1Department of Botany, MMV, Banaras Hindu University, Varanasi, India
2Department of Chemistry, Institute of Science, Banaras Hindu University, Varanasi, India
Article Publishing History
Received: 11/04/2020
Accepted After Revision: 25/05/2020
Dillenia indica is an important medicinal tree. Its leaf and bark extracts are being used as medicine due to the presence of several pharmaceutically important phytochemicals, but presence of phytochemicals in its flowers is still unexplored and there is utmost need to check medicinal efficacy of flower extract. Phytochemical screening of flower extract showed the presence of coumarins, steroids, saponins, flavonoids. GCMS study was done for the identification of bioactive compounds from the methanolic extract of flowers, which showed the presence of various phytochemicals such as Tetrabutyl Titanate, Fucoxanthin, Chromone, Beclomethasone, Betamethasone Acetate, Demecolcine etc. Most of these compounds have many pharmacological activities such as antibacterial, antimicrobial, cancer radiotherapy, cytotoxic activity, antioxidant etc., whereas some chemicals are of industrial importance. Some toxic chemicals were also reported. Present work shows pharmaceutical importance of flowers of Dillenia indica.
GCMS, Plant extract, Phytochemical, Secondary metabolites
Gupta A, Kumari N, Tiwari I. Phytochemical Screening and GC-MS Analysis of Flower Extract of Dilleniaindica. Biosc.Biotech.Res.Comm. 2020;13(2).
Gupta A, Kumari N, Tiwari I. Phytochemical Screening and GC-MS Analysis of Flower Extract of Dilleniaindica. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/2SZg47D
Copyright © Gupta et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Dillenia indica is commonly known as Elephant Apple, belongs to family Dilleniacae. It is an ethnomedical plant and is being used for the treatment of severe disease (Shahin et al, 2015). Its stem bark, leaves, fruit and seed extracts show the presence of various active compounds such as polyphenols, tannins, alkaloids, steroids, saponins and flavonoids. Extracts of Dillenia have shown antileukemic, antioxidant, anti-inflammatory, antiproliferative, antidiabetic, hepatoprotective, antimicrobial and other pharmaceutically important activities (Gandhi & Mehta, 2013). Due to over exploitation, it is considered as rare plant in Egypt (Khalifa and Loutfy, 2006) and as an endangered plant in China (Qin et al, 2017). It is indigenous to Indonesia. It also occurs in Bhutan, Malaysia, China, Sri Lanka, Myanmar, Nepal, Philippines, Vietnam and Bangladesh. In India, it is distributed in Assam, North Bengal, Bihar, Orissa, Madhya Pradesh and Gujarat. However, no qualitative and quantitative studies were made for the extract of flowers. Present work shows GCMS study of floral extract of Dillenia indica for detailed information of phytochemicals.
Gas chromatography-mass spectrometry (GCMS) is widely used in pharmaceutical industries for the identification and quantification of secondary metabolites (Rukshana et al, 2017). It is considered sensitive and suitable method for the analysis of natural organic substances. The analysis provides the details of bioactive compounds present in the methanolic extract and retention time indicates the separation of compounds at different time interval. GCMS analysis of many plants has helped in the identification and characterization of phytochemicals present in their extracts (Socrates & Mohan, 2019; Eswaraiah et al. 2020).
Figure 1: (A) Tree of D.indica(B)Flower
MATERIAL AND METHODS
Flowers of Dillenia indica were collected from the plant growing in Ayurvedic garden, Dravyaguna, Institute of Medical Sciences, Banaras Hindu University, Varanasi (Fig.1).
Phytochemical Screening: Shade dried flowers were made into fine powder by mixer grinder. The powder of flower (5 gm) was added to 50 ml of distilled water and was boiled at 60o C for 10 min. Boiled extract was filtered with Whatman filter paper. Filtered solution was boiled till the formation of chocolate colored powder. This powdered plant extract was used for preliminary phytochemical screening. Aqueous plant extract was prepared by dissolving 25 mg of crude extract to 25 ml of double distilled water. Presence or absence of phytochemicals was observed by performing following tests with minor modifications:
For the test of Coumarins (Rizk, 1982), aqueous plant extract (2 ml) was added to 3ml of 10% NaOH. The confirmation of coumarins was observed by the change in colour. Formation of yellow colour shows the presence of coumarins. To detect the presence of Saponins, foam test was done (Kumar et al, 2009). In this test, plant extract (2 ml) was mixed in 6 ml of DDW and was shaken vigorously. Presence of foam is confirmation for saponins. To observe the presence of phenol (Gibbs, 1974) in plant extract, 2 ml of aqueous extract was added to 2 ml 5% of aqueous ferric Chloride (FeCl3). If deep blue or black color is observed, then it indicates the presence of phenol. Presence of Flavonoids was observed by Alkaline Reagent Test and 2 ml plant extract was taken and few drops of 1N NaOH were added. Presence of flavonoids will be observed if it turns to yellow color and becomes colorless after adding dilute HCl. For detection of Tannins (Braymer’s Test, Ugochukwu et al, 2013), 2 ml extract was added to 3 ml 10% alcoholic ferric chloride solution. Formation of blue or greenish color will indicate the presence of tannins. Test for Quinones (Ramya et al., 2015) was done by treating 2 ml plant extract with 4 ml of dilute Sodium hydroxide. If blue green or red color is formed, it will show the presence of quinone.
To see the presence of Phlobatannins (Precipitate Test, Auwal et al, 2014), 1ml of extract was treated with 2ml of 1% hydrochloric acid. Then mixture was boiled. Observation of red precipitation will confirm the presence of phlobatannins. In the test for Alkaloids (Mayer’s Test, Auwal et al, 2014), 2ml of extract was added to 0.5 ml of Mayer’s reagent. White creamy precipitate formation is indicative of the presence of alkaloids. Fehling solution test was done to observe the presence of carbohydrate. Mixture of equal volume of Fehling solution A and B was made. Extract (2 ml) was added to 2 ml of Fehling solution and then it was boiled in water bath for 30 minute. Red precipitate will indicate the presence of carbohydrate. For observing the presence of Anthocyanins (Paris and Moyse, 1969), 2 ml extract was treated with 2N Hydrochloric acid. If pink-red color appears, which turns into blue-violet after addition of Ammonia, then it shows the presence of anthocyanins. For detection of fatty acid (Ayoola, 2008), 1 ml of extract was mixed with 3 ml of ether. It was poured on the filter paper and was evaporated till the filter paper becomes dried. Transparent filter paper indicates the presence of fatty acid. For confirmation of Steroids (Salkowski reaction, Shear & Kramer, 1926), 1ml of extract was dissolved in 10 ml chloroform. Concentrated sulfuric acid was added by side of test tube wall, appearance of red and yellow color in the upper and lower layer, respectively along with green florescence indicates the presence of steroids.
Preparation of extract and methodology of GCMS: Shade dried flowers were made into fine powder by mixer grinder. Flower powder was incubated with 25ml 95% methanol and kept for 72 hour. Methanolic extract was filtered by using Whatman filter No.1 (pore size 0.4 µm). GCMS analysis of filtered extract was done to get the details of bioactive compounds.Methanolic extract was injected in the port of the Gas chromatography (GC) device. Here the extract got vaporized and spectral peaks of various phytochemicals was recorded on chromatogram. GCMS analysis of methanolic extract of flower of the plant was performed using a THERMOSCEINTIFIC Gas Chromatography-TRACE ULTRA VER: 1100. and mass spectrometry- TSQ Duo. The oven temperature was maintained at 220ºC at a rate of 6ºC/min and flow rate of carrier gas was adjusted at 1 ml/min. The column of the GC was TG-5MS. Different parameters of the column were as such- the length of the column: 30 m, the diameter: 0.25nm and the thickness of the film: 0.25µm. The GCMS programming were done as follows: Injector temperature 215o C, Transfer line 218o C, oven temperature program 80-280°C with ramping 5°C per min, carrier gas: Helium at 1.5 mL/min, individual components were identified by NIST MS 2.0 structural library.The split sampling technique was used to inject the sample in the ratio of 1:10.
The time elapsed between elution and injection was recorded as the “retention time”. The peak was measured from the base to the tip of the peak. Retention index of the compounds were identified by comparing the retention times and identification of each component was confirmed by the comparison of its retention index with data in the NIST library. Interpretation of Mass-Spectrum was carried out by using the database of the National Institute Standard and Technology (NIST) having more than 62,000 patterns. Spectrum of the known compound which are stored in NIST library was used to compare the spectrum of unknown component. The molecular weight, name, chemical structure and molecular formula of the components of the test materials were ascertained.
RESULTS AND DISCUSSION
GCMS is a technique that combines the separation of phytochemicals by gas chromatography and their detection by mass spectroscopy (Chauhan et al., 2014). Various parts of Dillenia indica i.e.fruit, leaf and bark have shown the presence of many primary and secondary metabolites (Barua et al, 2018). Present work shows preliminary screening of phytochemicals of aqueous flower extract of Dillenia indica and the presence of some very important phytochemicals such as coumarins, flavonoids, steroids and saponin were observed (table-1).
Table 1. Phytochemical analysis of D. indica flower
| S.N. | Phytochemicals | Flower |
| 1. | Tannins | – |
| 2. | Coumarins | + |
| 3. | Flavonoids | + |
| 4. | Steroids | + |
| 5. | Anthocyanin | – |
| 6. | Saponin | + |
| 7. | Quinones | – |
| 8. | Phlobatnins | – |
| 9. | Fatty Acid | – |
| 10. | Phenolics | – |
| 11. | Alkaloids | – |
| 12. | Carbohydrates | – |
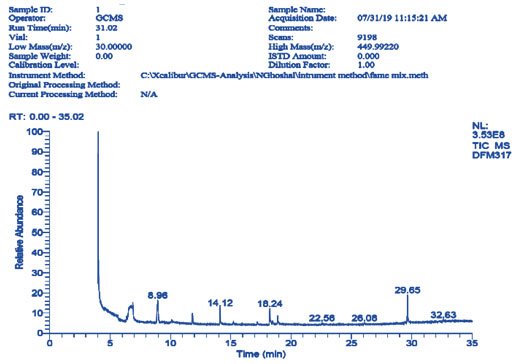
The peak in GCMS of methanolic extract of the flower of Dilleniaindica showed the presence of the secondary phytochemical compounds like phenolic and fatty acids and other medicinally important bioactive compounds.GCMS analysis of methanolic flower extract showed significant presence of 27 phytochemicals (Fig. 2 and Table-2). The most abundant 27 compounds found in the methanolic extract of flowers were TetrabutylTitanate, Fucoxanthin, Ergosta-5, Lauryl Acetate, Beclomethasone, Betamethasone Acetate, Demecolcine,Androstane-11,17-Dione, Ethyl Iso-Allocholate, 1,2-Cinnolinedicarboxylic Acid, 2,4-Imidazolidinedione, Benzene Propanoic Acid, α-D-Glucopyranosiduronic Acid, Lauryl Acetate, 3-Trifluoroacetoxypentadecane, 3-Trifluoroacetoxytridecane, Prednisolone Acetate, Chromone, Z-11-Pentadecenal, 17-Pentatriacontene, Acetic Acid, Isoxazole, Phthalic Acid, Diisooctyl Phthalate, Hexa-T-Butylselenatrisiletane, Normorphin, Prostaglandin. The structures of above compounds have been mentioned in Table-3. Many of above compounds are pharmaceutically important and their medicinal efficacies have been reported by many researchers as antibacterial, anti-inflammatory, and antiviral activities (Table 3). Some compounds show their industrial applications, whereas few compounds also show toxicity (Table 3).
Figure 2: GCMS analysis and Chromatogram of methanolic extract of D. indica Flower
GCMS is an integrative technique for separation, identification and quantification of chemicals in a given sample (Leary et al, 2019). Quantification is an important step for data analysis and different softwares are being used for the calculation of retention time corresponding to specific peaks (Johnsen et al, 2017). The technique has great applications in pharmaceutical industries as it helps in the identification of bioactive compounds as well as any impurities present in the plant extract (Chauhan et al., 2014). In present study, several compounds have been identified from flower extract. Pharmaceutical activities of many compounds have been reported earlier by several researchers (Table- 3), which indicates potential of flower for the production of medicines. Therefore, like leaves and bark, flowers of D. indica too has significant medical efficacy. Uses of other parts of Dillenia indica as traditional medicines are already in practice. Now, there is need to use flower extract as well.
Table 2. Bioactive compounds present in methanolic extract of D.indica Flowers
| S.N. | COMPOUND | M. W. | M. F. | %AREA | RT |
| 1. | TETRABUTYL TITANATE | 344.35 | C16H40O4Ti | 0.35 | 4.35 |
| 2. | ANDROSTANE-11,17-DIONE | 260.5 | C19H32 | 0.07 | 6.21 |
| 3. | ETHYL ISO-ALLOCHOLATE | 436.6 | C26H44O5 | 1.02 | 6.36 |
| 4. | ERGOSTA-5 | 394.6 | C28H42O | 1.02 | 6.36 |
| 5. | PROPANOIC ACID | 74.08 | C3H6O2 | 0.04 | 7.22 |
| 6. | 1,2-CINNOLINEDICARBOXYLIC ACID | 173.17 | C10H7NO2 | 0.04 | 7.22 |
| 7. | 2,4-IMIDAZOLIDINEDIONE, 5-[3,4-BIS[(TRIMETHYLSILYL)OXY]PHENYL]-3-METHYL-5-PHENYL-1-(TRIMETHYLSILYL)- | 516 | C25H40N2O4Si3 | 0.05 | 7.29 |
| 8. | FUCOXANTHIN | 658.91 | C42H58O6 | 0.09 | 7.36 |
| 9. | BENZENE PROPANOIC ACID | 152.19 | C9H12O2 | 0.13 | 7.46 |
| 10. | α-D-GLUCOPYRANOSIDURONIC ACID | 208.17 | C7H12O7 | 0.13 | 7.46 |
| 11. | LAURYL ACETATE | 228.37 | C14H28O2 | 0.47 | 8.34 |
| 12. | 3-TRIFLUOROACETOXYPENTADECANE | 324.4 | C17H31F3O2 | 0.15 | 9.29 |
| 13. | 3-TRIFLUOROACETOXYTRIDECANE | 296.37 | C15H27F3O2 | 0.15 | 9.29 |
| 14. | PREDNISOLONE ACETATE | 402.5 | C23H30O6 | 0.19 | 11.21 |
| 15. | CHROMONE | 146.14 | C9H6O2 | 0.19 | 11.21 |
| 16. | BECLOMETHASONE | 408.9 | C22H29ClO5 | 0.06 | 11.34 |
| 17. | Z-11-PENTADECENAL | 224.38 | C15H28O | 0.19 | 13.49 |
| 18. | BETAMETHASONE ACETATE | 434.5 | C24H31FO6 | 0.21 | 16.49 |
| 19. | 17-PENTATRIACONTENE | 490.9 | C35H70 | 0.1 | 20.27 |
| 20. | DEMECOLCINE | 371.4 | C21H25NO5 | 0.1 | 23.06 |
| 21. | ACETIC ACID | 60.052 | CH3COOH | 0.13 | 23.28 |
| 22. | ISOXAZOLE | 69.06 | C3H3NO | 0.02 | 26.22 |
| 23. | PHTHALIC ACID | 166.14 | C8H6O4 | 6.67 | 29.65 |
| 24. | DIISOOCTYL PHTHALATE | 390.55 | C24H38O4 | 6.67 | 29.65 |
| 25. | HEXA-T-BUTYLSELENATRISILETANE | 505.90 | C24H54SeSi3 | 0.04 | 29.93 |
| 26. | NORMORPHINE | 271.31 | C16H17NO3 | 0.05 | 29.98 |
| 27. | PROSTAGLANDIN | 354.5 | C20H34O5 | 0.04 | 33.04 |
Table. 3 Compound Structures with their Biological activities
CONCLUSIONS
In the present investigation, 27 bioactive compounds have been identified from the methanolic extract of D. indica by GC-MS. The presence of various bioactive compounds in D. indica proved pharmaceutical and medicinal importance. However, further research is needed in order to analyze its bioactivity and toxicity profile.
ACKNOWLEDGEMENTS
The authors are grateful to Department of Botany, Banaras Hindu University, Varanasi, for providing institutional and technical support.
Conflict of interest: The authors declare no conflict of interest.
REFERENCES
Adams NP, Bestall JC & Jones P (2002) Beclomethasone versus budesonide for chronic asthma. Cochrane Database of Systematic Review, 1:CD003530.
Alvaro G, Décor A, Fontana S, Hamprecht D, Large C & Marascosa A(Nov.15, 2012) Imidazolidinedione derivatives. PCT/EP2010/068946.
Anonymous (2018) Safety data sheet: Trifluoroacetone, Solvay Fluorides, Houston, USA.
Auwal MS, Saka S, Mairiga IA, Sanda KA, Shuaibu A & Ibrahim A(2014) Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). Veterinary Research Forum, 5(2):95- 100.
Ayoola GA, Coker HAB, Adesegun SA, Adepoju–Bello AA, Obaweya K, Ezennia EC and Atangbayila TO (2008), phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in South Western Nigeria. Trop. J. Pharm. Res.7: 1019-1024.
Bang DY, Lee IK & Lee B-M (2011) Toxicological characterization of phthallic acid. Toxicological Research, 27: 191- 203.
Barua CC, Yasmin N & Buragohain L (2018) A review update on Dillenia indica, its morphology, phytochemistry and pharmacological activity with reference to its anticancer activity. MOJ Bioequivalence & Bioavailability,5(5):244-254.
Burkoth TL, Taskovich LT, Crisologo NM, Beste R, Hamlin RD, Gale RM, Lee ES & Yum S (1996) Skin permeation enhancer comprising glycerol monolaurate and lauryl acetate. WO 96/40259.
Chauhan A, Goyal MK & Chauhan P (2014) GC-MS technique and its analytical applications in science and technology. Journal of Analytical and Bioanalytical Techniques, 5(6):1000222.
Cortesia C, Vilcheze C, Bernut A, Contreras W, Gomez K, de Waard J, Jacobs WR, Kremer JL & Takiff H(2014) Acetic acid, the active component of vinegar, is an effective tuberculocidal disinfectant. American Society for Microbiology, 5(2):e00013.
Enema OJ, Umoh UF, Thomas PS, Adesina SK & Eseyin OA (2019) Phytochemical and antioxidant studies of leaf of Tetrapleura tetraptera (Schun and Thon) Taubert (Mimosaceae). British Journal of Pharmaceutical and Medical Research, 4(3): 1865-1875.
Eswaraiah G, Peele KA, Krupanidhi S, Indira M, Kumar RB & Venkateswarulu TC (2020) GC–MS analysis for compound identification in leaf extract of Lumnitzera racemosa and evaluation of its in vitro anticancer effect against MCF7 and HeLa cell lines. Journal of King Saud University-Science, 32(1), pp.780-783.
Fattahi MJ & Mirshafiey A (2012) Prostaglandins and rheumatoid arthritis. Arthritis, 2012: 239310.
Fernandes C, Martins L, Devito L, Tsuribe P, Seraiva N, Garcia J & Landin- Alvarenga F (2007) The use of Demecolcine for chemical enucleation of equine oocytes destined to nuclear transfer. Biology of Reproduction,77: 129-130.
Gandhi D & Mehta P (2013) Dillenia indica And Dillenia pentagyna Roxb: Pharmacognostic, Phytochemical and Therapeutic aspects. J App Pharm Sci., 3(11):134–142.
Gibbs RD (1974) Chemotaxonomy of Flowering Plants. McGill Queen’s University Press, Montreal and London.
Glare PA, Walsh TD & Pippengar CE (1990) Normorphine, a neurotoxic metabolite. The Lancet, 355(8691): 725-726.
Gonalez- Garcia RA, McCubbin T, Navone L, Stower C, Nielsen LK & Marcellin E (2017) Microbial propionic acid production. Fermentation, 3(2): 21.
Hamalainen E, Fotsis T & Adlercreutz H (1991) A gas chromatographic method for the determination of neutral steroid profile in urine, including studies on the effect of oxytetracycline administration on these profiles in men. Clinica Chimica Acta, 199(2): 205- 220.
Hassan SS, Ibrahim SK & Mohammed MS (2016) Synthesis, spectral study and theoretical treatment of some mixing ligand complexes of Quinaldic acid and 1,10-Phenathroline. Baghdad Science Journal, 13(2): 320-330.
Hussein HM, Hameed, IH & Ibraheem OA (2016). Antimicrobial Activity and spectral chemical analysis of methanolic leaves extract of AdiantumCapillus-Veneris using GC-MS and FT-IR spectroscopy. International Journal of Pharmacognosy and Phytochemical Research, 8(3), 369-385.
Johnsen LG, Skou PB, Khakimov B & Bro R (2017) Gas chromatography- mass spectrometry data processing made easy. Journal of Chromatography A, 1503: 57-64.
Karpiński TM & Adamczak A (2019) Fucoxanthin – An Antibacterial Carotenoid. Antioxidants, 8(8), 239.
Keirse MJNC (1992) 6 Therapeutic uses of Prostaglandins. Bailliere’s Clinical Obstetrics and Gynaecology, 6(4): 787-808.
Khalifa SF & Loutfy MH(2006). Ornamental Cultivated Plant Collection: The First Occasion of the First International Conference on Strategy of Botanic Gardens, pp: 61.
Kumar AR, Ilavarasn T, Jayachandran M, Decaraman P, Aravindhan N, Padmanaban & Krishnan MRV (2009) Phytochemical investigation on a tropical plant. Pak. J. Nutri.,8:83-85.
Leary PE. Kammrath BW, Lattman KJ & Beals GL (2019) Deploying portable gas chromatography- mass spectrometry (GC-MS) to military users for the identification of toxic chemical agents in theater. Applied Spectroscopy, 73(8):841-858.
Lou X & Cassidy S. (2010) Regioselective synthesis of α-d-glucopyranosiduronic acid derivatives and biological test against bacterial Staphylococcus aureus and Salmonella agona. Science China Chemistry, 53(4), 877-883.
Malathi K & Ramaiah, S (2017). Ethyl iso-allocholate from a medicinal rice Karungkavuni inhibits dihydropteroate synthase in Escherichia coli: A molecular docking and dynamics study. Indian Journal of Pharmaceutical Sciences, 78(6), 780-788.
Mei SJ (2006) Pyrimidine substituted benzenepropanoic acid derivative, its preparation method and use in curing polycystic kidney disease. PAT: CN101054372.
Miller RJ, Jolles, C & Rapoport H (1973). Morphine metabolism and normorphine in Papaver somniferum. Phytochemistry, 12(3): 597-603.
Oguri K, Kuo CK & Yoshimura H (1989) Synthesis and analgesic effect of normorphine -3 and -6-glucuronides. Chemical & Pharmaceutical Bulletin, 37(4):955-957.
Paris R & Moyse H (1969) Precis de medicinale, Paris, Masson.
Qin HN, Yang Y & Dong SY (2017)A list of threatened species of higher plants in China. Biodiversity,25: 697–744.
Ramaya I, Arunadevi S & Vidhya A (2015) Screening of phytochemicals and testing the antimicrobial activity of different parts of Erigeon and its essential oil. International Journal of Current Microbiology and Applied Science, 4(8):372-378.
Rizk AM (1982) Constituents of Plants Growing in Qatar I.A. Chemical Survey of Sixty Plants. Fitotrapia. 52:35.
Rukshana MS, Doss A & Kumari PRTP (2017) Phytochemical screening and GC-MS analysis of leaf extract of Pergularia daemia (Forskk)Chiov. Asian Journal of Plant Science and Research, 7(1):9-15.
Saillenfait A-M, Sabate J-M, Robert A, Cossec B, Roudot A-C, Denis F & Burgart M(2013) Adverse effects of diisooctyl phthalate on the male rat reproductive development following prenatal exposure, Reproductive Toxicology, 42:192- 202.
Shahin SM, El- Fouly AS & Abdel- Moniem AM (2015) Seeds of elephant apple (Dillenia indica L) response to some pre- germination treatments. Scientific J. Flowers & Ornamental Plants, 2(1): 39-50.
Shear MJ & Kramer B (1926) Fractionation of irradiated cholesterol..I.Chemical observations. Journal of Biological Chemistry, 71: 213-220.
Shokoohi- Rad S, Zeinab S, Javaheri H, Malekabad FZ, Khakshoor H & Daluee MK (2018) Effects of preoperative doses of betamethasone acetate 0.1% on dry eye control after cataract surgery. Indian Journal of Ophthalmology, DOI:10.4103/ijo.IJO_618_10
Socrates SH & Mohan SC (2019) Phytochemical analysis of flower extracts of different Cassia species by using gas chromatography- mass spectrometry, Int.J.Biol.Chem,13 :1-11.
Stanley (2008) Ocular clinical pharmacology, In: Maddison J, Page SW & Church DB (eds) Small Animal Clinical Pharmacology (Second Edition), Elsevier, pp 557-573.
Tawfik HA, Ewies EF & El- Hamouly WS (2014) Synthesis of chromones and their applications during the last ten years, International Journal of Research in Phamacy and Chemistry, 4(4):1046- 1085.
Togashi K, Hewett DG, Whitaker DA, Hume GE, Francis L & Appleyard MN(2004) The use of acetic acid in magnification colonoscopy. Gastrointestinal Endoscopy, 59:96.
Ugochukuwu SC, Arukwe UJ & Ifeanyi O (2013) Preliminary phytochemical screening of different solvent extracts of stem bark and roots of Dennetia tripetala Baker. Asian Journal of Plant Science and Research, 3(3):10-13.
Malathi, K & Ramaiah S (2017). Ethyl iso-allocholate from a medicinal rice Karungkavuni inhibits dihydropteroate synthase in Escherichia coli: A molecular docking and dynamics study. Indian Journal of Pharmaceutical Sciences, 78(6), 780-788.
Wang H-Y (2013) Conversion of microalgae to jet fuel: Process design and simulation, Thesis, The Gene and Linda Voiland School of Chemical Engineering and Bioengineering, Washington State University, USA.
Zhang H & Zhang XT (2014) Modification and dyeing of silk fabric treated with tetrabutyl titanate by hydrothermal method, Journal of Natural Fibres, 11: 25- 38
Zimecki M, Bachor U & Maczynski M(2018) Isoxazole derivatives as regulators of immune functions. Molecules, 23(10):2724.