1Department of Botany, Patliputra University, Patna, Bihar – 800020, India.
2Department of Botany, College of Commerce, Arts & Science, Patliputra University,
Patna, Bihar-800020, India.
Corresponding author email:annisingh65@gmail.com
Article Publishing History
Received: 03/01/2025
Accepted After Revision: 22/03/2025
Over the past few years, the attention of people has changed from man-made (synthetic) to natural medicines. Withania somnifera’s phytochemical analysis has identified the presence of withanolides, which are pharmacologically active steroidal lactones. The two main withanolides that were isolated from Withania somnifera in India were withanolide D and withaferin A, both of which demonstrated cytotoxic and anticancer effects. The present investigation aimed to evolve a standard phytochemical profiling of Withania somnifera for the presence of withaferin A. The dried root sample was minced and ground with a mechanical grinder. The bioactive reference standard withaferin A was used. Methanol was used to extract the active fraction. By comparing the spectra of standard withaferin A with the root powder of Withania somnifera extracted using methanol, the peak purity of withaferin A was determined.
The calibration curve was established for peak area vs concentration of Withaferin A. From the standard stock solution, six different concentrations (0.0 ppm, 1.25 ppm, 250 ppm, 5.0 ppm, 10.0 ppm, and 20.0 ppm) were used for preparing six six-point calibration curves. The comparative analysis of standard withaferin A and the sample has been determined. The sample (Withania somnifera root powder) and standard solutions were run in methanol mobile phase systems. It was found that after running the sample and standard withaferin A, it had a good resolution, a sharp and symmetrical peak at retention times 13.685 min. and 13.600 min.
respectively. Estimation of Withaferin A can be used by the pharmaceutical industry as an appropriate bioactive marker compound for phytochemical profiling and quality control of Withania somnifera. Phytochemical profiling of Withania somnifera is essential to assess the quality, purity, efficacy, and safety of the bioactive component (withaferin A). The HPLC chromatogram of Withania somnifera root powder extract corresponding to standard Withaferin A was shown at a retention time of 13.685 min, at 227 nm wavelength. The quantitative evaluation of Withaferin A present in the sample was 0.10%.
High Performance Liquid Chromatography, Qualitative Determination, Quantitative
Determination, Withaferin A, Withania Somnifera.
Anni. Fazal M.Phytochemical Profiling of Withania Somnifera for Qualitative and Quantitative Study of Bioactive Component Withaferin a by High-Performance Liquid Chromatography. Biosc.Biotech.Res.Comm. 2025;18(1).
Anni. Fazal M.Phytochemical Profiling of Withania Somnifera for Qualitative and Quantitative Study of Bioactive Component Withaferin a by High-Performance Liquid Chromatography. Biosc.Biotech.Res.Comm. 2024;18(1). Available from: <a href=”https://shorturl.at/H3P9r“>https://shorturl.at/H3P9r</a>
INTRODUCTION
Over the past few years, the attention of people has changed from manmade (synthetic) to natural medicines. With increasing demand and acceptance of natural products, it is essential to maintain their quality for the benefit of human beings (Sharma et al., 2007). Many quality control tools are at hand, which are used to confirm the quality of herbal drugs before being marketed and consumed by consumers and patients. Both quantitative & qualitative data are essential for deciding the quality of them. The analytical technique HPLC (high-performance liquid chromatography) is commonly used for qualitative and quantitative determinations of natural bioactive fractions for quality control (Balekundri and Mannur, 2020).
Ashwagandha, or Withania somnifera (L.) Dunal, is a typical adaptogen that has long been utilized in Ayurvedic therapy in India. Withania somnifera‘s primary active ingredients are withanolides, and the root is frequently used as a medication with a variety of pharmacological actions that can be used to treat skin cancer, diabetes mellitus, insomnia, and neurasthenia. (Xiaoxing Liu et al , 2024)
Ashwagandha (Withania somnifera) is most precious herbaceous plant in the conventional systems of Indian medicine having many valuable effects (Gupta and Rana, 2007). This plant is also called “Indian Winter cherry” or “Indian Ginseng” (Singh et al., 2011). It is one of the xeromorphic plants that grew up in desiccated and rainforest area (Meher et al., 2016).
The active chemical component of Withania somnifera is withaferin A (Saleem et al., 2020). It has life life-persisting, regenerating effect and is a beneficial therapy for cancer cells (Joshi, 2016).
Some marketed preparations of Withania somnifera are Ashwagandharista, Himalaya ashwagandha, Stresswin, Stresscom, MuscleBlaze Ashwagandha 1000mg Tablet, Inlife Ashwagandha Capsules, Himalaya massage oil, Ancient Apothecary, KSM 66 Ashwagandha, Vigomax, Baidyanath Ashwagandha Amrita 450 ml, Vital plus, Amrutha kasthuri and Brento etc. are available in market.
somnifera’s phytochemical analysis identified withanolides, which are pharmacologically active steroidal lactones, (Lavie, et al 1972, Glotter, et al 1973).A class of alkaloids called withanine, which was separated from the plant’s roots, makes up 38% of the alkaloids’ total weight, (Atal et al 1975)
The two main withanolides that were isolated from W. somnifera in India were withanolide D and withaferin A, both of which demonstrated cytotoxic and anticancer effects. (Yoshida, et al 1979).The steroidal lactones known as withanolides were present in the methanolic extract.(Lavie et al 1964) ( Lavie, 1966)(Lavie and Kirson, 1968).Depending on their geographic distribution, distinct chemotypes of Withania somnifera had varying amounts of substituted steroidal lactones. ( Schmelzer et al 2008)(Lavie, et al 1972)
Lately, demand for Ayurvedic medicine has risen more in recent years in the world market because the bioactive fraction of Withania somnifera (Withaferin A) are nontoxic,safe and cost effective with fewer side effects (Arun et al 2013).
According to demand and popularity of Ayurvedic preparation, it is important to develop Phytochemical profiling of the bioactive fraction of Withania somnifera for safety, efficacy, and quality control of Ayurvedic preparation. Phytochemical profiling provides more safety and increase the faith of customers in ayurvedic preparations.It is challenging to initiate the quality control parameters for phytochemical profiling due to the multifaceted nature and in-built variability of the bioactive fraction constituents of Withania somnifera ( withaferin A).
The present investigation aimed to evolve a standard phytochemical profiling of Withania somnifera for the presence of withaferin A.
MATERIAL AND METHODS
Plant sample: Dried Ashwagandha (Withania somnifera) root samples were obtained from the Government Ayurvedic College and Hospital Herbarium, Patna, Bihar, . Dried root sample was minced and ground with a mechanical grinder (Hanil Co. Seoul, South Korea) into a mesh size 120 mm and stored at 4 °C, until further analysis.
Reagents and Standards: The bioactive reference standard withaferin A was purchased from ELECTROCRAFTS (FBD), Faridabad, Haryana, India. All chemicals and solvents used were of analytical grade or HPLC grade and obtained from E-Merck and other reputed companies.
HPLC Analysis
Instrumentation
HPLC instrumentation & chromatographic conditions: HPLC analysis was carried out on Shimadzu Model No. CBM-20A facilitated with Shimadzu’s Lab Solutions software. Shimadzu High Performance Liquid Chromatographic system was equipped with a quaternary pump, Auto autosampler, an oven, and a detector. Separation was achieved on RP-C18, a model LC-20AD of length 150mm column. The mobile phase was methanol and used in an isocratic mode with a flow rate of 1.8ml/min, 10μl of the test sample (triplicate) was injected to HPLC system.
Autosampler Model SIL-20AC, Sample Rack 1.5 mL 105 vials, Rinsing Volume 500 uL, Needle Stroke 52 mm, Control Vial Needle Stroke 52 mm, Rinsing Speed: 35 uL/sec, Sampling Speed: 15 uL/sec , Purge Time: 25.0 min, Rinse Dip Time : 3 sec and Cooler Temperature 150C Was used. Oven Model CTO-10ASvp, Oven Temperature 32 °C, Maximum Temperature 850C was used.
PDA Model SPD-M20A, Lamp D2, Start Wavelength 190 nm, End Wavelength 800 nm and Cell Temperature 400C was used.
Standard preparation: Stock solution of standard compound (Witheferin A) was prepared by dissolving 1.0 mg standard in 1.0 ml methanol. The stock solution as per requirement was diluted further.
Table1. Six-point calibration table
| serial No. | Concentration(ppm) | Peak area |
| 1 | 0 | 0 |
| 2 | 1.25 | 2431.12 |
| 3 | 2.5 | 5026.89 |
| 4 | 5 | 11385.1 |
| 5 | 10 | 23141.02 |
| 6 | 20 | 38015.15 |
Sample preparation: 19.7mg powdered Ashwagandha root was dissolved in 10.0 ml of methanol. From this solution 0.1ml was taken and dissolve in 0.9ml methanol.
The following optimal chromatographic settings were used to achieve good separations and an appropriate retention duration of Withaferin A in isocratic elution(Meena et al., 2021): –
Column: C18 ,150mm.
Detection: 227 nm wavelength.
Detector: Photodiode Array (PDA).
Mobile phase: Methanol
Flow rate: 1.8 ml/min.
Injection volume: 10 μl.
Mode of Operation: Isocratic elution.
Retention Time (Std.): 13.600
Retention Time (Test): 13.685
Run time: 30 min
RESULTS AND DISCUSSION
Calibration curve: From the standard stock solution, six different concentrations (0.0 ppm, 1.25 ppm, 250 ppm, 5.0 ppm, 10.0 ppm, and 20.0 ppm) were used for preparing six point calibration curve. Each of the standard solutions were run through the HPLC, and the respective peak areas were recorded. A calibration curve was created for the peak area versus Withaferin A concentration. Calibration peak summary is presented in Table 1, and the calibration curve is sketched in Figure 1.
Figure1: HPLC Calibration curve of withaferin A standard
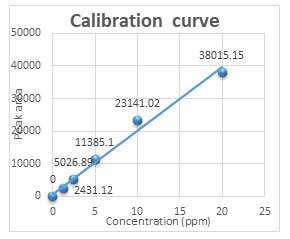
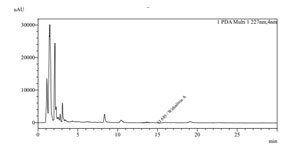
Isocratic elution of the formulation extract produced a distinctive HPLC chromatogram with a smooth, clean baseline and good resolution where the marker peak was easily detected. In the chromatogram, the marker chemical Withaferin A is seen at retention times of 13.600 for the standard and 13.685 for the sample. The calibration curve, which was linear (r2 = 0.9853) in the concentration range of 0 ppm to 20 ppm, was created by measuring the peak area. Figure 2 displays the HPLC chromatogram of the sample that corresponds to standard Withaferin A at a wavelength of 227 nm and a retention period of 13.685 min.
Figure 2: HPLC chromatogram of sample
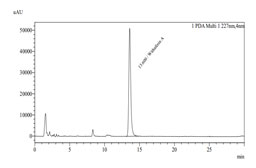
Quantitative estimation of withaferin A in sample by HPLC: By using methanol as mobile phase a sharp, reproducible and symmetric peak at retention time 13.685 mins. for standard and 13.600 mins. for sample (Fig.3) were obtained. Using the below mentioned formula, the quantitative assessment of Withaferin A in the sample was determined to be 0.10%.

Figure 3: HPLC chromatogram of standard withaferin A
Comparative analysis of standard withaferin A with the sample by HPLC: By comparing the peak obtained for standard withaferin A and that obtained for standard sample, it was found that the peak obtained for standard withaferin A and that for standard sample overlapped each other at retention time 13.6. This indicates that the sample contain withaferin A because at the retention time 13.6 standard sample showed a peak that is matched with the standard withaferin A peak. The comparative analysis of standard withaferin A and standard sample is depicted in figure 4. Zoomed view of comparative analysis of standard withaferin A and standard sample was depicted in figure 5.
Figure4: Comparative HPLC chromatogram of sample (red) and standard
withaferin A(black) at retaintion time 13.6
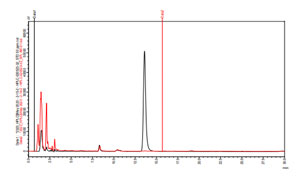
Figure 5: Zoomed view of comparative HPLC chromatogram of sample (red) and standard
withaferin A (black) at retention time 13.6
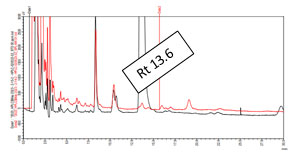
The sample and standard solutions were run in methanol mobile phase systems. It was found that after running the sample and standard Withaferin A, a good resolution, sharp and symmetrical peak at retention times 13.685 min. and 13.600 min. respectively was obtained.
CONCLUSION
Development of standard and reliable quality protocols for the Soxhlet extracted root powder of Withania somnifera by using modern techniques of analysis is extremely important. The generated HPLC result with bioactive marker compound will be used as a consistent analytical tool in the routine quality control of Withania somnifera. Estimation of Withaferin A can be used by pharmaceutical industry as an appropriate bioactive marker compound for phytochemical profiling and quality control of Withania somnifera. Phytochemical profiling of Withania somnifera is essential in order to assess the quality, purity, efficacy, and safety of the bioactive component (withaferin A). The HPLC chromatogram of Withania somnifera root powder extract corresponding to standard Withaferin A was shown at a retention time of 13.685 min, at 227 nm wavelength. The quantitative evaluation of Withaferin A present in the sample was 0.10%.
Declarations
Funding statement: No funding was received from any agency
Data availability statement: Data included in the article /referenced in the article.
Declaration of interest statement: The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Head, Department of Botany, Patliputra University, Patna, Bihar, Head, Department of Botany, College of Commerce, Arts & Science, and Principal, College of Commerce, Arts & Science, Patna, Bihar for their constant encouragement and providing laboratory and other facilities.
REFERENCES
Arun Rasheed, K.V. Satyanarayana, P.S.G. and M.S.R. (2013) ‘Chemical and pharmacological standardization of Ashwagandhadi lehyam: an ayurvedic formulation’, Journal of Complementary and Integrative Medicine [Preprint]. Available at: https://doi.org/http://doi.org/10.1515/jcim-2012-0026.
Atal CK, Dhar KL, Gupta OP, R.K. (1975) ‘Pharmacognosy and phytochemistry of Withania somnifera (Linn.) Dunal (Ashwagandha)’, Central Council for Research in Indian Medicine and Homeopathy, New Delhi., 11.
Balekundri, A. and Mannur, V. (2020) ‘Quality control of the traditional herbs and herbal products: a review’, Future Journal of Pharmaceutical Sciences, 6(1). Available at: https://doi.org/10.1186/s43094-020-00091-5.
Lavie, D, I. Kirson, E. Glotter, D.R. and Z.S. (1972) ‘Crystal and molecular structure of withanolide E, a new natural steroidal lactone with a 17α-side-chain’, Journal of the Chemical Society, Chemical Communications [Preprint], (15). Available at: https://doi.org/https://doi.org/10.1039/C39720000877.
Lavie D, S.G. and E.G. (1966) ‘Constituents of Withania somnifera Dun. Part VI. The stereochemistry of withaferin A’, Journal of the Chemical Society C: Organic, pp. 1753–1756. Available at: https://doi.org/10.1039/J39660001753.
Lavie, D I Kirson, E.G. (1968) ‘Constituents of Withania somnifera part X. The structure of withanolide D.’, Israel Journal of Chemistry, 6(5), pp. 671–678. Available at: https://doi.org/10.1002/ijch.196800085.
Glotter E David Lavie, I Kirson , A.A. (1973) ‘Constituents Of Withania-Somnifera Dun .13. Withanolides Of Chemotype-Iii’, Tetrahedron, 29(10), pp. 1353–1364. Available at: https://ezproxy.weizmann.ac.il/login?url=http://dx.doi.org/10.1016/S0040-4020(01)83156-2.
Schmelzer, GH A. Gurib-Fakim, R. Arroo, C.H. Bosch, A. de Ruijter, M.S.J. Simmonds, R.H.M.J. Lemmens, L.P.A.O. (2008) ‘Plant Resources of Tropical Africa 11(1) : Medicinal plants 1’, medicinal plants Backhuys Publishers, Wageningen, p. 630. Available at: https://edepot.wur.nl/417238.
Gupta, G. and Rana, A. (2007) ‘PHCOG MAG.: Plant Review Withania somnifera (Ashwagandha): A Review Girdhari’, Withania somnifera, 1(1), pp. 129–136.
Joshi, K. (2016) ‘Studies of Ashwagandha (Withania somnifera Dunal) Anti-fatigue formula View project SAMRAS project View project’, International Journal of Pharmaceutical & Biological Archives, 7(January), pp. 1–11. Available at: https://www.researchgate.net/publication/303343480.
Lavie, D., Glower, E. and Shvo, Y. (1964) ‘Constituents of Withania sornnifera Dun. 111. The Side Chain of Withaferin A’, 30, pp. 1774–1778.
Meena, A.K. et al. (2021) ‘Estimation of Withaferin-A by HPLC and standardization of the Ashwagandhadi lehyam formulation’, Heliyon, 7(2), p. e06116. Available at: https://doi.org/10.1016/j.heliyon.2021.e06116.
Meher, S.K. et al. (2016) ‘ Uses of Withania somnifera (Linn) Dunal (Ashwagandha) in Ayurveda and its Pharmacological Evidences ’, Research Journal of Pharmacology and Pharmacodynamics, 8(1), p. 23. Available at: https://doi.org/10.5958/2321-5836.2016.00006.9.
Mitsuzi Yoshida, Akio Hoshi, Kazuo Kuretani, Masaji Ishiguro, N.I. (1979) ‘Relationship Between Chemical Structure And Antitumor Activity Of Withaferin A Analogues’, Journal of Pharmacobio-Dynamic ., 1979(2), pp. 92–97. Available at: https://doi.org/10.1248/bpb1978.2.92.
Saleem, S. et al. (2020) ‘Withania somnifera L.: Insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective’, Iranian Journal of Basic Medical Sciences, 23(12), pp. 1501–1526. Available at: https://doi.org/10.22038/ijbms.2020.44254.10378.
Sharma, V. et al. (2007) ‘A validated and densitometric HPTLC method for the quantification of withaferin-A and withanolide-A in different plant parts of two morphotypes of Withania somnifera’, Chromatographia, 66(9–10), pp. 801–804. Available at: https://doi.org/10.1365/s10337-007-0396-2.
Singh, N. et al. (2011) ‘An overview on Ashwagandha: A Rasayana (Rejuvenator) of Ayurveda’, African Journal of Traditional, Complementary and Alternative Medicines, 8(5 SUPPL.), pp. 208–213. Available at: https://doi.org/10.4314/ajtcam.v8i5S.9.
Xiaoxing Liu, Chunyu Chen, Yingying Lin, Yanhong Liu, Shaochun Cai, Dongcui Li, Li Li, Peigen Xiao, F.Y. (2024) ‘Withania somnifera root extract inhibits MGO-induced skin fibroblast cells dysfunction via ECM-integrin interaction’, Journal of Ethnopharmacology Volume 323, 6 April 2024, 117699, 323, p. 117699. Available at: https://doi.org/https://doi.org/10.1016/j.jep.2023.117699.


