1Biochemistry Department, School of Medicine, Kashan University of Medical Sciences, Kashan, Iran
2Radiopharmacy Department, School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
3Department of Pharmaceutics & Medical Nanotechnology, School of Pharmacy, Pharmaceutical Branch of Azad University, Tehran, Iran
4Herbal medicine research center (HMRC) and Department of Pharmaceutics. Branch of Pharmaceutical Sciences. Islamic Azad University, Tehran, Iran
Corresponding author Email: mostafa.saffary@gmail.com
Article Publishing History
Received: 10/01/2017
Accepted After Revision: 21/03/2017
According to progressive trend of herbal application in modern medicine, various studies has been shown several properties for Rosemary essential oil (EO) such as antioxidant, anti-bacterial, anti-cancer and anti-inflammatory effect. Owing to liposomal benefits such as increased solubility, enhanced performance and increased stability of its content, the main objective of this study was to design nanoliposomes containing rosemary essential oils to achieve more efficacy. Nano-liposomes containing EO was prepared by three different methods including thin film hydration, sonication and extrusion methods. The physical properties of nanoliposomes such as particle size, poly dispersity index, zeta potential, encapsulation efficiency and release profile of EO were studied. The mean size of liposomes containing essential oils of rosemary prepared by sonication method was 162 nm which, is greater than extruder method (470 nm) and liposomes were prepared by thin film hydration were about one µm. Encapsulation efficiency was higher in sonication method rather that extrusion method. Both methods lead to spherical particles. Release of EO from liposomes in aqueous media was negligible (P value > 0.05). It was found that the method of liposomes preparation, cholesterol concentration and essential oil concentration is effective on the size and encapsulation efficiency of nanoliposome.
Nanoliposome, Rosemary Essential Oil, Thin Film Hydration, Extrusion, Sonication
Arabi M. H, Chabok H, Mirzapour A, Ardestani M. S, Mostafa M. Preparation of Nanoliposomes Containing Rosmarinus Officinalis L Essential Oil: a Comparative Study. Biosc.Biotech.Res.Comm. 2017;10(1).
Arabi M. H, Chabok H, Mirzapour A, Ardestani M. S, Mostafa M. Preparation of Nanoliposomes Containing Rosmarinus Officinalis L Essential Oil: a Comparative Study. Biosc.Biotech.Res.Comm. 2017;10(1). Available from: https://bit.ly/2Y5Wyek
Introduction
Plants have been the basis for medical treatments through much of human history, and such traditional medicine is still widely practiced today. Modern medicine make use of many plant-derived compounds as the basis for evidence-tested pharmaceutical drugs, and phytotherapy works to apply modern standards of effectiveness testing to herbs and medicines that are derived from natural sources. There are many forms in which herbs can be administered, in many cases essential oils are used as medicine. An essential oil is a concentrated hydrophobic liquid containing volatile aroma compounds from plants. An oil is “essential” in the sense that it contains the characteristic fragrance of the plant that it is taken from (Oxford dictionary, 2014). Essential oils are complex blends of a variety of volatile molecules such as terpenoids, phenol-derived aromatic components, and aliphatic components have been great interest in various industries such as food and medicine (Bilia et al., 2014).
Rosmarinus officinalis L that belongs to the family Lamiaceae (Lamiaceae) and have at least 1% (volume / weight) volatile oil Rosemary commonly used in the food industry as spice and flavors. Rosemary officinalis’s essential oil possess a verity of properties include antioxidant, antimicrobial, antitumor anti-HIV, anti-inflamation, analgestic effect and etc Peng et al., 2007). The most important constituents of the Iranian Rosemary are 1,8-cineole (23.47%), á-pinene (21.74%), berbonone (7.57%), camphor (7.21%) and eucalyptol (4.49%) (Altinier et al., 2007, Honorio et al., 2015).
Rosemary essential oil used in various commercial products on the market, Including bath additive for support the function of the skin and auxiliary treatment in conditions of exhaustion, ointment for the symptomatic treatment of muscle and joint pain, solutions for stimulation of circulation (Jalali-Heravi et al., 2011).
Studies have shown that a new drug delivery methods such as encapsulation can reduce volatility, increase solubility in water, increase dilution for use in the end product and the efficacy of essential oils (Sherry et al., 2013). Nanocarriers can be structured by a great variety of material and designs (Saraf, 2010). Liposomes are bilayer vesicles that obtained from association of amphiphilic lipids that can carry up hydrophilic, hydrophobic and amphiphilic compounds. Nanoliposomes according to the method and ingredients can have different shapes and sizes (Moghimipour et al., 2012; Mozafari et al., 2010). The aim of this study was encapsulation of Rosemary EO oil in nanoliposome that done by three different methods including thin film hydration, extruder and sonication. Then prepared liposomes, will compared for their physicochemical properties.
Material and Methods
Soybean lecithin, cholesterol and DOTAP taken from Sigma–Aldrich (Steinheim, Germany), Merck KGaA (Darmstadt, Germany) and Lipoid GmbH (Ludwigshafen, Germany), Rosemary essential oil (taken from Barij Essence Kashan, Iran), PBS were purchased from Sigma–Aldrich (Steinheim, Germany).
Cholesterol, phosphatidyl choline and rosemary essential oil in several ratios of essential oil to total lipids, and several molar ratio of cholesterol: lecithin and lipid composition(DOTAP), were solved in chloroform/ ethanol (2: 1, v/v) inside the 500 ml round-bottom flask then by using rotary evaporator in constant temperature above the lipid phase transition temperature (Tc), under negative pressure and high vacuum for 2 hour organic solvent was removed and a thin layer was formed in bottom flask. Then the obtained lipid film was hydrated by Phosphate buffered saline (PBS pH: 7.4) for 1.5 hour at temperatures above Tc and in 120 rpm of speed.
Initial ratio of essential oils/total lipid was 1/3, 1/4 and 1/5. Molar ratio of Cholesterol: Phosphatidyl Choline was 1: 1, 1: 2 and 1: 3 in 100 mM total lipid concentration.
In the preparation of Cationic liposomes containing essential oil, phosphatidyl choline (E80), 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), and cholesterol (Chol) at a molar ratio of E80:DOTAP:Chol = 1:1:1 Formulation was used (Table1).
| Table 1: Composition of cationic liposomes formulation | ||
| Formulation name
|
Chol: PC: DOTAP | Rosemary essential oil/total lipid |
| H1
|
1:1:0 | 1/3 |
| H2 | 1:1:0 | 1/4 |
| H3 | 1:1:0 | 1/5 |
| H4 | 1:2:0 | 1/3 |
| H5 | 1:3:0 | 1/3 |
| H6 | 1:1:1 | 1/3 |
Liposomes obtained from thin film hydration method was passed through Nucleopore polycarbonate filters (0.2 and 0.1 μm in series, Whatman, UK) under strong pressure at above the lipid transition temperature in three stages by using the extruder device.
Liposomes obtained from thin film hydration method were sonicated by probe sonicator (Hielscher UP400S, Germany) at 70 Watt amplitude for 30 minutes.
The size and polydispersity (PDI) of formed liposome was measured by Dynamic light scattering in a Zetasizer Nano ZS (Malvern ZEN 3600). The samples were diluted with phosphate buffer saline and measurement was triplicate.
Charge on loaded vesicles surface and average zeta potential is measured by zeta potential analyzers Zetasizer instrument (Malvern ZEN 3600) (Kraft et al., 2014).
Encapsulation Efficiency was determined by centrifugation techniques (Bhatia et al., 2014). In brief, liposomes containing essential oil was isolated from unencapsulated materials by using centrifuge at 10000rpm for 10 minutes (laboratory centrifuge Hettich Universal 320 R). The liposomes were destroyed in ethanol 90% (Merck,Germany) and encapsulated essential oil content was measured by using UV/VIS spectrophotometer at 270 nm of wavelength.
The morphology of liposomal formulation were Studied by Scanning electron microscopy (SEM) using an AIS2100 (Seron Technology, South Korea).The release study was performed by using dialysis membranes method. In summary, the 1000 μL of the 14.20 mg/mL essential oil encapsulated liposomal samples were inside the dialysis bag (MWCO 12kDa, Thermo Fisher Scientific). The dialysis system was suspended in a release volume of 100 mL PBS at 37°C and rotated at 100 rpm (1:100 dilution between donor and acceptor compartments). At scheduled intervals, 1 ml of the release medium was collected for the UV spectrophotometric assay. The same volume of fresh PBS buffer at the same temperature was added immediately to maintain constant release volume. The length of the dialysis tubing was kept consistent for all methods to ensure that the surface area available for dialysis remained constant. To ensure that dilution between the donor and acceptor compartments provided sink conditions, a 1:100 dilution study was conducted and release volume was set at 100 mL PBS.
Results are presented as mean ± SEM. Statistical analysis was performed using SPSS Software (version 22). The mean values were compared by one-way analysis of variance (ANOVA) followed by the post-hoc test. The differences of P < 0.05 were considered as statistically significant. Results and Discussion
The results showed that nanoliposomes containing essential oils obtained from sonicator method are smaller than extruder method. The average size of nanoliposomes containing essential oil of three methods were as follows: Thin Film> Extruder>sonicator. It was also found that the concentration of essential oils affect the size of the liposomes and nanoliposomes with lowest essential oils have smaller size. On the other hand, it was found that the ratio of cholesterol to total lipid in the same concentration of Essential oil also affects the particle size and in higher ratio of cholesterol to total lipid, smaller nanoliposome can be achieved (Table 2).
The results showed that there was not significant change on the PDI concentrations by changing the
concentration of essential oil. But as the concentration of cholesterol increased in both sonication and extrusion methods, PDI was increased. EO containing liposomes prepared by thin films have the highest PDI (0.9), but in comparison the sonication and extrusion showed no significant difference (0.2; P value > 0.05). Add DOTAP to the lipid composition of liposomes in comparison with the same concentration of essential oils and cholesterol showed not-significant difference, as shown in Table 2.
Zeta Potential
Nano-liposomes containing DOTAP have a zeta potential of +17 mV. Other liposomes due to the lack of charging ingredients, showed neutral charge.
Results showed that the preparation method of nanoliposomes is effective on encapsulation of essential oil. Nanoliposome prepared by sonicator and extrusion have the maximum and Minimum of Essential oil load respectively. Also increasing concentration of essential oils is increasing its load into liposomes. On the other hand, it was found that the percentage of cholesterol is effective in loading of essential oil and increasing the percentage of cholesterol lead to increase the loading of the essential oil (Table 2).
| Table 2: Characterization of Rosemary EO containing nanoliposomes | |||||||||
| Formulation | Extrusion method | Sonication method | Thin film hydration method | ||||||
| MD(nm) | PDI) | EE (%) | MD(nm) | PDI | EE (%) | MD(nm) | PDI | EE (%) | |
| H1
|
358.6±
1.3 |
0.46 | 83 ± 6.3 | 122 ±
3.2 |
0.35 | 98.9 | 1833.5 ±
926.5 |
0.51
|
100 |
| H2 | 346.9 ±
9.3 |
0.3 | 67.4 ± 6.9
|
104.33 ± 3.4
|
0.22 | 99.03 | 2747.5 ±
1632.5 |
0.50
|
100 |
| H3 | 230.9±
8 |
0.26 | 41.9 ± 4.3 | 92.36 ± 4.5 | 0.24 | 99.3 | 2453.9 ±
2186.1 |
0.65
|
100 |
| H4 | 366.9 ± 9 | 0.19 | 64.7 ± 3.3 | 154 ± 3.2 | 0.15 | 95.3 | 1757.5 ±
332.5 |
0.77
|
100 |
| H5 | 469.7 ± 2.5 | 0.19 | 47.7 ± 6.3 | 162 ± 4.3 | 0.12 | 96.3 | 1053
37 |
0.64 | 100 |
| H6 | 871.3 ±
14 |
0.29 | 97.5 ± 2.5
|
133 ±
1.0 |
0.23 | 95.3 | 2720 ±
11 |
0.71 | 100 |
| MD: Mean diameter; PDI: Poly dispersity index; EE: encapsulation efficacy;
Mean ± standard error of mean (SEM) |
|||||||||
The Morphology of Nanoliposomes
SEM Images of nanoliposome shown in Figure 1. As can be seen all containing particles are spherical shape and a coherent. They are homogeneous in size and shape.
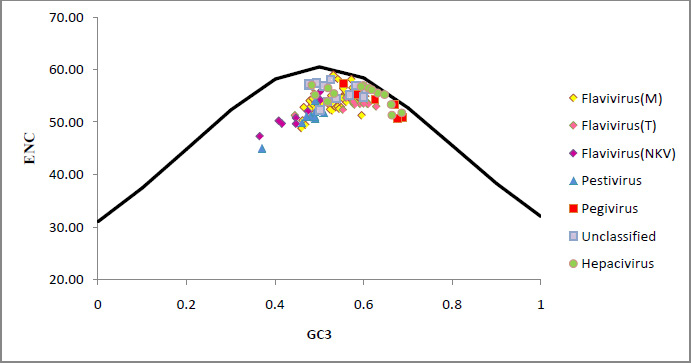 |
Figure 1a: The SEM image of nanoliposomes containingrosemary essential oils; prepared by sonication method (A) and extrusion method(B) |
 |
Figure 1b |
As showed in the Figure 2, Essential oil concentrations within solvent is relatively stable. It indicates lack of EO leakage from liposomes over time. After initial release of less than 1 % of total EO no more release could be seen. This behavior is very desirable in storage stability of nanoliposomes. It is important that liposomes can protect and keep inside their cargo before reach target site.
 |
Figure 2: Release curve for Rosemary Essential Oil from H3 nanoliposomes containing Rosemary essential oils in hours |
Discussion
Essential oils and their components are volatile and sensitive to environmental factors such as light, heat, pH and oxidation. Encapsulation of essential oils reduced degradation and increased its stability before arriving target site.
Compare liposomes with different ratio of essential oil/ total lipid showed that the essential oils influence the size of nanoliposomes and increasing in Rosemary essential oil concentrations, increased the size of the liposomes. Probably due to the arrangement of essential oil into the lipid bilayer of liposomes fusion of bilayer fragment increase and larger liposomes would formed. Also, results revealed that by increasing the concentration of essential oils, encapsulation efficiency is increased (Stimac et al., 2017). The results of this study as well as previous studies conclude that the method of preparation of liposomes is effective on the size and encapsulation efficiency (Stimac et al., 2017; Saffari et al., 2013). In comparison with the three methods of nanoliposome preparation: thin film hydration, sonication and extrusion, the lowest and largest size of the liposomes was related to sonication and thin film hydration method but the Essential loaded into the liposomes is more in the sonication method compare to extrusion method one.
Against studies that showed use of sonication method reduces the encapsulation efficiency in nanoliposomes production and may also lead to damage liposomes phospholipid and loaded compounds (Stimac et al., 2017; Khatibi et al., 2015) this study doesn’t show a significant reduction in encapsulation efficiency in preparation of liposome by sonication method.
Poly dispersity index is an index of particle size distribution in a system, which low level of that, reflects the uniformity of particle diameter distribution. PDI less than 0.1, indicating homogeneous particle diameter and the value of that greater than 0.3 indicates heterogeneous distribution of particle diameter (Sinico et al., 2005). In previous studies it has been reported that essential oil containing liposome have wider size distribution than liposomes without essential oils (Ruozi et al., 2005). Comparisons between different concentrations of essential oil in the preparation of liposomes showed that change in oil concentration had not a significant effect on the liposome size distribution. (P value: 0.178) in preparation of rosemary containing liposomes.
Also in the comparison between three methods of liposome preparation, Method of preparation of liposomes did not have significant effect on PDI, However, thin film hydration method was highest PDI.
Contrary to Ortan et al. reports, the results of this study showed that cholesterol help to entrapment of essential oil into nanoliposome structure, (Ortan et al., 2009) Unlike previous studies that had been done on MLV liposome, this study showed that in both methods of extrusion and sonication, in the presence of essential oil, increasing the concentration of cholesterol reduces the size of nanoliposomes (Arriaga et al., 2009; Detoni et al., 2009).
In analyze of SEM image of Nanoliposome containing Rosemary Essential Oil, particles had monodispersity and spherical structure. This can reveal that critical packing parameter of our composition is suitable for EO containing liposomes.
The release study showed that essential oil release from nanoliposome was very low during 24 hours incubation time, which is probably due to lipophilic properties of essential oil. Results showed impression of multiple factor must be considered in this regard as mentioned by different works (Fathi Moghaddam et al., 2008; Rezaee et al., 2015). EO can be trapped well inside the liposomes. Adding DOTAP to the lipid composition of liposomes enhances the encapsulation Efficiency of essential oil and on the other hand, increase the size of the liposomes.
In brief, this study showed that the method of preparation of nanoliposomes containing Rosemary essential oil has been effective on the particle size, dispersity and encapsulation of essential oil. The study also found that changes in formulation, percentage of cholesterol, addition of ionic lipid and using different ratio of essential oil can cause changes in the physicochemical properties of EO containing nanoliposomes. Present study also, revealed that due to lipophilic and connection with nanoliposomes rosemary EO have fewer tendency to release in physiological environments. To determine the efficiency and effectiveness of drug delivery of liposomes, in-vivo studies on animal models and MIC studies are useful.
Acknowledgements
The authors would like to thank Ms. Zahra Abbasian & Ms. Astaraki for their valuable technical assistance. This research was performed with support from research Center of Kashan University of Medical Sciences.
Conflict of interest
There is no conflict of interest.
Abbreviations
EO: Essential Oil Tc: Transition temperature,PBS: Phosphate Buffered Saline, DOTAP: 1, 2-dioleoyl-3-trimethylammonium-propane, PC: Phosphatidyl Choline , Chol: Cholesterol, PDI: Polydispersity Index, EE: Encapsulation Efficiency SEM: Scanning Electron Microscopy
References
- Altinier G, Sosa S, Aquino RP, Mencherini T, Della Loggia R, Tubaro A.(2007) Characterization of topical antiinflammatory compounds in Rosmarinus officinalis L. J Agric Food Chem 55-1718-23.
- Arriaga LR, López-Montero I, Monroy F, Orts-Gil G, Farago B, Hellweg T. (2009) Stiffening effect of cholesterol on disordered lipid phases: a combined neutron spin echo+ dynamic light scattering analysis of the bending elasticity of large unilamellar vesicles. Biophysical journal. 96(9):3629-37.
- Bhatia A, Kumar R, Katare OP. (2004)Tamoxifen in topical liposomes: development, characterization and in-vitro evaluation. J Pharm Pharm Sci..7(2):252-9.
- Bilia AR, Guccione C, Isacchi B, Righeschi C, Firenzuoli F, Bergonzi MC.(2014) Essential Oils Loaded in Nanosystems: A Developing Strategy for a Successful Therapeutic Approach. Evid Based Complement Alternat Med,
- Detoni C, Cabral-Albuquerque E, Hohlemweger S, Sampaio C, Barros T, Velozo E.(2009) Essential oil from Zanthoxylum tingoassuiba loaded into multilamellar liposomes useful as antimicrobial agents. Journal of microencapsulation. 2009;26(8):684-91.
- Fathi Moghaddam H, Shafiee Ardestani M, Saffari M, Navidpour L, Shafiee A, Rahmim A. (2008) Dopaminergic but not glutamatergic neurotransmission is increased in the striatum after selective cyclooxygenase-2 inhibition in normal and hemiparkinsonian rats. Basic & clinical pharmacology & toxicology 103(4):293-296.
- Honorio VG, Bezerra J, Souza GT, Carvalho RJ, Gomes-Neto NJ, Figueiredo RC. (2015) Inhibition of Staphylococcus aureus cocktail using the synergies of oregano and rosemary essential oils or carvacrol and 1,8-cineole. Frontiers in Microbiology. 6:18-27.
- Jalali-Heravi M, Moazeni RS, Sereshti H(2011) Analysis of Iranian rosemary essential oil: application of gas chromatography-mass spectrometry combined with chemometrics. J Chromatogr A. 1218(18):2569-76.
- Khatibi SA, Misaghi A, Moosavy M-H, Amoabediny G, Basti AA. (2015) Effect of Preparation Methods on the Properties of Zataria multiflora Boiss. Essential Oil Loaded Nanoliposomes: Characterization of Size, Encapsulation Efficiency and Stability. Pharmaceutical Sciences. 20:141.
- Kraft JC, Freeling JP, Wang Z, Ho RJ. (2014) Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. Journal of pharmaceutical sciences. 2014;103(1):29-52.
- Moghimipour E, Aghel N, Mahmoudabadi AZ, Ramezani Z, Handali S. (2012) Preparation and characterization of liposomes containing essential oil of Eucalyptus camaldulensis leaf. jundishapur journal of natural pharmaceutical products. 7(3):117-22.16.
- Mozafari M.(2010) Nanoliposomes: preparation and analysis. Liposomes: Methods and Protocols, Volume 1: Pharmaceutical Nanocarriers. p. 29-50.
- Ortan A, Câmpeanu G, Dinu-Pirvu C, Popescu L. (2009) Studies concerning the entrapment of Anethum graveolens essential oil in liposomes. Roum Biotechnol Lett. 14:4411-7.
- Oxford English Dictionary, (2014) NEssential oil. (online, American English ed.) 2014;07-21.
- Peng CH, Su JD, Chyau CC, Sung TY, Ho SS, Peng CC.(2007) Supercritical Fluid Extracts of Rosemary Leaves Exhibit Potent Anti-Inflammation and Anti-Tumor Effects. Bioscience, Biotechnology, and Biochemistry. 71(9):2223-32.
- Rezaee S, Khalaj A, Adibpour N, Saffary M. (2015) Correlation between lipophilicity and antimicrobial activity of some 2-(4-substituted phenyl)-3 (2H)-isothiazolones. DARU Journal of Pharmaceutical Sciences. 17(4):256-263.
- Ruozi B, Tosi G, Forni F, Fresta M, Vandelli MA. (2005) Atomic force microscopy and photon correlation spectroscopy: two techniques for rapid characterization of liposomes. European Journal of Pharmaceutical Sciences. 25(1):81-9.
- Saffari M, Tamaddon AM, Shirazi FH, Oghabian MA, Moghimi HR.(2013) Improving cellular uptake and in vivo tumor suppression efficacy of liposomal oligonucleotides by urea as a chemical penetration enhancer.; The journal of gene medicine 15(1):12-19.
- Saraf S. (2010) Applications of novel drug delivery system for herbal formulations. Fitoterapia. 81(7):680-9.
- Sherry M, Charcosset C, Fessi H, Greige-Gerges H. (2013) Essential oils encapsulated in liposomes: a review. Journal of liposome research. 23(4):268-75.
- Sinico C, De Logu A, Lai F, Valenti D, Manconi M, Loy G. (2005) Liposomal incorporation of Artemisia arborescens L. essential oil and in vitro antiviral activity. European Journal of Pharmaceutics and Biopharmaceutics. 59(1):161-8.
- Stimac A, Sekutor M, Mlinaric´-Majerski K, Frkanec L, Frkanec R.(2017) Adamantane in Drug Delivery Systems and Surface Recognition. Molecules. 22:297.


