1Program of Food Sciences and Nutrition, Turabah University College,
Taif University, P.O.Box 11099,Taif 21944, Saudi Arabia.
2Department of Biotechnology, Taif University, P.O.Box 11099,Taif 21944, Saudi Arabia.
3Department of Biology, University College of Taymaa, University of
Tabuk, P.O. Box 741, Tabuk 71491, Saudi Arabia.
4Department of Biology, Turabah University College, Taif University,
P.O.Box 11099,Taif 21944, Saudi Arabia.
5Department of Clinical Laboratory Sciences, Turabah University College,
Taif University, P.O.Box 11099,Taif 21944, Saudi Arabia.
6Department of Chemistry, Turabah University College, Taif
University, P.O.Box 11099,Taif 21944, Saudi Arabia.
7Department of Basic Sciences, Deanship of Preparatory Year and Supporting Studies, Imam
Abdulrahman Bin Faisal University, P.O. Box 1982, Dammam 34212, Saudi Arabia.
8Department of Pathology, Umm Al- Qura University. Saudi Arabia.
Corresponding author email: o.saeeed@tu.edu.sa
Article Publishing History
Received: 29/06/2021
Accepted After Revision: 15/09/2021
The present study showed the dose beneficial of Helianthus annuus L. on 5-7 month old Balb/c mice, using different important parameters like physiological, biochemical, haematological and histopathological as indicators. The effects of Helianthus annuus seeds were investigated with the dose concentrations of 25% and 50% for 21 day supplementation, which were compared with mice fed on normal standard pelleted food. The results of the study suggested that the physiological parameters considering body weight gain was reduced with a dose-dependent concentration of Helianthus annuus seed as compared to the controls, with the order of 50% (-1.10±0.22) < 25% (-1.05±0.43) < control (1.88±0.63).
The other parameters; fluid intake, urinary output, food intake, faecal weight, and dry weight did not show much differences with increasing dose concentration of 50%, with increasing supplementation in the treatment days. The effect of the drug did not show much differences in all parameters including liver function test and histopathological aspects. It is concluded that the seed of Helianthus annuus showed a beneficial effect in weight loss, the high amount of seed may be beneficial to reduce weight, is good for diabetic conditions, and results in accordance with physiological, biochemical haematological parameters supporting the kidney histopathological data as well. Hence, the use of Helianthus annuus seed extract as a drug among young people should be encouraged for obesity and its management.
Helianthus Annuus L, Physiological, Biochemical, Haematological, Histopathological, Dose Toxicity.
Abushal S.A, Baty R.S, Ahmed O, Alqadria N, Elsayed S, Alotaibi S.H, Omer H, Suliman L, Nasir O. Protective Effect of Helianthus annuus seeds on Renal and Liver Functions of Healthy Mice. Biosc.Biotech.Res.Comm. 2021;14(3).
Abushal S.A, Baty R.S, Ahmed O, Alqadria N, Elsayed S, Alotaibi S.H, Omer H, Suliman L, Nasir O. Protective Effect of Helianthus annuus seeds on Renal and Liver Functions of Healthy Mice. Biosc.Biotech.Res.Comm. 2021;14(3). Available from: <a href=”https://bit.ly/3gZciac“>https://bit.ly/3gZciac</a
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Obesity, which is an excessive fat accumulation in the body, significantly impairs health, leads to the onset of other diseases such as diabetes, hypertension, arthritis, atherosclerosis, and cardiovascular diseases (Akil & Ahmad 2011). Moreover, the prevalence of cancer due to less physical activity has been also reported for the last many decades, and it has been a serious issue globally. Major problem is the treatment of obesity, which depends on lifestyle related physical activity. Synthetic drugs cause severe side effects, hence natural herbs are of good choice, due to few or no harmful effects.
Natural compounds such as plant extracts containing phytochemicals are leading medicines used from ancient to modern science due to less adverse effects as compared to synthetic drugs. Phytochemicals protect the plants and act as a defense mechanism against predators. Secondary metabolites such alkaloids, flavonoids, saponins, terpenoids, tannins have been used in treating different chronic diseases (Seca & Pinto 2019, Shirsath & Goswami 2020).
Free radical production in the body is the major area of concern as its progression leads to complications and forming of several pathological conditions, (Lobo, 2010). Antioxidant capacity of the plants leads to scavenging of the free radicals, (Engwa, 2018). In contrast, some studies have shown that phytochemicals are toxic to humans and animals, and some are lethal, (Halliwell, 2007 and Bode & Dong 2015). Helianthus annuus is a common sunflower, belonging to the genus Helianthus, which is widely grown for edible oil and fruits used for health and nutrition.
Seed extract preparations have many nutritional values (Pal, 2011), recent research has exposed a high risk of aflatoxins in sunflower seeds (Mmongoyo, et al., 2017). Although the seeds of sunflower showed beneficial effects, but also have adverse effects, consuming large amounts of seeds can lead to many health problems like impairing the kidney function. Sunflower seeds possess a large amounts of phosphorus which consumed in large quantities and can impair kidney functions (Guo et al., 2017).
Consuming more of it can increase the weight gain, which can cause rashes on the skin due to the presence of selenium (Nordberg et al., 2014), can also cause chronic fatigue and mood swings. It elevates sodium in the blood, which can elevate blood pressure which in turn causes the risk of heart conditions (National Research Council. 1983). It has the property of dermatitis (Hausen & Spring 1989). Respiratory allergy was observed with pollen allergens (Ghosh0 et al., 2015). The other adverse effects were headache and constipation (Leverrier et al., 2019). The other findings state that oral toxicity of leaf extract in high doses elicits hepatic, testicular, and nephrotic disorders ( Guo, et al., 2017, Onoja 2018, Puga et al., 2019).
The present research was initiated to observe the role of the seed extract of this plant in weight loss. By observing other parameters (physiological, biochemical, haematological, and histopathological) it has been attempted to ensure that the drug does not affect the other organs of the mice. Hence, the present study was aimed to study the weight loss by Helianthus annuus L. seed extract in balb/c mice.
MATERIAL AND METHODS
The seeds of Helianthus annuus L. were assessed in normal old wild-type balb/c, mice of (5-7) months old (n=18), pursued from King Fahd Center for Medical Research, KAU University, Jeddah, Saudi Arabia. All mice were housed under controlled environmental conditions (22-24°C, 50-70% humidity, and a 12-h light/dark cycle). Helianthus annuus L. seeds were purchased from local market of Mecca, Saudi Arabia, and identified by nutritional speciation.
The outer layers of seeds Helianthus annuus L. were removed, grained very fine weight of different concentration, one group 25%, the other group 50% powder of Helianthus annuus L seeds, and mixed with normal standard pelleted food (C1310, Altromin, Heidenau, Germany), for the comparison with control group which had access to normal standard pelleted food (C1310, Altromin, Heidenau, Germany) throughout the study period.
The mixed food was dried and then given to the mice. All animal experiments were conducted according to the guidelines of the local and international law for the care and welfare of animals. The effects of Helianthus annuus L seeds were investigated during the first 7 ,21 days after supplementation, all mice were put in metallic cages for 4 day (adaption one days and 3 days for sample collection) for determination of food, fluid intake and urine output, the body weight of all mice were measured daily.
On the last day of experiment, blood samples from all animals were taken by puncturing the retro-orbital plexus using di ethyl ether (Roth, Karlsruhe, Germany) and blood was withdrawn into the blood collecting tubes as required for different biochemicals.For the biochemistry of plasma and urine, the concentrations of Na+, and K+ were measured by flame photometry (AFM 5051, Eppendorf, Germany).
Plasma and urinary creatinine concentrations were measured using (kinetic method), urinary urea concentrations were measured by (UREA, colormetric method), blood cholesterol (CHOD PAP method), AST GOT, ALT GPT, HDL and LDL Cholesterol (direct method) and Triglycerides (GOPt method) were measured using kits from BIOLABO, (Les Hautes Rivers, 02160, Maizy, France), www.biolabo.fr and all measurements were carried according to manufacturing requirements.
Measurements of plasma cholesterol were measured using Erba Cholesterol Kit (CHOD-PAP Method), both with the help of Chem 5 Plus-V2 Auto-analyser (Erba Mannhein, Germany).Fasting and non-fasting blood glucose concentrations were measured using a glucometer (Accutrend, Roche, Mannheim, Germany), after fasting the mice for 8 hour.
The complete blood picture (CBC), analysis, packed cell volume, blood hemoglobin concentration, and white blood cell count were determined using an electronic hematology particle counter (MDM 905 from Medical Diagnostics 140 Marx; Butzbach, Germany) equipped with a photometric unit for determination of hemoglobin.
The histological analysis were done on the last days after taking the blood samples from all mice, then sacrificed and kidney and liver organs were removed and processed for further histological analysis by the method of John & Alan (1999). The stained sections were viewed and evaluated for pathological changes using a light microscope (Nikon, Eclipse i80). The required images were taken in different magnifications with Nikon mounted digital camera (OXM 1200C, Nikon, Japan).
To see the significant difference between the control group and mice who had food supplementation of Helianthus annuus L seeds, all values were expressed as mean ± S.E.M. and statistical analysis was performed by one-way analysis of variance (ANOVA) using GraphPad Prism 8 Software, version 8-4-3(686), San Diego California USA. The results with a probability factor of P < 0.05 were significantly considered (Khan et al., 2019).
RESULTS AND DISCUSSION
Results of the present study, the physiological parameters considering body weight are shown in Table 1 and Fig. 1, with dose-dependent concentrations of Helianthus annuus seed extract, the weight gain was less comparable to control. the gradual increase in weight was observed in controls from the start with (25.65±0.33to 26.72±0.42) on 7th day, hence the body weight gain was (1.07±0.17), whereas on 21st day, the increased weight were (27.53±0.70), from the baseline (25.65±0.33), hence the weight increased is (1.88±0.63), similarly when treated with different concentrations of Helianthus annuus seed extract, there were t slight reduction in body weight after 7 days from baseline (24.96±0.22), to 24.55±0.20) after 25% of Helianthus annuus seed extract supplementation, but on day 21st there were reduction in body weight (24.96±0.22 to 23.90±0.53) compared to control.
Moreover the same was noticed after 50% of Helianthus annuus seed extract supplementation on day 7th (24.39±0.43) and day 21st (23.82±0.53) compared to baseline body weight (24.92±0.34).There were significant reduction in body weight gain after 25% and 50% Helianthus annuus seed extract supplementation with (-1.05±0.43) and (-1.10±0.22), respectively as compared to body weight gain in control group after 21 days (1.88±0.63), showing the beneficial effect of Helianthus annuus seed extract supplementation on body weight reduction.
Table .1 Effect 25% of Helianthus annuus seeds supplementation on body weight after 7 and 21 days compared to control group.
| Control | 25% of Helianthus annuus L seeds | 50% of Helianthus annuus L seeds | |
| Body weight,
at start/g. |
25.65±0.33 | 24.96±0.22 | 24.92±0.34 |
| Body weight,
after 7 day/g. |
26.72±0.42 | 24.55±0.20** | 24.39±0.43** |
| Body weight,
after 21 day/g. |
27.53±0.70 | 23.90±0.53** | 23.82±0.53** |
| Body weight, gain
after 7 days/g. |
1.07±0.17 | -0.41±20*** | -0.53±0.23*** |
| Body weight, gain
after 21 days/g. |
1.88±0.63 | -1.05±0.43** | -1.10±0.22*** |
Arithmetic means ± standard error of Arithmetic means ± standard error of (25 and 50% ) of Helianthus annuus seeds after 7 and 21 days of supplementation compared to control.**indicates highly significant (P<0.0001), **indicates extremely significant (P<0.00001), between control and (25 and 50% ) of Helianthus annuus seed and after 7 ,21 days ,.
Figure 1: Effect (25 and 50%) of Helianthus annuus seeds supplementation on body weight at start (a), after 7days (b), after 21 days (c), body weight gain after 7 days (d) and body weight gain after 21 days (e) days compared to control group.
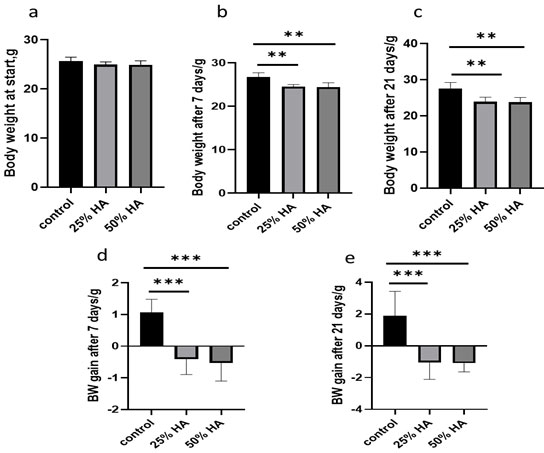
The metabolic cage results show the effects of Helianthus annuus seed supplementation (25%,50%), on food, fluid intake, urinary output, faecal wet and dry weight as compared to the control group (Table 2 for 7 days, and 21 days in Table 3). Observation shows no statistical differences in all groups of mice, and only urinary outputs were little reduced after Helianthus annuus seed treatment, (25%, 50%) but did not reach a significant difference.
Table 2. shows the effect of (25 and 50%) Helianthus annuus seed supplementation on food intake, fecal wet, dry weight, fluid intake, urinary output, as compared to the control group after 7 days.
| Control | 25% of Helianthus annuus seeds | 50% of Helianthus annuus seeds | |
| 7th day | |||
| Food Intake, g/24 h. | 1.45±0.15 | 1.43±0.20 | 1.41±0.04 |
| Fecal wet weight, g/24h. | 0.50±0.10 | 0.48±0.09 | 0.47±0.01 |
| Fecal dry weight, g/24h. | 0.37±0.01 | 0.35±0.01 | 0.33±0.02 |
| Fluid Intake, ml/24h. | 2.34±0.06 | 2.15±0.16 | 2.09±0.03 |
| Urine output, ml/24h. | 1.02±0.08 | 0.85±0.13 | 0.72±0.19 |
Arithmetic means ± standard error of (25 and 50% ) of Helianthus annuus seeds after 7 days supplementation compared to control.
Table 3: Effect of (25 and 50%) Helianthus annuus seed supplementation on food intake, fecal wet, dry weight, fluid intake, urinary output, as compared to the control group after 21 days.
| Control | 25% of Helianthus annuus seeds | 50% of Helianthus annuus seeds | |||
| 21st day | |||||
| Food intake, g/24 h. | 1.50±0.06 | 1.40±0.02 | 1.37±0.07 | ||
| Fecal wet weight, g/24 h. | 0.45±0.05 | 0.38±0.02 | 0.36±0.02 | ||
| Fecal dry weight, g/24 h. | 0.34±0.01 | 0.32±0.02 | 0.30±0.01 | ||
| Fluid intake, (ml/24h. | 2.25±0.20 | 2.09±0.22 | 2.05±0.14 | ||
| Urine output. ml/24 h. | 0.82±0.09 | 0.74±0.19 | 0.69±0.04 | ||
Arithmetic means ± standard error of (25 and 50% ) of Helianthus annuus seeds after 7 days supplementation compared to control.
Table 4. Effect of (25 and 50) Helianthus annuus seed supplementation on urinary Na+, K+ and Ca2+ as compared to the control group after 21 days of treatment.
| Control | 25% of Helianthus annuus seeds | 50% of Helianthus annuus seeds | |
| Urine [Na+], mmol/24 h. | 220.75±5.04 | 226.87±8.43 | 237.17±8.23 |
| Urine [K+], mmol/24 h. | 697.29±23.09 | 701.60±2.79 | 717.20±10.75 |
| Calcium [Ca2+], mmol/24 h. | 7.54±0.19 | 7.72±0.17 | 7.89±0.34 |
| [Na+]plasma , mM. | 139.00±1.39 | 142.64±1.69 | 141.80±1.49 |
| [K+]plasma, mM. | 4.36±0.12 | 4.32±0.22 | 4.29±0.21 |
| [Ca2+]plasma , mM. | 2.23±0.05 | 2.11±0.03 | 2.21±0.16 |
Arithmetic means ± standard error of (25 and 50% ) of Helianthus annuus L seeds after 7 days supplementation compared to control
Table 4 results showing the Effect of different concentrations of Helianthus annuus L seeds supplementation on urinary Na+, K+, and Ca2+ concentrations compared to control group. Observing the above, results mentioned in Tables 1 and 2 acts in accordance with Table 4, showing that the biochemical results of urine comply with the above results, with normal levels of urine Na+, Urine K+ and calcium. Blood examination also showed normal levels of Na+, K+, and Ca2+. The effect of different concentration of Helianthus annuus L. seed extract supplementation on 21st day as compared with control group, Table 5, shows that normalized levels after Helianthus annuus L. seed extract supplementation of 25% and 50% respectively compared to control group.
Table 5. Effect of (25 and 50%) Helianthus annuus L seed supplementation on blood urea nitrogen, urinary creatinine, plasma creatinine concentration, glomerular filtration rate and normalization 24-h creatinine clearance as compared with control group after 21 days of treatment.
| Control | 25% of Helianthus annuus seeds | 50% of Helianthus annuus seeds | |
| Blood Urea Nitrogen,(mg/dl. | 17.85±1.43 | 17.99±1.35 | 17.38±1.32 |
| Urinary creatinine, mg/dl. | 36.34±2.24 | 35.42±3.66 | 35.18±2.08 |
| [creatinine]plasma , mg/dl. | 0.29±0.02 | 0.28±0.02 | 0.28±0.03 |
| Glomerular filtration rate (GFR), µl/min. | 3.66±0.22 | 3.84±0.38 | 3.95±0.15 |
| Normalized 24-h creatinine clearance, mL/min/g body weight. | 11.15±2.40 | 11.96±2.03 | 12.21±2.41 |
Arithmetic means ± standard error of (25 and 50% ) of Helianthus annuus L seeds after 7 days supplementation compared to control.
The biochemical results in Table.6, show the effect of different concentrations of 25% and 50% of Helianthus annuus seed supplementation as compared to control group. Fasting blood glucose was little reduced in 25% as compared with 50% of Helianthus annuus seeds supplementation. The liver function of Alkaline Phosphatase, Alanine Aminotransferase (ALT), Aspartate Aminotransferase, Bilirubin, Cholesterol, High-density lipoproteins, and Triglycerides, were all in normal range. However, that the high HDL- cholesterol indicates to lower the risk of heart stroke, it is generally visualized that it reduces ischemic stroke in elderly people.
Table 6. showing the effect of (25 and 50%) of Helianthus annuus seed supplementation on fasting blood glucose, Alkaline Phosphatase, Alanine Aminotransferase, Aspartate Aminotransferase, Bilirubin, Cholesterol, High-density lipoproteins, Triglycerides and Uric Acid as compared with control group after 21 days of treatment.
| Control | 25% of Helianthus annuus seeds | 50% of Helianthus annuus seeds | |
| Fasting blood glucose, mg/dl. | 97.60±4.09 | 91.33±14.11 | 95.67±9.83 |
| Alkaline Phosphatase (AP), U/L. | 96.67±2.06 | 76.48±13.43 | 68.57±17.27 |
| Alanine Aminotransferase (ALT), U/L. | 39.35±4.69 | 42.37±13.04 | 38.97±6.50 |
| Aspartate Aminotransferase (AST), U/L. | 47.18±3.03 | 39.55±7.78 | 38.00±5.75 |
| Bilirubin, mg/dl. | 0.26±0.02 | 0.23±0.01 | 0.26±0.03 |
| Cholesterol, mg/dl. | 31.77±7.06 | 28.97±4.11 | 30.83±11.72 |
| High-density lipoproteins (HDL), mg/dl. | 24.83±0.98 | 21.30±3.14 | 28.17±8.58 |
| Triglycerides, mg/dl. | 46.57±4.49 | 47.50±14.51 | 43.67±9.31 |
Arithmetic means ± standard error of (25 and 50%) of Helianthus annuus L seeds after 7 days supplementation compared to control.
Table 7, shows the effect of Helianthus annuus L seed supplementation on complete blood count (CBC) as compared with control group (WBC, LYM, GRA, MID, and MPV) were in normal range only there were slight reductions in hemoglobin level compared to control group but it’s not significant.
Table 7. Effect of (25 and 50%) Helianthus annuus L seeds supplementation on Complete Blood Count (CBC), as compared with control group after 21 days of supplementation
| Control | 25% of Helianthus annuus L seeds | 50% of Helianthus annuus L seeds | |
| 21st | |||
| White Blood Cells (WBC), 10^3/ml. | 7.25±0.32 | 7.68±0.36 | 7.89±0.53 |
| Lymphocyte (LYM),10^3/ml. | 5.23±0.27 | 5.85±0.37 | 5.68±0.24 |
| Lymphocyte (LYM), %. | 91.32±0.83 | 90.90±2.27 | 89.48±1.19 |
| Granulocytes (GRA),10^3/ml. | 0.40±0.03 | 0.47±0.06 | 0.43±0.06 |
| Granulocytes (GRA), %. | 2.93±0.22 | 3.02±0.32 | 3.13±0.37 |
| Monocytes (MID), 10^3/ml. | 0.57±0.09 | 0.47±0.05 | 0.58±0.09 |
| Monocytes (MID), %. | 5.68±0.71 | 6.35±0.57 | 5.45±0.28 |
| Hemoglobin (Hb), g/l. | 13.92±0.27 | 13.33±0.29 | 13.35±0.24 |
| Mean Corpuscular Volume (MPV), fl. | 7.42±0.29 | 7.61±0.41 | 7.93±0.54 |
Arithmetic means ± standard error of (25 and 50%) of Helianthus annuus L seeds after 7 days supplementation compared to control.
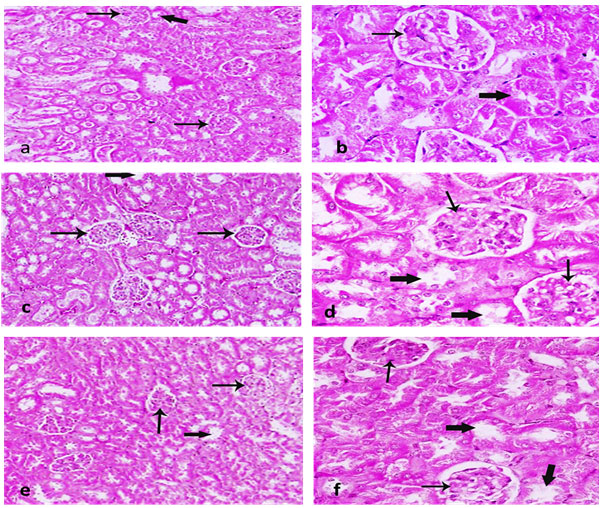
Figure 2: shows kidney sections with glomerulus, renal tubules (A, B) photo with the Control group. Whereas large cells were observed due to inflammation (C, D) after 25% of Helianthus annuus seed supplementation. Further, little shrinkage in the cell structure was observed (E, F) after 50% of Helianthus annuus seed supplementation.
Figure 2: Section of kidney showing Glomerulus (thin arrow), Renal tubules (thick arrow). (a, b) photo of the control group. (c, d) after 25% of Helianthus annuus L seed supplementation and (e, f) after 50% of Helianthus annuus L seed supplementation
Supportive effect of 25% and 50% Helianthus annuus L. seed extract in BALB/c mice of 5-7 months old with different parameters like physiological, biochemical, haematological and histopathological studies was investigated. Physiological parameters in Table .1 and Fig.1 considering body weight showed the reduction of weight with the increasing dose concentration of Helianthus annuus L. seed extract with increasing days of supplementation (Leverrier et al., 2019).
Table.2 with 25% Helianthus annuus L. seed extract showed no difference on food and fluid intake was observed, but there were reduced urine output (ml/24h) Fecal wet weight (g/24h) and Fecal dry weight (g/24h) was reduced with increasing supplementation of days, the same parameters in Table.3 the Effect of Helianthus annuus L seed supplementation (50%) for 7th and 21st day; Food intake, fecal wet and dry weight was also reduced with increasing the days of dose supplementation (Blicharska, et al., 2014).Due to the less fluid intake, the animal could be in a dehydrated state (Puga et al., 2019).
Table 4. results showing mild elevated results of Urine Na+ (µmol/24h), Calcium (mg/dl), Urine K+ (µmol/24h), Uric acid (mg/dL) with the high dose Effect of different concentrations of Helianthus annuus L. seed extract supplementation compared to control group. Mild increase in WBC in accordance with Granulocytes (GRA) % is associated with the stress response of the animal with little output of fluids, which could also be due to inflammatory results (Nishitani & Sakakibara 2014).
Effect of different concentrations of Helianthus annuus L seed supplementation on liver function test (ALT, AST, and ALT) and fasting and random blood glucose concentrations as compared with control group after 21 days of treatment (Table.6) showing normal results. Bilirubin was reduced in 25% conc., with not much difference compared to control. Cholesterol was reduced in both conc. High-density lipoproteins were improved in 50% dose conc., with the decline in Triglycerides showing blood lipid profile improvement (Leverrier et al., 2019).
Table.7 showing Increase levels of WBC, LYM, GRA, and MID suggest the increase due to the response of infection. The Mean Corpuscular Volume increase can also be the cause of anemia with the deficiency of B vitamins, namely B-12 and foliate. Hemoglobin was not much significantly reduced, whereas large inflamed cells were observed after 25% and 50% of Helianthus annuus L. seed supplementation. Free radicals are generated in many ways of mechanism, which reacts in damaging cellular substances such as nucleic acids, protein, and lipids. Studies have shown the scavenging activity of Helianthus annuus L., this plant showed scavenging and anti- inflammatory activities (Guo et al., 2017).
The effects shown in this research could be caused due to phytoconstituents present in the plant, the secondary metabolites such as alkaloids, tannins, flavonoids, terpenoids, saponins which were also reported (Dwivedi & Sharma 2014). These secondary metabolites are well known for its scavenging of free radicals and anti-inflammatory function. Treatment of 25% and 50% Helianthus annuus L. seed extract has resulted in weight loss in BALB/c mice of 5-7 months old without affecting the others parameters.
CONCLUSION
The study of this research concluded that Helianthus annuus L seed extract observed in this investigation on BALB/c mice has shown beneficial effects on animals. Can be used for a long period of time for weight management with well diagnostic condition, hence will be effective for diabetic patients. Can be used for weight loss management and can be considered for obese patients should be explored for clinical efficacy and safety of this plant extract as anti-obesity.
ACKNOWLEDGEMENTS
The authors acknowledge the help of Dr. Mobark and Dr.Yasreeb, Alkhram, Saudi Arabia, in sample analysis and special thanks to Dr. Meher for English Editing of the manuscript.
Funding:This study was supported by all authors.
Declarations:Author(s) declare that all works are original and this manuscript has not been published in any other journal.
Data Availability :All data sheet are available on request from corresponding author.
Conflict of Interest: Authors declares no conflicts of interests to disclose.
Ethical Clearance Statement: The Current Research Work Was Ethically Approved by the Institutional Review Board (IRB) of Turabah University Saudi Arabia.
REFERENCES
Akil, L., & Ahmad, H. A. (2011). Relationships between obesity and cardiovascular diseases in four southern states and Colorado. Journal of health care for the poor and underserved, 22(4 Suppl), 61.
Blicharska, E., Komsta, Ł., Kocjan, R., Gumieniczek, A., Kloc, A., & Kaźmierczak, J. (2014). Determination of microelements in sprouts grown on metal-enriched solutions by ion chromatography. Acta Chromatographica, 26(4), 739-747.
Bode, A. M., & Dong, Z. (2015). Toxic phytochemicals and their potential risks for human cancer. Cancer prevention research, 8(1), 1-8.
Dwivedi, A., & Sharma, G. N. (2014). A review on Heliotropism plant: Helianthus annuus L. The journal of Phytopharmacology, 3(2), 149-155.
Engwa, G. A. (2018). Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. Phytochemicals: source of antioxidants and role in disease prevention, 49-73.
Ghosh, N., Sircar, G., Saha, B., Pandey, N., & Bhattacharya, S. G. (2015). Search for allergens from the pollen proteome of sunflower (Helianthus annuus L.): a major sensitizer for respiratory allergy patients. PloS one, 10(9), e0138992.
Guo, S., Ge, Y., & Jom, K. N. (2017). A review of phytochemistry, metabolite changes, and medicinal uses of the common sunflower seed and sprouts (Helianthus annuus L.). Chemistry Central Journal, 11(1), 1-10.
Halliwell, B. (2007). Dietary polyphenols: good, bad, or indifferent for your health?. Cardiovascular research, 73(2), 341-347.
Hausen, B. M., & Spring, O. (1989). Sunflower allergy On the constituents of the trichomes of Helianthus annuus L.(Compositae). Contact Dermatitis, 20(5), 326-334.
Khan, I.A., Jahan, P., Hasan, Q., Rao, P. (2019). Genetic confirmation of T2DM meta-analysis variants studied in gestational diabetes mellitus in an Indian population. Diabetes Metab Syndr 13 (1):688-694.
Leverrier, A., Daguet, D., Calame, W., Dhoye, P., & Kodimule, S. P. (2019). Helianthus annuus Seed Extract Affects Weight and Body Composition of Healthy Obese Adults during 12 Weeks of Consumption: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients, 11(5), 1080.
Lobo, V., Patil, A., Phatak, A., & Chandra, N. (2010). Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy reviews, 4(8), 118.
Mmongoyo, J. A., Wu, F., Linz, J. E., Nair, M. G., Mugula, J. K., Tempelman, R. J., & Strasburg, G. M. (2017). Aflatoxin levels in sunflower seeds and cakes collected from micro-and small-scale sunflower oil processors in Tanzania. PloS one, 12(4), e0175801.
Moe, S. M. (2008). Disorders involving calcium, phosphorus, and magnesium. Primary Care: Clinics in Office Practice, 35(2), 215-237.
National Research Council. (1983). Selenium in Nutrition: Revised Edition. National Academies Press.
Nishitani, N., & Sakakibara, H. (2014). Association of psychological stress response of fatigue with white blood cell count in male daytime workers. Industrial health, 52(6), 531-534.
Nordberg, G. F., Fowler, B. A., & Nordberg, M. (Eds.). (2014). Handbook on the Toxicology of Metals. Academic press.
Onoja, S. O., Udem, S. C., Anaga, A. O., & Asuzu, I. U. (2018). Acute and chronic toxicity studies of hydromethanol leaf extract of Helianthus annuus Linn. in rats. Asian Pacific Journal of Tropical Medicine, 11(9), 534.
Pal, D. (2011). Sunflower (Helianthus annuus L.) Seeds in health and nutrition. In Nuts and Seeds in Health and Disease Prevention (pp. 1097-1105). Academic Press.
Puga, A. M., Lopez-Oliva, S., Trives, C., Partearroyo, T., & Varela-Moreiras, G. (2019). Effects of Drugs and Excipients on Hydration Status. Nutrients, 11(3), 669.
Seca, A. M., & Pinto, D. C. (2019). Biological Potential and Medical Use of Secondary Metabolites.
Shirsath, N. R., & Goswami, A. K. (2020). Natural Phytochemicals and Their Therapeutic Role in Management of Several Diseases: A Review. Current Traditional Medicine, 6(1), 43-53.
Song, S. H., Park, D. H., Bae, M. S., Choi, C. Y., Shim, J. H., Yoon, G., … & Cho, S. S. (2018). Ethanol extract of Cudrania tricuspidata leaf ameliorates hyperuricemia in mice via inhibition of hepatic and serum xanthine oxidase activity. Evidence-Based Complementary and Alternative Medicine, 2018.


