of extracted HBsAg is highest in the urea treated supernatant (0.073mg/ml) but the % purity (0.0091%) of the extracted HBsAg is lower then control sample. The ability of the chaotropic salts and PEG600 to decrease the HCP and to increase the percent purity of the HBsAg is confirmed by SDS PAGE results. Though the quality of the SDS gel is not good but still we can conclude: The band intensity of the low molecular weight impurities (< 20kd) is very faint in the PEG600 treated supernatant. The urea treated supernatant showed the high band density of low mole wt (< 20kd) as well as high mole wt (>30kd) impurities. While the band intensity of low mole wt (< 20kd) and high mole wt (>30kd) impurities are faint in lithium chloride treated supernatant as compare to the control sample.
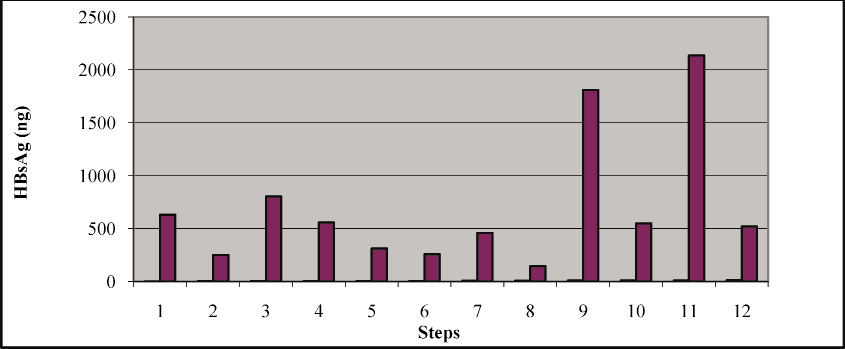 |
Figure 3: Effect of Triton X-100 and Tween 20 on the extraction of HBsAg |
A lot of HBsAg seems to be losing because of insufficient extraction method. Most of the HBsAg is bound to the membrane and if its not extracted properly its going to be waste and at the end it will have negative effect on the over all product recovery. The presence of non-ionic detergents seems to have positive effect on removing the membrane bound HBsAg. This positive effect helps to increase the HBsAg content in the supernatant which is the starting material for further purification. In order to address this issue the following experiments was conducted. This was studied in three stages: 1. HBsAg content was checked estimated in whole cell lysate, 2. HBsAg was estimated in supernatant and 3. The content of HBsAg was also estimated in the pellet which going to be waste.
First, cell lysate was treated with 0.1% Triton X-100 and Tween-20 separately. Surprisingly the supernatant treated with Triton X-100 was showing approximately 25% increased of HBsAg content in comparison with control untreated sample. The same types of results were also reported by (Levine et al., 1987). While the sample treated with Tween 20 was not showing any significant increase in HBsAg content in comparison to the control sample.
Second, the supernatant from the control sample was treated with Triton X-100 and Tween 20 separately. The content of HBsAg was increased by 25% because of the treatment of Triton-X-100 (from 250ng-310ng). But the Tween 20 was not showing any effect (250ng- 257ng) (Levine et al. 1987 also reported the same types of results.
Third, the pellet was suspended into the 0.1%, 0.5% and 1.0% triton X-100 and tween -20 separately. The treatment of Triton –X100 from 0.1, 0.5-1.0% also released the supernatant 459, 812 and 2135ng respectively. HBsAg is present in the pellet and it will be extracted with treatment with triton X-100 this type of work was also presented by (Simons et al.1973; Levine et al. 1987, Skelly et al. 1981). Pellets treated with 0.1% triton X-100 and supernatant of 0.1 % tritonX-100 were showing 459 ng and 310 ng this will give total of 769 ng which is almost equal to 804ng in sample 3 that cell homogenate treated with 0.1% triton X-100. The tween -20 treated samples were didn’t show much difference even after increasing the concentration from 0.1-1. %. The overall recovery can be increased by increasing the concentration of Triton X-100 during the extraction as this experiment proves that a lot of membrane bound HBsAg is going waste in the form of pellet. It has been reported that 90% of HBsAg is present in cell bound form and only 10% can be detected in the supernatant (Bitter et al.1988, Levine et al. 1987; Skelly et al., 1981).
This can also be explained that HBsAg remains in two forms such as immunoreactive which is detected in the supernatant and non-immunoreactive form which is cell bound. After treating with triton X-100 it becomes immunoreactive and detected in the supernatant. Wijnendaele, et al. (1987) also reported the use of triton x-100, which helps in HBsAg particle formation (22nm particles), and this might be the reason after treatment the cell lysate it shows increased reactivity and content of HBsAg which is absent in the control sample.
Conclusion
Yeast is having several advantages over prokaryotic system for expressing heterologus proteins and vaccines. HBsAg is expressed and remained bounded into the membrane. To get the maximum yield it is good to have high expression but not necessary. At the same time one should have an efficient and robust purification scheme to recover maximum from available starting material. We established the cell lysis procedure using the dynomill, glass beads and agitation speed and dry cell weight. But this not the optimum if some one work very closely there is lot of scope to improve the cell lysis step. This step where one can have a very strong control to limit the loss of host cell proteins by limited cell lysis. if cell lysis is less then the final yield will definitely hamper. If the more lysis the quality of final product will have some quality issues.
Uses of chaotropic agents didn’t improve the recovery this may be due to they are not suitable for membrane bound proteins. Sodium chloride in the presence of PEG gives very good results; a kinetics studied can be performed to enrich the HBsAg using different concentrations of Sodium chloride and PEG. This may help in pelleting out the host cell protein as much as possible which definitely help in purification and it will have positive impact on quality of HBsAg. There is lot of scope for increasing the recovery by re-extracting the HBsAg from the cell debries which is going as waste in the form of pellet. We have demonstrated the effect of Triton X-100 for selective removal of HBsAg, this step can be further improved by executing series of experiments with different concentration of Troton X-100 which will increase the final yield.
References
Craig, J. William S. Siegel, Robert S. 1991. Purification of hepatitis proteins. United States Patent 5004688.
Craig, William S, San Diego, Calif, July 28, 1987. Purification of Pichia produced lipophilic proteins. US Patent 4,683,293.
Cregg J. M. S. Siegel, Robert S. (1987)High-Level Expression and Efficient Assembly of Hepatitis B Surface Antigen in the Methylotrophic Yeast, Pichia pastoris” Biotechnology, vol. 5, No. 5, pp. 479-485, New York, U.S.
DeRizzo, E., R. Pandey, C. Wallis, and J. L. Melnick (1972). Concentration and purification of hepatitis B antigen with polyethylene glycol and polyelectrolyte 60, a cross-linked copolymer of isobutylene maleic anhydride, Infect. Immun. 6:335-338
Feliu JX, Cubarsi R.,Villaverde1 A. (1998) Optimized Release of Recombinant Proteins by Ultrasonication of E. coli Cells. Biotechnol Bioeng. 58: 536–540.
Gerd Gellissen. Hansenula polymorpha (2012) biology and applications. Google book P.171. Publisher Willey Blackwell, 1st edition . ISBN No. 3527303413.
Howard C. R., Young P. R., Lee S., Dixon J.,Zuckerman A J.,McAleer W. J. and Lehman E. D. (1986) Hepatitis B surface antigen polypeptide micelles from antigen expressed in Saccharomyces cerevisiae. Journal of Virological Methods. Volume 14, Issue 1, Pages 25-3.
Hsieh, Jih-han (Parsippany, NJ), Shih, Shu-ching (York Avenue, NY), Chi, Wei-kuang (Taipei, TW (1995,). Isolation of Hepatitis B surface antigen from transformed yeast cells. US patent 5462863.
Hurni, William M. Kubek, Dennis J, Rienstra, Mark S ,Scolnick (1993). Edward M. Purification of recombinant hepatitis B surface antigen, Biotechnol and Bioeng. 58: 581–584.
Vnek J and Alfred M.prince.( 1997) Large-Scale Purification of Hepatitis B Surface Antigen, Journal of clinical Microbiology, p. 626-63.1
Knorr, D. Shetty, K. J. Kinsella E. (1979).Enzymatic Lysis of Yeast Cell Walls. Biotech. and Bioeng. 21(11). 2011-2021.
Levine, Howard,L.(1987)Lysis method and buffer for extraction of hepatitis B surface antigen from yeast cells. World intellectual propertyorganization.WO1987001128A1.
Michel De Wilde, Glabais,Joseph Cohen,Brussels.Jan.2,(2001). Hybrid protein between CS from plasmodium and HbsAg. US Patent 6169171B1.
Nirmala Bardiya, (2006) Expression in and purification of Hepatitis B surface antigen (S-protein) from methylotrophic yeast Pichia pastoris, Anerobe 12, (4) 194-203.
Páez R., A. Agraz, L. (2004). The recovery of the hepatitis B virus surface antigen (HBsAg) from a recombinant P. pastoris strain disruption and precipitation studies Acta Biotechnology volume 13 issue 2. (p 117-122).
Pointek Michael, Wenige Michael Nov2, (2000) Method for obtaining recombinant HBsAg. US Patent No. 6428984.
Skelly, J., Howard, C. R. & Zuckerman, A. J. (1981) Nature (London): 290, 51-54.
Wijnendaele V., Frans, Simonet, Guy (1987). Method for the isolation and purification of hepatitis B surface antigen using polysorbate. US Patent 4649192.
Wijnendaele, Frans V. Gilles, Daniel Simonet, Guy (1989). Process for the extraction and purification of proteins from culture media producing them. United States Patent 4857317.
Zhang, N. Gardner, D C.Oliver, S G. Stateva, LI. Genetically (1999)Controlled Cell Lysis in the Yeast Saccharomyces cerevisiae. Biotech and Bioengineering. 5;64(5):607-15.


