¹ Department of Biotechnology and Microbiology, Shri Maneklal M Patel Institute of
Science and Research, Kadi Sarva Vishwavidyalaya, Gandhinagar, India.
² Department of Post Graduate Diploma in Medical Laboratory Technology, Mehsana
Urban Institute of Sciences, Ganpat University, Mehsana Gujarat, India.
Corresponding Author email : adp04@ganpatuniversity.ac.in
Article Publishing History
Received: 14/07/2021
Accepted After Revision: 24/09/2021
The use of lignocellulosic material is the second-generation process of Bioethanol production and there is a sufficient supply of lignocellulosic materials in agricultural industries such as wheat bran, cotton ginning waste and sugarcane bagasse. The huge amount of waste generated from cotton ginning industry is a burning issue to dispose off, so in order to curb that issue, fermentative bioethanol production from cotton ginning waste using Aspergillus spp. is the best possible options to overcome it. It is also helpful in alleviating the global problem of greenhouse gases and decreasing the use of non-renewable energy with the generation of bio fuel using cotton ginning waste.
The key stages involved in Bioethanol production from lignocellulosic biomass consist of pretreatment of biomass, saccharification, fermentation, and Ethanol Production. In current research work, the combination of three different methods for pretreatment of cotton ginning wastes: acid, alkaline and acid-alkaline were evaluated for the conversion of cellulose and getting higher ethanol yield. After Pre-treatment, sugar i.e., cellulose was further exposed for enzymatic hydrolysis by the cellulase enzyme produced from Aspergillus Spp. using Solid State Fermentation (SSF). The saccharified substrate was then utilized as C source for the fermentation and production of Bioethanol.
The results of pre-treatment succeeded that acid pre-treated, alkaline pre-treated and acid-alkaline pre-treated substrates Cotton ginning wastes (CGW) released 172.8 µg/ml, 256 µg/ml and 140.8 µg/ml of sugar, respectively. The sugar released were then fermented with Saccharomyces cerevisiae using SHF method (Separate Hydrolysis and Fermentation). The amount of ethanol produced from Acid pre-treated, Alkaline pre-treated and Acid-Alkaline pre-treated Cotton ginning wastes (CGW) was 48 mg/ml, 48 mg/ml and 60 mg/ml, respectively. Thus, based on current experimental work it can be stated that the lignocellulosic biomass Cotton Ginning Wastes (CGW) can be converted into biofuel (bioethanol) using Separate Hydrolysis and fermentation process which is the best alternative source of fuel.
Bioethanol, Cotton Ginning Wastes, Fermentation, Hydrolysis, Lignocellulose.
Panchal A, Padhiar A, Mehta P. Pretreatment, Saccharification and Bioethanol Production from Lignocellulosic waste using Aspergillus spp. Biosc.Biotech.Res.Comm. 2021;14(3).
Panchal A, Padhiar A, Mehta P. Pretreatment, Saccharification and Bioethanol Production from Lignocellulosic waste using Aspergillus spp. Biosc.Biotech.Res.Comm. 2021;14(3). Available from: <a href=”https://bit.ly/3s7tizm“>https://bit.ly/3s7tizm</a>
Copyright © Panchal et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The cotton mills and cotton ginning industries worldwide are facing the biggest problem for the waste disposal and in maintaining environmental regulation, as enormous amount of cotton gin waste is created every day. The cotton crop cultivation generates two major types of residues: cotton plant trash (CPT) and cotton gin trash (CGT). Out of these two forms, CGT is very significant due to its higher production and difficulties in its disposal (Agblevor et al. 2006; Sharma and Chen 2008; Pla´cido et al. 2013; Chaudhary and Padhiar 2020).
Biological conversion of these cellulosic wastes into energy, food and chemicals at economic rate is beneficial to mankind (Arthe 2008). With the growth in population there is increasing demand of fuel, so converting such non feed material into bioethanol is of prime importance. As per the earlier research, the production of bioethanol was carried out from sugarcane and starchy feed stocks such as molasses, corn and potato etc.
These substrates are measured as the first-generation process but these processes require pointedly increased amount of cultivable land and increase in food prices leads to its unavailability. This raises the concern since the first-generation process for bioethanol production could not meet the global energy need (Yang et al. 2007; Mitchell 2008; Chaudhary and Padhiar 2020).
Thus, in the coming years focus is shifted towards the production of bioethanol from the non-food plant sources which are considered as the second-generation process. In this process majorly lignocellulosic biomass is used, which consist of major share of agricultural and forest wastes which are the most plentiful renewable biological resources (Splomon et al. 1999; Lin et al. 2006).
In the production of Biofuel using second generation process, the major steps involved are preparation of feedstock, pretreatment, saccharification, fermentation and bioethanol recovery (Saha 2004; Gamage et al. 2010). Feedstock is prepared by washing and complete drying of cotton ginning waste and further Acid Treatment, Alkaline Treatment and Acid-Alkaline Treatment were evaluated for cellulose conversion and yield of ethanol (Tyagi et al. 2019; Darwesh et al. 2020).
The current study is concerned with the production of cellulase using low-cost agro-industrial residue (CGW) as a substrate using SSF. Aspergillus spp. is selected for saccharification because of its rapid growth rate, easy preservation and high metabolic activity in production of cellulase enzyme. Pretreatment of CGW using different methods were carried out and Saccharomyces cerevisiae (MTCC No.172) was used for (SHF) separate hydrolysis and fermentation process for bio ethanol production. The estimation of substrate was carried out using Dinitro salicyclic acid (DNSA) method and Ethanol Estimation is carried out using Iodometric Titration (Miller 1959; Breuil and Saddler 1985; Darwesh et al. 2020).
So far, there are very few such study is conducted to demonstrate the production of bioethanol from cotton ginning waste. Thus, this work significantly represents that saccharified substrate cotton ginning waste is the finest alternate way for the bioethanol making using separate hydrolysis and fermentation method to overcome the increased use of non-renewable energy source and global warming.
MATERIAL AND METHODS
Isolated Aspergillus species was used for present study. It was grown and maintained at 28˚C on Rose Bengal agar plate. The culture was maintained on Rose Bengal agar slant and were stored at 4˚C for long term preservation (Chaudhary and Padhiar 2020).
For collection of the sample, CGW (Cotton ginning waste) was collected from sites of cotton ginning mills from kadi district Mehsana, India. The cotton ginning waste was initially washed with normal tap water in order to remove the soil and other impurities and placed in oven for complete drying (Chaudhary and Padhiar 2020). For the acid pretreatment of cotton ginning waste, 700 ml of 3% sulphuric acid was prepared in 2000 ml Erlenmeyer flask. The flask was added with 54 gm of processed cotton waste and further autoclave carried out at 121˚C for 15 minutes. The flasks containing the pre-treated substrate were then neutralized by washing with tap water till the pH of pre-treated substrate and tap water equalizes. The acid pre-treated samples were then dried separately for further analysis (Chaudhary and Padhiar 2020).
For the alkaline pretreatment of cotton ginning waste, 700 ml of 5% sodium hydroxide was prepared in 2000 ml Erlenmeyer flask. The flask was added with 54 gm of processed cotton waste and further autoclave carried out at 121˚C for 15 minutes. The flasks containing the pre-treated substrate were then neutralized by washing with tap water till the pH of pre-treated substrate and tap water became similar. The alkaline pre-treated samples were then dried separately for further analysis (Dimos et al. 2019). For the acid and alkaline pretreatment of cotton ginning waste, 700 ml of 3% Sulphuric acid and 5% sodium hydroxide both were prepared in 2000 ml Erlenmeyer flaks. The flasks were added with 54 gm of processed cotton waste and further autoclave carried out at 121˚C for 15 minutes.
The pre-treated substrate was then neutralized by washing with tap water till the pH of pre-treated substrate and tap water equalizes. The pre-treated samples were then dried separately for further analysis (Dimos et al. 2019; Chaudhary and Padhiar 2020). For the experimental design of solid-state fermentation (SSF) for enzyme production, preparation of Inoculum was carried out by inoculating previously preserved culture on fresh Rose Bengal agar slant and incubating at 30˚C for 3-7 days. The activated spores were harvested and suspended in sterile D/W (100 ml). 4.5 ml of spore suspension was then further inoculated into substrate flasks for enzyme production (Chaudhary and Padhiar 2016).
Extraction of enzyme from substrate flask is done by adding 5 ml of cold 0.05 M acetate buffer (pH 4.8). The homogenate was filtered using muslin cloth and then centrifuged at 5000 rpm at 4˚C for 15 minutes. The supernatant is used as a crude enzyme source and analyzed for carboxyl methyl cellulase (CMCase), filter paper activity (FPase) and β- glucosidase (Chaudhary and Padhiar 2016). For the saccharification of pre-treated substrates, about 5 gm of acid, alkaline and acid-alkaline pretreated cotton ginning waste taken into 100 ml Czapek Dox medium and 5 ml of the cellulase enzyme was inoculated which was extracted by SSF.
Flasks were incubated at 45˚C up to 10 days in shaking condition. Sugar content was analyzed using DNSA method after every 24 hours of interval till 10 days. An enzyme blank was prepared without adding any substrate (Venkatachalapathy et al. 2014). For the estimation of cellulase activity, cellulase activity was measured by increasing level of sugar using DNSA method. After 24 hrs., 5ml of broth was harvested from each cotton pretreated flasks. The broth was then filtered with Whatman filter paper no.1 and the filtrate were used for further sugar estimation (Chaudhary and Padhiar 2016).
For the isolation of yeast strain, the yeast strain of Saccharomyces cerevisiae (MTCC No.172) was used for this work. It was grown and maintained at 30˚C and 4˚C on Yeast Extract Potato agar plate and slants respectively (Guilherme et al. 2019). For ethanol Production by Saccharomyces cerevisiae using Separate Hydrolysis and Fermentation Method, fermentation was carried out in 100 ml volume with saccharified substrates (CGW). Enzymatic hydrolysate obtained after saccharification of acid, alkaline and acid-alkaline treated substrates (CGW) was decontaminated at 121˚C for 15 min. After disinfection, 9% inoculums of S. cerevisiae were added for Bioethanol production.
The process was carried out at 30˚C for 72 hrs., along with shaking condition at 100 rpm. After 24 hours of incubation, samples were retrieved to determine the concentration of ethanol produced through fermentation (Chandel et al. 2007). The substrate consumption was estimated using the Dinitro salicylic acid (DNSA) method and estimation of bio ethanol was carried out after 48 hrs. using Iodometric Titration method (Miller 1959; Breuil and Saddler 1985; Chaudhary and Padhiar 2020).
For the estimation of Ethanol, ethanol concentration was determined by Iodometric titration method. In this method K2Cr2O7 reacts with concentrated H2SO4 to liberate nascent oxygen which in turn oxidizes ethanol to acetic acid. Remaining amount of potassium dichromate reacted with potassium iodide to liberate iodine which was measured by titrating with 20% sodium thiosulphate using 1% starch as an indicator. The titration results were further used to calculate the ethanol concentration after fermentation process (Chaudhary and Padhiar 2020).
RESULTS AND DISCUSSION
Isolation of Aspergillus spp.: Isolation of fungus was carried out using RBA media. Morphological observation was done and Light green to dark green appearance of colony was observed after 3 to 4 days of incubation. Microscopic observation was done after mounting with lacto-phenol cotton blue dye using high power. Light violet-colored hyphae with dark violet color spores were observed after maturation (Chaudhary and Padhiar 2016; Sahu and Pramanik 2018).
Figure 1: Colonial Characteristics of Aspergillus. Spp.
Figure 2: Morphological Characteristics of Aspergillus Spp.
Cultural and morphological characteristics of yeast: The colony characteristics of the yeast culture were studied on Yeast extract potato dextrose agar plate which was shown in table:1. Microscopic observation of yeast under oil immersion lens was shown in fig:3. Yeast was further inoculated at 30˚C for 3 days to check ethanol production (Sahu and Pramanik 2018).
Table 1.
| Size | Shape | Margin | Elevation | Surface | Consistency | Opacity | Pigmentation |
| Medium | Round | Entire | Convex | Smooth | Moist | Opaque | White |
Figure 3: Morphological characteristics of isolated Yeast
Pre-treatment of CGW: The cotton ginning wastes was used as lignocellulosic wastes in the present study (Fig:4). Impurities of CGW was removed by water washing which was shown in fig:5. Acid pre-treatment was undertaken using 3% H2SO4 and alkaline pre- treatment was undertaken using 5% NaOH. Acid and Alkaline pretreatment of cotton ginning waste carried out using mixture of 3% H2SO4 and 5% NaOH. The resultant effect of pre-treatment on cotton ginning wastes was shown in figure 6,7 and 8 after pretreatment with acid, alkali and mixture of acid-alkali respectively (Dimos et al. 2019; Chaudhary and Padhiar 2020).
Figure 4: Raw Cotton

Figure 5: Water Washed Cotton

Figure 6: Acid treated Cotton

Figure 7: Alkaline treated Cotton

Figure 8: Acid and Alkaline treated Cotton

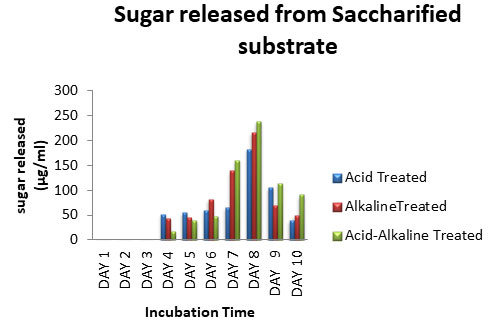
Saccharification of Pretreated CGW: Saccharification of treated CGW was carried out using Aspergillus spp. After every 24 hours, samples were harvested and analyzed for release of sugar up to 10 days. Results of the effect of time duration on the enzymatic saccharification of the acid, alkaline, and acid-alkaline pre-treated CGW were shown in Fig: 9. The result shown that up to 3 days of incubation enzyme was not able to secret sugar from the substrate. There was gradual increase in saccharification of CGW with respect to time, on 8thday maximum saccharification was observed from substrate.
When prolonged to 9th day, the yields of reducing sugar had decreased. The sugar released on 8th day from acid treated, alkaline treated and acid-alkaline treated CGW was 182.4 µg/ml, 216µg/ml and 238.4 µg/ml, respectively (Chaudhary and Padhiar 2016). The rate of sugar production decreased with increase in time after 8th day. Among different pretreatment combine acid –alkali treatment released maximum sugar after saccharification (Dimos et al. 2019; Chaudhary and Padhiar 2020).
Figure 9: Sugar released from saccharified substrate of CGW
Separate Hydrolysis and Fermentation (SHF) of cotton ginning waste (CGW): In SHF of CGW, first the enzymatic hydrolysis was carried out for 8 days at 45˚C temperature. On 8thday maximum reducing sugar was obtained from CGW through saccharification using Aspergillus spp. Enzymatic hydrolysate obtained after saccharification of acid, alkaline and acid-alkaline treated substrates (CGW) was inoculated with S. cerevisiae for fermentation. The process was conducted at 30˚C temperature using 100 rpm in shake flask method up to 48 hrs.
After 24 hours incubation, samples were retrieved to determine the concentration of ethanol produced. Figures 10 to 12 shows the sugar utilization pattern and ethanol accumulation in the SHF inoculated with S. cerevisiae using cotton ginning waste (Sahu and Pramanik 2018). At 48 hrs. maximum content of ethanol were achieved by using separate hydrolysis and fermentation (SHF) method (Chaudhary and Padhiar 2016; Sahu and Pramanik 2018; Chaudhary and Padhiar 2020).
Figure 10: Sugar Utilization and Ethanol production from Acid treated CGW.
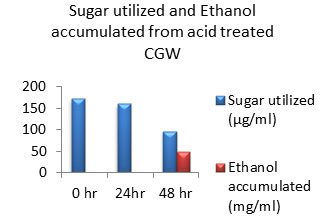
Figure 11: Sugar Utilization and Ethanol production from Alkaline treated CGW.
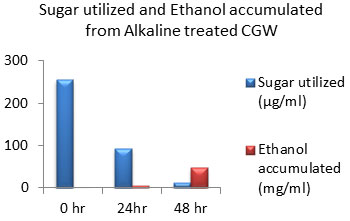
Figure 12: Sugar Utilization and Ethanol production from Acid-Alkaline treated CGW.
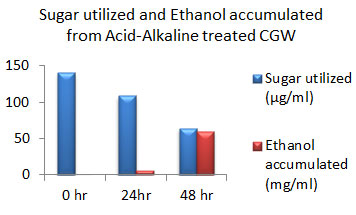
The amount of ethanol produced from Acid-pre-treated, Alkaline-pre-treated and Acid-Alkaline pre-treated Cotton ginning wastes (CGW) was 48 mg/ml, 48 mg/ml and 60 mg/ml respectively after 48 hours which was shown in the Fig: 10 to 12. After 24 hours amount of ethanol produced was very less as sugar utilization was also less.
After 48 hours of incubation sugar will be exhausted to negligible amount in Alkali pretreated sample which was shown in fig: 11. While in case of acid pretreated CGW available residual sugar was higher compared to alkali pretreated CGW which indicates that acid pretreated samples may releases high amount of 5 C sugar which may not be able to get converted into ethanol. Same result was observed in Acid-Alkaline pre-treated CGW shown in fig: 12.
The fermentation process can be economical if it can convert both the pentose and hexose sugars in the hydrolysate to bioethanol. Saccharomyces cerevisiae can ferment only hexose sugars into ethanol. In present study the quantity of residual sugar was higher and the concentration of ethanol obtained in SHF is lower due to temperature difference between Saccharification and Ethanol Production. Aspergillus culture gave maximum Saccharification at 50oC and S. cerevisiae gave maximum ethanol yield at 30oC.
Previous studies on fermentation using Saccharomyces cerevisiae for substrates like, groundnut hull and rice husk, the yields of 0.142g per gram from groundnut oil and 0.108g per gram from rice husk were stated (Srivastava et al. 1997). Similarly, the sugarcane leaf litter as feedstock produced 0.130 mg/L (alkaline pre-treated) and 0.335 mg/L (acid pre-treated) of ethanol. For substrate bagasse pith hydrolyzed by cellulase enzyme the bioethanol produced was 7.7% (v/v) (Iuliana 2009).
In one more research, 0.218g per gram yield of cellulosic waste mixture (office paper, newspaper and cardboard in 1:1:1 ratio) was testified (Iuliana et al. 2010). Acid-Alkaline pre-treated Cotton ginning wastes (CGW) proves better compare to only acidic or alkali pretreatment and these can be used for further scale up for bioethanol production (Chaudhary and Padhiar 2020).
Previously various research has been undertaken for the bioethanol production from diverse lignocellulosic wastes such as molasses, sugar cane bagasse, starchy biomass etc. and also various different pretreatment process such as hydrothermal process, hydrogen peroxide process etc. were used but such treatment process are very costly and also release harmful byproducts as well use of starchy feed stock increase its price and unavailability (Lin and Tanaka 2006; Pla´cido J et al. 2013; Dimos et al. 2019).
Thus, the results of the present work clearly revealed that the cellulosic Cotton Ginning Wastes (CGW) could be converted into bio ethanol with enzymatic hydrolysis followed by fermentation. Thus, through appropriate use of a pretreatment process and a renewable feedstock like lignocellulosic biomass for production of a biofuel (ethanol) it is likely to serve as a sustainable substitute for energy production and reducing the requirement for crude oil (Sahu and Pramanik 2018; Chaudhary and Padhiar 2020).
CONCLUSION
The finding of the present study clearly flourished that the lignocellulosic Cotton Ginning Wastes (CGW) can be used for bio ethanol production with enzymatic hydrolysis followed by fermentation process. Among all the pretreatments, Acid-alkaline pre-treated substrate can be used to produced more amount of ethanol i.e., 60 mg/ml in comparison to acid and alkaline pre-treated CGW substrate. Result of this preliminary work can be exploited for pilot scale production and must be scale up for efficient bio ethanol production. Therefore, using a suitable pretreatment process and a renewable feedstock like lignocelluloses biomass for production of bio ethanol with Separate hydrolysis and fermentation method is very successful and can be used as an energy efficient process for renewable fuel generation.
ACKNOWLEDGEMENTS
This study was supported by Shri Maneklal M Patel institute of Science and Research, KSV University, Department of Biotechnology and Microbiology. Authors are thankful for giving an opportunity to carry out this research work and supporting throughout the work.
Conflict of Interests: Authors declare no conflicts of interests to disclose.
REFERENCES:
Agblevor, F A, Cundiff, J S, Mingle, C, et al. (2006) Storage and characterization of cotton gin waste for ethanol production. Resources, Conservation and Recycling, 46(2), 198–216.
Arthe, R (2008). Production of bio-ethanol from cellulosic cotton waste through microbial extracellular enzymatic hydrolysis and fermentation. Electronic Journal of Environmental, Agricultural and Food Chemistry, 7(6), 2948–2958.
Breuil, C and Saddler, J N (1985). Comparison of the 3,5-dinitrosalicylic acid and Nelson-Somogyi methods of assaying for reducing sugars and determining cellulase activity. Enzyme and Microbial Technology, 7(7), 327–332.
Chandel, A K, Chan, E C, Rudravaram R, et al. (2007). Economics and environmental impact of bioethanol production technologies: An appraisal. Biotechnology and Molecular Biology Reviews, 2, 14–32.
Chaudhary K and Padhiar A (2016). Study on the cellulase activity of fungi by solid state fermentation using cellulosic wastes. International Journal of Science and Research 5(11), 1637-1642.
Chaudhary K, and Padhiar A (2020). Cellulase production by fungi from agro wastes under solid state fermentation. Biosc.biotech.res.comm, 13(3), 1495-1501.
Chaudhary KU, and Padhiar A (2020) Dilute acid pretreatment and enzymatic sachharification of cotton ginning waste. Indian Journal of Science and Technology 13(43), 4454-4464.
Osama M D, El-Maraghy S H, Abdel-Rahman H M and Rashed A (2019) Improvement of Paper Wastes Conversion to Bioethanol Using Novel Cellulose Degrading Fungal Isolate. Fuel, 262, 116518.
Guilherme A D A, Dantas P V F, Padilha C E A, et al. (2019). Ethanol production from sugarcane bagasse: Use of different fermentation strategies to enhance an environmental-friendly process. Journal of Environmental Management, 234, 44–51.
Dimos K, Paschos T, Louloudi A, et al. (2019). Effect of Various Pretreatment Methods on Bioethanol Production from Cotton Stalks. Fermentation, 5(1), 5.
Gamage J, Lam H and Zhang Z (2010). Bioethanol production from lignocellulosic biomass, A review. Journal of Biobased Materials and Bioenergy, 4(1), 3–11.
Iuliana L, Gigi C and Gabriela B (2010). The Plackett-Burman Model-An improved alternative to identify the significant factors implied in the Bioconversion of the complex Cellulosic Waste to Ethanol. Innovative Romanian Journal Food Biotechnology, 7, 55-60.
Leustean I (2009). Bioethanol from lignocellulosic materials. Journal of Agrolimentary process and Technologies, 15(1), 94-101.
Lin Y and Tanaka S (2006). Ethanol fermentation from biomass resources: Current state and prospects. Applied Microbiology and Biotechnology, 69(6), 627–642.
Miller G L (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428.
Mitchell D (2008). A note of rising food prices. Policy Research Working Paper, 4682, 1–21.
Plácido J, Imam T and Capareda S (2013). Evaluation of ligninolytic enzymes, ultrasonication and liquid hot water as pretreatments for bioethanol production from cotton gin trash. Bioresource Technology, 139, 203–208.
Sharma-Shivappa R R and Chen Y (2008). Conversion of cotton wastes to bioenergy and value-added products. Transactions of the ASABE, 51(6), 2239–2246.
Saha B C (2004). Lignocellulose biodegradation and applications in biotechnology. ACS Symposium Series. Washington, DC: American Chemical Society, pp 2–34.
Sahu S, and Pramanik K (2018). Evaluation and Optimization of Organic Acid Pretreatment of Cotton Gin Waste for Enzymatic Hydrolysis and Bioethanol Production. Applied Biochemistry and Biotechnology, 186(4), 1047-1060.
Solomon B O, Amigun B, Betiku E, et al. (1999). Optimization of cellulase production by Aspergillus flavus Linn isolate NSPR. Grown on Bagasse. JNSCHE, 16, 101, 61–68.
Srivastava S, Modi S, Garg D R et al. (1997). Production of ethanol from guava pulp by yeast strains. Bioresource Technology, 60, 263-265.
Tyagi S, Lee K J, Mulla S I, et al. (2019). Production of bioethanol from sugarcane bagasse: Current approaches and perspectives. Applied Microbiology and Bioengineering, 21–42.
Venkatachalapathy G, Krishnappa R K and Sirangala T G (2014). Estimation of Sugar and Eio ethanol from Different Decaying Fruits Extract. Advances in Applied Science Research, 5(1), 106–110.
Yang B and Lu Y (2007). The promise of cellulosic ethanol production in China. Journal of Chemical Technology and Biotechnology, 82(1), 6–10.


