1Department of Chemistry, Sri Aurobindo College, University of Delhi, New Delhi-110017,India.
2School of Life Sciences, Jawaharlal Nehru University, New Delhi-110067, India
Article Publishing History
Received: 05/12/2020
Accepted After Revision: 27/03/2021
Due to advancement and increasing development in industrialization, heavy metals especially Arsenic (As) may cause an environmental threat because of continuous release of effluents in ground water. Metallic As is hazardous in nature and have severe harmful outcomes on human, aquatic animals, plants and environment. As cause severe lethal impacts on the healthy human as well as environment after appearing into the chain of food. As is one of major cancer causing agent in humans. Though, development of new technology like nanotechnology gives hope for better techniques for As removal from waste water. Preparation of unique, novel and low cost of nanomaterials for environmental applications, detection of pollutant and other uses has drew attention for further considerable. On this note, zero valent iron and iron oxide nanoparticles are observed as the suitable materials for the As adsorption from waste water or ground water.
Electrical, ionic interaction, mechanical and physiochemical properties play key role in nanoparticle fabrication and control in desirable morphology. Iron oxide nanoparticles can also be used as catalyst, drug delivery carriers and contrasting agent. Different categories of iron oxide nanoparticles desired shape or topography, and size can be prepared by using different methodology such as sol-gel, co-precipitation, solvothermal reactions and iron oxide composites. Iron oxide nanoparticles have previously shown its efficiency, diversity and reusability in several areas including bio-imaging, drug or gene delivery, catalytic properties, immobilization of industrial important enzymes and removal of dye, phenol and toxic compounds. Present review is dedicated on the preparation of iron oxide nanoparticles and its composites for As metal removal.
Nanoparticles, Iron, Iron Oxide, Composites, Arsenic, Environment
Ravi R, Mishra A. Preparation of Iron Nanoparticles and Composites for Arsenic Removal: an Updated Review. Biosc.Biotech.Res.Comm. 2021;14(1).
Ravi R, Mishra A. Preparation of Iron Nanoparticles and Composites for Arsenic Removal: an Updated Review. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/3s2zAiL”>https://bit.ly/3s2zAiL</a>
Copyright © Ravi and Mishra This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
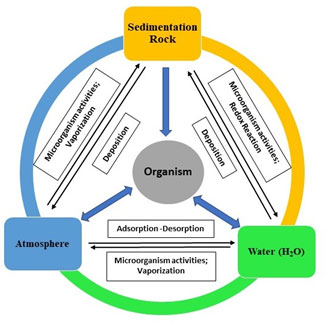
Arsenic occurs as in oxides form within dirt, dregs, aqueous solution, and ground water in several part of the globe (Nurmesniemi et al., 2010, Park et al., 2011). Naturally, arsenic occurs more than different 200 distinctive mineral structures. Arsenic appears around in the form of arsenates (60%), sulphides (20%) and sulphosalts, and consequently arsenide, arsenite, silicates and elemental arsenic are the left over 20% (McCarty et al., 2011, Chiban et al., 2012). The well-known Aresenic compounds that naturally occur are arsenite (As(III)) and arsenate (As(V)) (Drewniak et al., 2012). As(V) is the prevalent type of As existing under oxidized surroundings and present as oxyanions of arsenic acid, while As(III) occurs as arsenious acid under mild reducing environments (Fig.1) (Sinha et al., 2013, Campbell et al., 2014, Podgorski, et al., 2020).
Arsenic and related compounds have been recognized as potent carcinogen as per guideline of the International Agency for Cancer Research (IARC) (Karagas et al., 2015). Hydrothermal activity associated with granitic magma intrusion and orogenesis shapes the primary arsenic containing mineral arsenopyrite (Schindler et al.,2016). Due to the natural geological distribution in the bedrock, the presence of As in natural source water may appear (Bondu et al., 2016). It primarily presents as an inorganic as well as organic type in drinking H2O, particularly in surface water. If there is a higher concentration of sulphide ions is present in water, and it forms precipitate with arsenic in a reduction state (Bibi et al., 2017). The permissible limit of As in drinking water is 10 mg/L as per recommendation of World Health Organization (WHO) (Chen et al.,2017, Adimalla, et al.,2020).
Natural phenomena like minerals dissolution due to weathering, activity of different types of microbes, and complexing with organic materials may release arsenic into the aquatic environments (Tabelin et al., 2018). On the other hand, arsenic pollution in soils and surface water may result from human caused activities such as mineral mining and metallurgy productions, fuel burning and, the use of arsenic based pesticides (Zhang et al., 2018, Rahman et al.,2019). There are several techniques including process of oxidation, co-precipitation with other materials, ion-exchange, membrane filtration processes and adsorption via using matrix have been practiced to treating polluted groundwater or surface water (Karthikeyan et al., 2019).
Adsorption on matrix is a purely surface phenomenon which relied on the interaction among the adsorbent and adsorbate (Ravi et al., 2019, Sirajudheen et al., 2020). Robust particle such as nanomaterials with smaller size, high volume to surface area ratio, and enormous number of pores, can significantly decrease the concentration of arsenic from aqueous solution (Ahmad et al., 2020). The matrix applied for the removal of arsenic must be cost effective, simple to formulate, and abundant availability in nature, (Hasan et al., 2020). This review highlights the removal of As (III) and As (V) from H2O by adsorption process using iron nanoparticles and its composites.
Figure 1: Graphical representation of Geochemical Cycle of As in Environment (Hao et al., 2018)
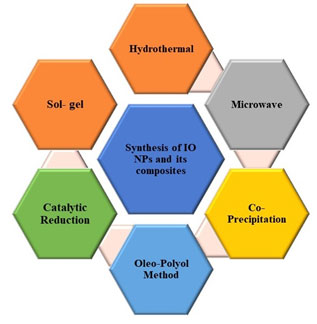
Synthesis of Iron Oxides NPs and Their composites: Currently, Iron NPs and their composites of different shapes and sizes is already applied successfully in several areas including living tissue imaging, farming applications and environmental applications such as removal of dyes, toxic compounds and toxic metals like mercury, lead, arsenic etc. (Tucek et al., 2014, Gangadhar, et al., 2020). The importance of magnetic nanoparticles for several of applications is because of their significant properties including stability in different conditions, biological compatibility, easy to prepare and process and reusability (Bohara et al.,2016).
Usually, there are two oxidation states of iron i.e. Fe 2+and Fe3+ present in iron oxides, possessing four and five unpaired electrons in 3d sub shell, respectively. Nassar (2012) claimed several categories of iron oxides for example hematite, maghemite, magnetite, and wustite, oxides/hydroxides of Fe such as goethite, akagneite, lepidocrocite, feroxyhyte, and hydroxides of iron viz. ferrihydrite and bernalite (Figure 2.). A brief outline of the different approaches used for the synthesis of iron nanoparticles or composite for arsenic removal is given below.
Sol–gel deposition: Sol- gel deposition is a one of the most used process for the preparation of nanostructured porous membranes, nanoscaled layers and coatings can be prepared via the production of Sol-gel (Nisticò et al., 2017). The initivial step involves a polymerization process that creates a suspension of colloid, or “sol,” of separate, homogenous dispersion of fine particles which is held in suspension after adding by the surfactant (Alehosseini et al., 2020). Further, the sol may be treated to remove the suspended particles, like casting or spin-coating on a substrate.
It is changed into a gel by chemical reactions so as to restrict the surfactant from making network of bound particles in the solution, which may lead to produce a class of superpolymer, a huge molecule in the form of 3D or, on the surface, a 2D complex i.e. the “gel.” Sol-gel thin film deposition process offers numerous benefits including processing at low temperature and effortless processing. Many researchers like Puscasu et al., (2016), Demirci et al., (2018), Yilmaz, et al., (2020) synthesized difference (Fe2O3) particles by sol-gel method.
Co-precipitation: The co-precipitation technique is most probable method for synthesizing magnetic nanoparticles due to easy to prepare and proficient chemical method (Bhateria et al., 2019). Fe3O4 is typically prepared by stoichiometric ratio of mixing of Fe2+ and Fe3+ solution in water (Sundar et al., 2020). The Fe3O4 is precipitated and predicted range of pH between 8 to 14. The structure and topography of the nanoparticles can be controlled by changing the concentration of respective salts, pH of aqueous solution, strength of ions and temperature (Díaz-Amaya et al., 2020).
Hydrothermal and Solvo-thermal synthesis: Hydrothermal process enables the solvents to heat up in a tightly packed container (bomb, autoclave, etc.) to reach a temperature beyond their boiling point (BP) (Biswas et al., 2017). When reaction takes place under definite conditions of temperature and pressure called as Solvo-thermal treatment and when H2O is applied as solvent acknowledged as hydrothermal (Guo et al., 2019). When water attained above the critical temperature and pressure is stated as supercritical and, as a liquid, exhibits the properties of both liquid and gas. In addition, to obtain hollow iron oxide NPs, the hydrothermal and solvo thermal way is a simple and traditional process, (Ounacer et al., 2020).
In a standard process, reagents are mixed together and continuous mixing by the help of stirrer with ferric salt as the iron supply, which gives homogeneous mixture is further moved to a steel autoclave lined with Teflon and heat sealed for 8-24 h at about 200°C. Organic solvent is used in a reaction mixture in its place of water in the solvothermal phase. Normally, hydrothermal process for synthesizing Fe3O4 using salt of ferrous, ferric and sodium hydroxide with a molar ratio of 1:2:8 to an autoclave and heat treatment at elevated temperatures (Bhateria et al., 2019).
Magnetic Iron nanocomposites: Several substances such as Ag, Si or Au may be applied to coat the external surface of Fe3O4 nanoparticles (Salem et al., 2019). These coatings on the external surface of the magnetic nanoparticle provide covalent binding positions for a specific ligand and also enhance the stability of the nanocomposites. The composition of magnetic nanoparticles is such a way where they have an outer shell which possess inorganic material while iron oxide is composing the inside center. For example, the alteration of electrolytes and pH is occupied by silica coating and therefore leads to a superior degree of robustness of nanoparticles (Hurtado et al., 2020).
Magnetic nanoparticles are doped with other metal(s) and other polymer(s) to form nanocomposites. Doping of iron oxides can be achieved by using oxidizing metallic agent such as manganese leading to precipitation and hydrolysis-precipitation processes. Bimetallic such as Fe-Mn, Fe-Ce and Fe-Cu and its oxides are insufficient patterns of metal doped iron oxides (Ma et al.,2020). These composites are very significant to prevent the step carried out in the pre-oxidation of arsenic using oxidants. The improved composites efficiently removed arsenic via oxidation and adsorption process (Priyadarshni et al., 2020).
Figure 2: Illustration of Synthesis of IO NPs and its Composites using different methods (Ravi et al., 2020)
Removal of As (III) and As (V): Iron based compounds for example hematite (α-Fe2O3), goethite (Mineral of the diaspore group, consisting of Fe3+ oxide-hydroxide), iron oxide coated nanomaterials and ferric hydroxide the ideal set of materials for As removal due to the minimum leaching of adsorbed As from the use adsorbent (Ghanizadeh et al., 2010). Iron nanoparticles possess magnetic properties which helps in smooth isolation of iron nanoparticles from H2O using powerful magnet (Gadad et al., 2014). Furthermore, Iron nanoparticles, zero-valent nanoparticles have also shown in different research papers for removal of metallic arsenic from H2O and industrial wastes or discharge to prove its efficacy for purification of water and environmental sustainability (Figure 3.) (Mosaferi et al., 2014).
Interaction of arsenate with iron oxides nanoparticles are established by the establishment of inner part of sphere and to a lower degree by weaker ionic exchange reactions phenomena. Luther et al., (2012) synthesized the Nanophase IO and shown its efficiency for the removal of As(III) and As(V). Maximum binding observed by the IO (Fe2O3 and Fe3O4) NPs were found 1.25 (mg/g), 8.19 (mg/g) for As(III) and 4.6 (mg/g), 6.7 (mg/g) for As(V) respectively at incubation period 1 hr. Graphene carbon nanotube-IO have shown significant absorption capacity for removal from As from dirty water because of high surface area to volume ratio and open porous structure (Vadahanambi et al., 2013, Mamaril et al., 2020).
Aredes et al., (2013) revealed that all natural IO NPs adsorbed arsenic at pH ranges from 4-11. Raul et al., (2014) synthesized IOH nanoflower and application in removal of As(III) (475 µg/g) from water. Bhowmick et al., (2014) fabricated the Mt-nZVI which shown very impressive result at pH 7.0 on both As(III) and As(V) 59.9 and 45.5 mg/g for As(III) and As(V) respectively. Devi et al., (2014) used IO coated sand for the As (III) removal from drinking water. Qi et al., (2015) reported that bimetallic oxide NPs like (Fe-Mn) remove 39.1 mg/g and 54.2 mg/g of As(V) and As (III) respectively.
Composite γ-Fe2O3@CTF (Leus et al., 2018) shows excellent removal for both form of arsenic (As(III) (198.0 mg g−1) and As(V)(102.3 mg g−1). Mishra et al., (2019) formulated aero gel based cerium doped IO for the As(III) removal and the maximum efficacy was 263 mg/g . Recently Dong et al., (2020) have formulated cellulosed nanocrystal IO and used this for the removal As(III) and As(V) adsorption. Which shows at pH levels of 7 and 3 CN/IO removed 13.866 mg/g and 15.712 mg/g of As(III) and As (V) from H2O respectively. Table I. shows synthesis of iron nanoparticles and its composites for As removal.
Figure 3: Mechanistic representation of Arsenic removal using IO NPs under aerobic condition (Hao et al., 2018)
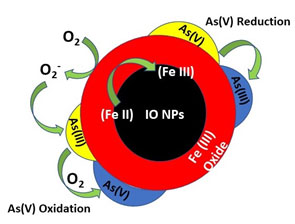
Table 1 . Removal of Arsenic As (III) and As (V) by Iron NPs and its composite
| S.N. | Nanoparticle Matrix | Isotherm | pH | Removal of Arsenic | Ref. | |
| As (III)
(mg/g) |
As (V)
(mg/g) |
|||||
| 1 | Bare NZVI | Freundlich | 7 | 3.5 | – | Kanel et al.,2005 |
| 2 | Bare NZVI | Langumir | 7 | – | 38.2 | Yuan et al., 2006 |
| 3 | γ-Fe2O3 | Langumir | 7 | – | 2.9 | Park et al., 2009 |
| 4 | α-Fe2O3 | – | 7 | 95 | 47 | Tang et al.,2011 |
| 5 | Fe3O4– γ-Fe2O3 nanoparticles | Langumir | 2.0 | 3.69 | 3.71 | Chowdhury et al.,2011 |
| 6 | γ-Fe2O3 | Langumir | 3-11 | 74.83 | 105.25 | Lin et al. 2012 |
| 7 | γ -Fe2O3 | Langumir | 6.6 | 47 | 95 | Prucek et al., 2013 |
| 8 | Β-FeOOH/GO
Ns |
Langumir | – | 77.50 | 45.70 | Ming et al. 2015 |
| 9 | Fe-NN/BFs | – | 7 | 70.22 | 93.94 | Wei et al., 2019 |
| 10 | IO gel | Langumir | 7 | 35.75 | – | Otero-González et al., 2020 |
| 11 | RH+IO | Langmuir | 7 | 82 | – | Pillai et al., 2020 |
| 12 | BT-FeN | Langmuir | 7 | – | 18.98 | Kamat et al., 2020 |
| 13 | OL-FeNP | Langmuir | 7 | – | 32.05 | |
| 14 | GT-FeNP | Langmuir | 7 | – | 13.70 | |
| 15 | PL-FeNP | Langmuir | 7 | – | 11.65 | |
| 16 | EL-FeNP | Langmuir | 7 | – | 39.84 | |
| 17 | HFOR | Langmuir | 5-7 | 41.6 | 71.5 | Liu et al., 2020 |
| 18
|
Iron Oxide Composite | Langmuir | 6 | 83.84 | – | Ramasubbu et al., 2020 |
CONCLUSION
The present scenario of nanotechnology related with preparation and application of iron nanoparticle in the light of As removal has been outlined and reviewed. Iron oxide have exclusive magnetic and physiochemical characteristics which could be harness to use in environmental applications. Nanostructure iron oxide materials have outstanding capacity for the get rid of arsenic contaminants from water. Presently, this is similarly significant to find new ideas for enhancing the stability and biocompatibility of iron nanoparticles and composites to serve the purpose of environmental applications. In addition, iron oxide nanoparticles and its composites are observed to be the very good absorbents for arsenic removal.
ACKNOWLEDGEMENTS
Authors are thankful to Department of Chemistry, Sri Aurobindo College, University of Delhi and Jawaharlal Nehru University, New Delhi India for providing the facilities. We are also thankful to Mr. Shubhankar Singh for helping us in the image preparation.
REFERENCES
Adimalla, N., & Taloor, A. K. (2020). Hydrogeochemical investigation of groundwater quality in the hard rock terrain of South India using Geographic Information System (GIS) and groundwater quality index (GWQI) techniques. Groundwater for Sustainable Development, 10, 100288.
Ahmad, H., Zhao, L., Liu, C., Cai, C., Ma, F. (2020). Ultrasound assisted dispersive solid phase microextraction of inorganic arsenic from food and water samples using CdS nanoflowers combined with ICP-OES determination. Food Chemistry, 128028.
Alehosseini, E., Jafari, S. M. (2019). Micro/nano-encapsulated phase change materials (PCMs) as emerging materials for the food industry. Trends in Food Science & Technology, 91, 116-128.
Aredes, S., Klein, B., Pawlik, M. (2013). The removal of arsenic from water using natural iron oxide minerals. Journal of Cleaner Production, 60, 71-76.
Bibi, S., Kamran, M. A., Sultana, J., Farooqi, A. (2017). Occurrence and methods to remove arsenic and fluoride contamination in water. Environmental chemistry letters, 15(1), 125-149.
Biswas, B., Kumar, A. A., Bisht, Y., Singh, R., Kumar, J., Bhaskar, T. (2017). Effects of temperature and solvent on hydrothermal liquefaction of Sargassum tenerrimum algae. Bioresource Technology, 242, 344-350.
Bhateria, R., Singh, R. (2019). A review on nanotechnological application of magnetic iron oxides for heavy metal removal. Journal of Water Process Engineering, 31, 100845.
Bhowmick, S., Chakraborty, S., Mondal, P., Van Renterghem, W., Van den Berghe, S., Roman-Ross, G., Iglesias, M. (2014). Montmorillonite-supported nanoscale zero-valent iron for removal of arsenic from aqueous solution: Kinetics and mechanism. Chemical Engineering Journal, 243, 14-23.
Bohara, R. A., Thorat, N. D., Pawar, S. H. (2016). Role of functionalization: strategies to explore potential nano-bio applications of magnetic nanoparticles. RSC advances, 6(50), 43989-44012.
Bondu, R., Cloutier, V., Rosa, E., Benzaazoua, M. (2016). A review and evaluation of the impacts of climate change on geogenic arsenic in groundwater from fractured bedrock aquifers. Water, Air, & Soil Pollution, 227(9), 296.
Campbell, K. M., Nordstrom, D. K. (2014). Arsenic speciation and sorption in natural environments. Reviews in Mineralogy and Geochemistry, 79(1), 185-216.
Chen, J., Wu, H., Qian, H., Gao, Y. (2017). Assessing nitrate and fluoride contaminants in drinking water and their health risk of rural residents living in a semiarid region of Northwest China. Exposure and Health, 9(3), 183-195.
Chiban, M., Zerbet, M., Carja, G., Sinan, F. (2012). Application of low-cost adsorbents for arsenic removal: A review. Journal of Environmental Chemistry and Ecotoxicology, 4(5), 91-102.
Chowdhury, S. R., Yanful, E. K., Pratt, A. R. (2011) Arsenic removal from aqueous solutions by mixed magnetite–maghemite nanoparticles. Environmental earth sciences, 64(2), 411-423.
Demirci, S., Yurddaskal, M., Dikici, T., Sarıoğlu, C. (2018). Fabrication and characterization of novel iodine doped hollow and mesoporous hematite (Fe2O3) particles derived from sol-gel method and their photocatalytic performances. Journal of hazardous materials, 345, 27-37.
Devi, R. R., Umlong, I. M., Das, B., Borah, K., Thakur, A. J., Raul, P. K., Singh, L. (2014). Removal of iron and arsenic (III) from drinking water using iron oxide-coated sand and limestone. Applied Water Science, 4(2), 175-182.
Díaz-Amaya, S., Zhao, M., Allebach, J. P., Chiu, G. T. C., Stanciu, L. A. (2020). Ionic Strength Influences on Biofunctional Au-Decorated Microparticles for Enhanced Performance in Multiplexed Colorimetric Sensors. ACS Applied Materials & Interfaces, 12(29), 32397-32409.
Dinçer Yilmaz, N. E., & Karakaş, G. (2020). Effect of Drying Conditions on the Characteristics and Performance of B/Fe2O3 Nano-Composites Prepared by Sol-Gel Method. Central European Journal of Energetic Materials, 17(1).
Dong, F., Xu, X., Shaghaleh, H., Guo, J., Guo, L., Qian, Y., Wang, S. (2020). Factors influencing the morphology and adsorption performance of cellulose nanocrystal/iron oxide nanorod composites for the removal of arsenic during water treatment. International journal of biological macromolecules, 156, 1418-1424.
Drewniak, L., Maryan, N., Lewandowski, W., Kaczanowski, S., Sklodowska, A. (2012). The contribution of microbial mats to the arsenic geochemistry of an ancient gold mine. Environmental Pollution, 162, 190-201.
Gadad, A. P., Kumar, S. V., Dandagi, P. M., Bolmol, U. B., Pallavi, N. P. (2014). Nanoparticles and their therapeutic applications in pharmacy. International Journal of Pharmaceutical Sciences and Nanotechnology, 7(3), 2509-2019.
Gangadhar, L., Reddy, K. B., Garg, A. P., & Sana, S. S. (2020). Green Synthesis of Bio-polymer Composites of Iron for Pharmaceutical Applications. J Nanomed Nanotech, 11, 551.
Ghanizadeh, G., Ehrampoush, M., & Ghaneian, M. (2010). Application of iron impregnated activated carbon for removal of arsenic from water. Journal of Environmental Health Science & Engineering, 7(2), 145-156.
Guo, H., Hu, Y., Zhang, X., Zhang, R., Hou, D., Sui, Y., Wu, L. (2019). Facile one-step hydrothermal synthesis of Na3V2 (PO4) 2F3@ C/CNTs tetragonal micro-particles as high performance cathode material for Na-ion batteries. Frontiers in Chemistry, 7, 689.
Hao, L., Liu, M., Wang, N., Li, G. (2018). A critical review on arsenic removal from water using iron-based adsorbents. RSC advances, 8(69), 39545-39560.
Hasan, M. M., Hasan, M. N., Awual, M. R., Islam, M. M., Shenashen, M. A., Iqbal, J. (2020). Biodegradable natural carbohydrate polymeric sustainable adsorbents for efficient toxic dye removal from wastewater. Journal of Molecular Liquids, 114356.
Hurtado, Y., Franco, C. A., Riazi, M., Cortés, F. B. (2020). Improving the stability of nitrogen foams using silica nanoparticles coated with polyethylene glycol. Journal of Molecular Liquids, 300, 112256.
Kamath, V., Chandra, P., Jeppu, G. P. (2020). Comparative study of using five different leaf extracts in the green synthesis of iron oxide nanoparticles for removal of arsenic from water. International Journal of Phytoremediation, 1-17.
Kanel, S. R.; Manning, B.; Charlet, L.; Choi, H. (2005) Removal of arsenic (III) from groundwater by nanoscale zero-valent iron. Environ. Sci. Technol. 39,1291-1298.
Karagas, M. R., Gossai, A., Pierce, B., Ahsan, H. (2015). Drinking water arsenic contamination, skin lesions, and malignancies: a systematic review of the global evidence. Current environmental health reports, 2(1), 52-68.
Karthikeyan, P., Meenakshi, S. (2019). Synthesis and characterization of Zn–Al LDHs/activated carbon composite and its adsorption properties for phosphate and nitrate ions in aqueous medium. Journal of Molecular Liquids, 296, 111766.
Leus, K., Folens, K., Nicomel, N. R., Perez, J. P. H., Filippousi, M., Meledina, M., Du Laing, G. (2018). Removal of arsenic and mercury species from water by covalent triazine framework encapsulated γ-Fe2O3 nanoparticles. Journal of hazardous materials, 353, 312-319.
Lin, S., Lu, D., Liu, Z., (2012) Removal of arsenic contaminants with magnetic γ-Fe2O3 nanoparticles. Chem. Eng. J. 211-212, 46-52.
Liu, B., Liu, Z., Wu, H., Pan, S., Cheng, X., Sun, Y., Xu, Y. (2020). Effective and simultaneous removal of organic/inorganic arsenic using polymer-based hydrated iron oxide adsorbent: Capacity evaluation and mechanism. Science of The Total Environment, 742, 140508.
Luther, S., Borgfeld, N., Kim, J., Parsons, J. G. (2012). Removal of arsenic from aqueous solution: a study of the effects of pH and interfering ions using iron oxide nanomaterials. Microchemical Journal, 101, 30-36.
Ma, P., Liu, Q., Liu, P., Li, H., Han, X., Liu, L., & Zou, W. (2020). Green synthesis of Fe/Cu oxides composite particles stabilized by pine needle extract and investigation of their adsorption activity for norfloxacin and ofloxacin. Journal of Dispersion Science and Technology, 1-18.
Mamaril, G. S. S., de Luna, M. D. G., Bindumadhavan, K., Ong, D. C., Pimentel, J. A. I., & Doong, R. A. (2020). Nitrogen and fluorine co-doped 3-dimensional reduced graphene oxide architectures as high-performance electrode material for capacitive deionization of copper ions. Separation and Purification Technology, 117559.
McCarty, K. M., Hanh, H. T., Kim, K. W. (2011). Arsenic geochemistry and human health in South East Asia. Reviews on environmental health, 26(1), 71-78.
Ming, L.C., Yan, S., Chun, B.H., Chen, L., Jian, H.W., (2015) Akaganeite decorated graphene oxide composite for arsenic adsorption/removal and its pro concentration at ultra-trace level. Chemosphere 130, 52-58.
Mosaferi, M., Nemati, S., Khataee, A., Nasseri, S., Hashemi, A. A. (2014). Removal of Arsenic (III, V) from aqueous solution by nanoscale zero-valent iron stabilized with starch and carboxymethyl cellulose. Journal of Environmental Health Science and Engineering, 12(1), 74.
Nassar, N. N., Hassan, A., Carbognani, L., Lopez-Linares, F., Pereira-Almao, P. (2012). Iron oxide nanoparticles for rapid adsorption and enhanced catalytic oxidation of thermally cracked asphaltenes. Fuel, 95, 257-262.
Nisticò, R., Scalarone, D., Magnacca, G. (2017). Sol-gel chemistry, templating and spin-coating deposition: A combined approach to control in a simple way the porosity of inorganic thin films/coatings. Microporous and Mesoporous Materials, 248, 18-29.
Nurmesniemi, H., Pöykiö, R., Watkins, G., Dahl, O. (2010). Total and extractable heavy metal, phosphorous and sulfur concentrations in slaker grits from the causticizing process of a pulp mill for use as a soil amendment. Chemical Speciation & Bioavailability, 22(2), 87-97.
Otero-González, L., Mikhalovsky, S. V., Václavíková, M., Trenikhin, M. V., Cundy, A. B., Savina, I. N. (2020). Novel nanostructured iron oxide cryogels for arsenic (As (III)) removal. Journal of hazardous materials, 381, 120996.
Ounacer, M., Essoumhi, A., Sajieddine, M., Razouk, A., Costa, B. F. O., Dubiel, S. M., Sahlaoui, M. (2020). Structural and Magnetic Studies of Annealed Iron Oxide Nanoparticles. Journal of Superconductivity and Novel Magnetism, 1-13.
Park, H., Myung, N. V., Jung, H., Choi, H. (2009). As (V) remediation using electrochemically synthesized maghemite nanoparticles. Journal of Nanoparticle Research, 11(8), 1981.
Park, J. H., Lamb, D., Paneerselvam, P., Choppala, G., Bolan, N., Chung, J. W. (2011). Role of organic amendments on enhanced bioremediation of heavy metal (loid) contaminated soils. Journal of hazardous materials, 185(2-3), 549-574.
Pillai, P., Kakadiya, N., Timaniya, Z., Dharaskar, S., Sillanpaa, M. (2020). Removal of arsenic using iron oxide amended with rice husk nanoparticles from aqueous solution. Materials Today: Proceedings.
Podgorski, J., & Berg, M. (2020). Global threat of arsenic in groundwater. Science, 368(6493), 845-850.
Priyadarshni, N., Nath, P., & Chanda, N. (2020). Sustainable removal of arsenate, arsenite and bacterial contamination from water using biochar stabilized iron and copper oxide nanoparticles and associated mechanism of the remediation process. Journal of Water Process Engineering, 37, 101495.
Prucek, R., Tuček, J., Kolařík, J., Filip, J., Marušák, Z., Sharma, V. K., Zbořil, R. (2013). Ferrate (VI)-induced arsenite and arsenate removal by in situ structural incorporation into magnetic iron (III) oxide nanoparticles. Environmental science & technology, 47(7), 3283-3292.
Puscasu, E., Sacarescu, L., Lupu, N., Grigoras, M., Oanca, G., Balasoiu, M., Creanga, D. (2016). Iron oxide-silica nanocomposites yielded by chemical route and sol–gel method. Journal of Sol-Gel Science and Technology, 79(3), 457-465.
Qi, J., Zhang, G., & Li, H. (2015). Efficient removal of arsenic from water using a granular adsorbent: Fe–Mn binary oxide impregnated chitosan bead. Bioresource Technology, 193, 243-249.
Rahman, Z., Singh, V. P. (2019). The relative impact of toxic heavy metals (THMs)(arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environmental monitoring and assessment, 191(7), 419.
Ramasubbu, D., Mahalingam, M., Vincent, J. (2020). Arsenic removal using Prosopis spicigera L. wood (PsLw) carbon–iron oxide composite. Applied Water Science, 10(9).
Raul, P. K., Devi, R. R., Umlong, I. M., Thakur, A. J., Banerjee, S., Veer, V. (2014). Iron oxide hydroxide nanoflower assisted removal of arsenic from water. Materials Research Bulletin, 49, 360-368.
Ravi, R., Iqbal, S., Ghosal, A., Ahmad, S. (2019). Novel mesoporous trimetallic strontium magnesium ferrite (Sr0. 3Mg0. 7Fe2O4) nanocubes: A selective and recoverable magnetic nanoadsorbent for Congo red. Journal of Alloys and Compounds, 791, 336-347.
Ravi R, Mishra A., (2020). Preparation of Bimetallic and Trimetallic Nanomaterials and their Role in Waste Water Treatment: A Review. Biosc.Biotech.Res.Comm.;13(3), 1566-1575.
Salem, M. A., Elsharkawy, R. G., Ayad, M. I.,Elgendy, M. Y. (2019). Silver nanoparticles deposition on silica, magnetite, and alumina surfaces for effective removal of Allura red from aqueous solutions. Journal of Sol-Gel Science and Technology, 91(3), 523-538.
Schindler, C., Hagemann, S. G., Banks, D., Mernagh, T., Harris, A. C. (2016). Magmatic hydrothermal fluids at the sedimentary rock-hosted, intrusion-related Telfer gold-copper deposit, Paterson Orogen, Western Australia: pressure-temperature-composition constraints on the ore-forming fluids. Economic Geology, 111(5), 1099-1126.
Sinha, D., Biswas, J., Bishayee, A. (2013). Nrf2-mediated redox signaling in arsenic carcinogenesis: a review. Archives of toxicology, 87(2), 383-396.
Sirajudheen, P., Karthikeyan, P., Ramkumar, K., Meenakshi, S. (2020). Effective removal of organic pollutants by adsorption onto chitosan supported graphene oxide-hydroxyapatite composite: A novel reusable adsorbent. Journal of Molecular Liquids, 114200.
Sundar, S., Kwon, S. J., Venkatachalam, G. (2020). Magneto-biosensor for the detection of uric acid using citric acid-capped iron oxide nanoparticles. Journal of nanoscience and nanotechnology, 20(4), 2144-2153.
Tabelin, C. B., Igarashi, T., Villacorte-Tabelin, M., Park, I., Opiso, E. M., Ito, M., Hiroyoshi, N. (2018). Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Science of the Total Environment, 645, 1522-1553.
Tang, W., Li, Q., Gao, S., Shang, J.K., (2011) Arsenic (III, V) removal from aqueous solution by ultrafine α-Fe2O3 nanoparticles synthesized from solvent thermal method. J. Hazard. Mater. 192, 131-138.
Tucek, J., Kemp, K. C., Kim, K. S., & Zboril, R. (2014). Iron-oxide-supported nanocarbon in lithium-ion batteries, medical, catalytic, and environmental applications. ACS nano, 8(8), 7571-7612.
Vadahanambi, S., Lee, S. H., Kim, W. J., & Oh, I. K. (2013). Arsenic removal from contaminated water using three-dimensional graphene-carbon nanotube-iron oxide nanostructures. Environmental science & technology, 47(18), 10510-10517.
Wei, Y., Wei, S., Liu, C., Chen, T., Tang, Y., Ma, J., Luo, S. (2019). Efficient removal of arsenic from groundwater using iron oxide nanoneedle array-decorated biochar fibers with high Fe utilization and fast adsorption kinetics. Water Research, 167, 115107.
Yuan, C., Lien, H. L. (2006) Removal of arsenate from aqueous solution using nanoscale iron particles. Water Qual. Res. J. Can,41, 210-215.
Zhang, X., Wei, S., Sun, Q., Wadood, S. A., Guo, B. (2018). Source identification and spatial distribution of arsenic and heavy metals in agricultural soil around Hunan industrial estate by positive matrix factorization model, principle components analysis and geo statistical analysis. Ecotoxicology and Environmental Safety, 159, 354-362.


