Department of Zoology, B N College, Patna University , Patna 800004, Bihar India.
Corresponding author email: sblal1990@gmail.com
Article Publishing History
Received: 14/04/2024
Accepted After Revision: 25/06/2024
Catharanthus roseus Linn., often known as Shadabahar, is a medicinal herb whose leaf extract has traditionally been used to prevent type 2 diabetes. The study’s objectives were to identify lead small molecules as diabetic medications phytocompounds using molecular docking, as well as to determine the bioactive pharmacokinetics of plants and ADMET (absorption–distribution–metabolism–excretion–toxicity) characteristics. In silico analysis of ADMET properties of four phytocompounds and a common synthetic medicine using PkCSM software, as well as receptor-ligand binding energy along with interaction studies through molecular docking for phytocompounds found in Catharanthus roseus Linn. on tyrosine phosphatase 1B or TP1B (PDB ID: 2BGD) followed by Thioredoxin interacting protein or TXNIP (PDB ID: 4LL1) as a causative agent for T2D was done.
The molecular docking was carried out using AutoDock 4.2 to determine the optimal binding affinity & energy. The molecular interaction was visualised using the molecular graphics laboratory (MGL)/ Chimaera X tool. Petunidin, Hirsutidin, Catharanthine, and Vindoline from Catharanthus roseus & Rosiglitazone demonstrated satisfactory findings on the numerous parameters used to evaluate the ADMET qualities.The molecular docking revealed that Hirsutidin had a lower binding energy (-7.62 Kcal/mol) on the TP1B receptor than Catharanthine on the TXNIP receptor (-5.8 Kcal/mol) when compared with synthetic medicines Rosiglitazone which had binding energy (-7.12 Kcal/mol) & (- 4.75 Kcal/mol) on the TP1B and TXNIP receptors. Finally, the predictions indicated that Hirsutidin or Catharanthine might represent a promising lead option for T2D prevention. It is proposed that the current prediction be validated with experimental toxicology & pharmacological assays in the future.
Catharanthus Roseus; Admet Properties; Molecular Docking; Tyrosine Phosphatase 1b; Thioredoxin Interacting Protein
Gupta M. L, Lal S. B. Pharmacokinetic and ADMET properties of bio actives from Catharanthus roseus and its associated molecular docking against Thioredoxin-interacting protein and Protein tyrosine phosphatase 1B for management of Type 2 Diabetes. Biosc.Biotech.Res.Comm. 2024;17(2).
Gupta M. L, Lal S. B. Pharmacokinetic and ADMET properties of bio actives from Catharanthus roseus and its associated molecular docking against Thioredoxin-interacting protein and Protein tyrosine phosphatase 1B for management of Type 2 Diabetes. Biosc.Biotech.Res.Comm. 2024;17(2). Available from: <a href=”https://shorturl.at/NrEpj“>https://shorturl.at/NrEpj</a>
INTRODUCTION
The term “diabetes mellitus” refers to a collection of metabolic diseases characterised by elevated blood glucose levels brought on by deficiencies in either the secretion or the function of insulin (Ganie and Kotwal, 2012). Based on its pathophysiology, diabetes mellitus can be divided into three groups (IDF , 2013). An auto-immune response is the cause of type 1 diabetes ( Liu ,et al.; 2017). Another subtype found in pregnant women is gestational diabetes mellitus. Insulin sensitivity and declining beta cell insulin production are the root causes of type 2 diabetes (Yoshihara , et al.; 2014). Numerous recorded pieces of evidence support the idea that pancreatic beta cell loss plays a part in the onset of diabetes, (Alhawiti ,et.al;2017 Sanyaolu et al 2023).
Thioredoxin-interacting protein, or TXNIP for short, is a protein that regulates metabolism and is present in key organs such as the liver, skeletal muscles, and adipose tissues. TXNIP are proteins that belong to the alpha arrestin family. TXNIP interacts with Thioredoxins (TXN1 and TXN2), causing them to become less active. Through controlling adipogenesis, peripheral glucose absorption, beta cell activity, and other processes, TXNIP is crucial for the regulation of glucose and lipid metabolism. Insulin sensitivity is decreased and pancreatic beta cell death is caused by overexpression of TXNIP (Alhawiti , et al; 2017).
The TXNIP protein binds to TXN1, interfering with TXN1’s capacity to decrease oxidised protein, leading to oxidative stress and increased apoptotic possibilities. After migrating to the mitochondria, TXNIP competes with apoptosis signal regulating kinase 1 (ASK-1) to interact with TXN2, causing ASK-1 to be released. ASK-1 frequently binds to TXN2, inhibiting its activity (Yoshihara , et al.; 2014). Pancreatic beta cells’ apoptotic signalling cascade is triggered when the released ASK-1 is phosphorylated and activated as a result of TXNIP activity. Diabetes is primarily caused by beta cell dysfunction and reduced insulin production (Wondafrash , et al; 2020 Sanyaolu et al 2023).
A non-transmembrane enzyme called protein-tyrosine phosphatase 1B (PTP1B) is present on the endoplasmic reticulum (ER). Its importance comes from the fact that it negatively regulates both insulin and leptin signalling. Insulin receptor substrate proteins, also known as PTP1B, are the main substrates of the insulin receptor (IR) and are dephosporylated, or to put it another way, free of a phosphate group. PTP1B in leptin removes phosphate from JAK2, a tyrosine kinase known as Janus kinase 2. It has lately been discovered to be an important factor in the development of tumours and has been connected more directly to breast cancer. It is also considered a possible pharmacological target since its blockage may prevent type 2 diabetes, obesity, and some types of cancer. Because of its highly conserved positively charged active site pocket, protein tyrosine phosphatase 1B (PTP1B) is a useful target for the treatment of type 2 diabetes and obesity.
However, PTP1B is difficult to target for drug discovery. Significant progress has been achieved in the creation of highly potent and specific PTP1B inhibitors that bind to both the active and catalytic sites. To enhance the pharmacological characteristics of PTP1B inhibitors, a number of approaches are being investigated (Rohan et al 2011). In addition to being a therapeutic target for the treatment of insulin resistant conditions including obesity and type 2 diabetes mellitus, protein tyrosine phosphatase 1B (PTB1B) is becoming more and more important in the pathogenesis of the resistance to insulin in diabetes mellitus (Radhika et al,2012). Moreover, PTP1B contributes to the suppression of leptin and insulin signalling. Consequently, PTP1B inhibitors offer therapeutic potential for the treatment of Type II diabetes as well as obesity. There is persuasive evidence that small molecule PTP1B inhibitors may be helpful in managing insulin resistance early on, resulting in a T2DM and obesity preventive strategy (Rao et al 2006).
According to Ayurvedic research, the flower of Catharanthus roseus is said to treat diabetes. Several laboratory investigations have shown that Catharanthus roseus extracts derived from various plant components, such as the root, leaf, flower, and stem, have hypoglycemic action. Throughout India, people have been using the ancient medicinal plant Catharanthus roseus to cure diabetes. Patients with diabetes mellitus are given various plant components, such as leaves, flowers, and stems ( Jayanthi et al 2010). Flowers of Catharanthus roseus include numerous bioactives such as Petunidin, Hirsutidin, Catharanthine, and Vindoline. Bioactives are a category of natural compounds with varying polyphenolic structures that have anti-oxidant, anti-diabetic, and anti-aging properties (Saul et al 2009).
Other phenolic compounds, including petunidin, are naturally occurring polyphenols that preserve pancreatic beta cells, promote their growth, lower beta cell death, and reduce oxidative stress, all of which aid in the prevention and management of Type 2 Diabetes Mellitus ( Sun, et al; 2020). Identifying targets and predicting innovative medications In-silico approaches have been quite important (Wadood et al., 2013). With the use of bioinformatics tools and AutoDock Tools to assess the docking score, the current work examined the bioactive from Catharanthus roseus inhibitory effect on TXNIP and PTP 1B protein.
TXNIP and PTP 1B protein retrieval, Ligand preparation and active site prediction: The crystal structure of the TXNIP and PTP 1B proteins was obtained from the Protein Data Bank of the Research Collaboratory for Structural Bioinformatics (www.rcsb.org/pdb). ADT was used to add hydrogen bonds & Kollman charges to the obtained protein structure. The structures of Petunidin, Hirsutidin, Catharanthine, and Vindoline, as well as the control medication Rosiglitazone, were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov) in canonical SMILES. ChemSketch was used to build and optimise 3D structures. The compound structures were stored as MDL mol files (.mol). The MDL mol files were then translated to PDB format via the Open Babel molecular converter ( Boyle et al 2011).
Evaluation of Molecular Properties and Ligands ADMET prediction studies for the chemicals: The compounds’ physiochemical and pharmacological characteristics were examined using the the “SwissADME” an implement offered by Swiss Institute of Bioinformatics ( www.swiss adme.ch) for Properties such as molecule size, rotatable bond, logP, and hydrogen bond donor and acceptor properties were calculated. The selected ligands were examined for membrane permeability, bioavailability, distribution, metabolism, and adsorption (Lipinski’s rule of 5) ( Lipinski et al 2001, Jarrahpour, et al 2012). Compound pharmacokinetic properties was predicted using the web server PkCSM (unimelb.edu.au)( Pires et al 2015). It expects several parameters based on specific qualities.
These factors include absorption, distribution, metabolism, and excretion, which help us determine a drug’s pharmacokinetics. Drug absorption can be anticipated using variables such as CaCO2 and skin permeability, intestinal absorption, and P-glycoprotein substrate or inhibitor. The drug’s distribution was predicted using factors that include volume distribution (VD), permeability of the central nervous system (CNS), and the blood brain barrier (BBB). Metabolism was determined using the Cytochrome P450 model. The drug’s excretion was measured by its total clearance, renal substrate
Molecular docking: To determine the affinity, the AutoDock Tool was used to dock Petunidin, Hirsutidin, Catharanthine, and Vindoline from C. roseus, as well as the control Rosiglitazone, against TXNIP and PTP 1B proteins (GM Morris et al, 1998). Kohlman charges and polar hydrogen atoms were introduced to the 3D TXNIP and PTP 1B macromolecular structure. The protein-ligand interaction of , Hirsutidin, Petunidin, Catharanthine, Vindoline and the control Rosiglitazone against TXNI and PTP 1B P protein was visualised with Molecular graphics laboratory (MGL)/Chimera X tool.
The structures of ligand under investigation were processed in Chimera to obtain a minimum energy conformer through 10 steepest descent steps followed by 100 conjugate gradient steps. The stabilized ligand conformers were also saved in .pdb format. The protein-ligand docking complexes were performed using AutoDock 4.2 (Forli et al.2016,Morris et al 2009). The LGA method was employed. A grid point spacing of 1.000 Å was set, centered on x= 86, y= 86, and z= 86Å for the TXNIP protein docking. And a grid point spacing of 0.500 Å was set, centered on x= 60, y= 60, and z= 60Å for the PTP1B protein docking. The coordinates of central grid point of maps (-14.025, 1.890, 40.242 Å) for TXNIP protein docking and (-1.591, 62.698, 2.975 Å) was used for PTP1B (2BGD) protein docking.
RESULTS AND DISCUSSION
Table 1. PubChem id , Molecular Formula and Smiles of the bio
actives of Catharanthus roseus & Rosiglitazone
| Compound | PubChem id | Molecular Formula | Smiles |
| Petunidin
|
441774 | C16H13O7+ | COC1=CC(=CC(=C1O)O)C2=[O+]C3=CC(=CC(=C3C=C2O)O)O
|
| Hirsutidin
|
441694 | C18H17O7+ | COC1=CC(=C2C=C(C(=[O+]C2=C1)C3=CC(=C(C(=C3)OC)O)OC)O)O |
| Catharanthine
|
5458190 | C21H24N2O2 | CCC1=CC2CC3(C1N(C2)CCC4=C3NC5=CC=CC=C45)C(=O)OC
|
| Vindoline
|
260535 | C25H32N2O6 | CCC12C=CCN3C1C4(CC3)C(C(C2OC(=O)C)(C(=O)OC)O)N(C5=C4C=CC(=C5)OC)C
|
| Rosiglitazone
|
77999 | C18H19N3O3S | CN(CCOC1=CC=C(C=C1)CC2C(=O)NC(=O)S2)C3=CC=CC=N3
|
Molecular compound selected for the Phyto chemical from the Catharanthus roseus have less than 500 daltons of molecular weight which is in accordance with the Lipinski rule of five and is also comparable with the control drug Rosiglitazone. Octanol- water partition coefficient (log P) was found to be less than 5 for all Phyto compounds used in this study. Number of hydrogen bond acceptors for Petunidin, Hirsutidin, and Rosiglitazone was calculated to be as 6 and 3 hydrogen bond acceptors for Catharanthine and 8 hydrogen bond acceptors Vindoline. Catharanthine, Vindoline and Rosiglitazone have only one hydrogen bond donor whereas Petunidin and Hirsutidin have 5 and 3 donors bond respectively. Surface Area was least for Petunidin and highest for Vindoline satisfying the Lipinski rule of five for prediction as an ideal drug candidate for treatment of T2D.
Figure 1: Molecular formula and 2D Structure of the bio actives
of Catharanthus roseus & Rosiglitazone
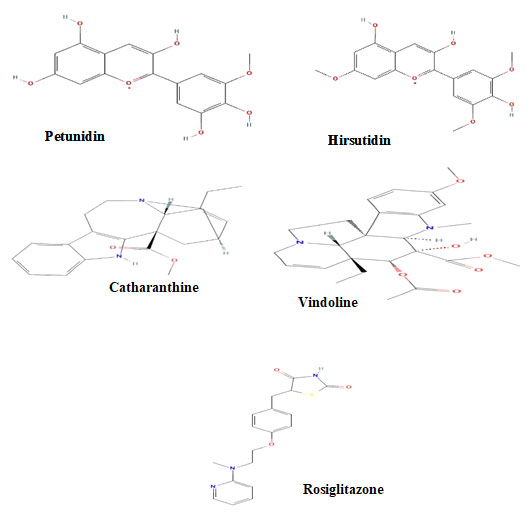
Figure 2: The ribbon shaped 3D structure of the Thioredoxin
interacting protein & Tyrosine phosphatase
Molecular properties of the bio actives of Catharanthus roseus & Rosiglitazone
Table 2. Molecular properties of the bio actives of Catharanthus roseus & Rosiglitazone
| MOLECULAR PROPERTIES | |||||
| PROPERTY | Petunidin
|
Hirsutidin
|
Catharanthine
|
Vindoline
|
Rosiglitazone
|
| Molecular Weight | 317.273 | 345.327 | 336.435 | 456.539 | 357.435 |
| LogP | 2.9175 | 3.5235 | 3.1753 | 1.6413 | 2.4909 |
| #Rotatable Bonds | 2 | 4 | 2 | 4 | 7 |
| #Acceptors | 6 | 6 | 3 | 8 | 6 |
| #Donors | 5 | 3 | 1 | 1 | 1 |
| Surface Area | 129.425 | 142.793 | 147.394 | 193.630 | 150.126 |
ADMET properties of the bio actives of Catharanthus roseus & Rosiglitazone: ADMET properties of the bioactives such as Petunidin, Hirsutidin, Catharanthine, and Vindoline from Catharanthus roseus & Rosiglitazone , showed satisfactory results on the various parameters used to evaluate the ADMET properties. Absorption parameter Catharanthine violated the Caco2 permeability as it have 1.114 log cm/s permeabilities which should be not more than 0.90 log cm/s. Intestinal absorption (human) is grater than 30% for all the bioactive such as Petunidin, Hirsutidin, Catharanthine, and Vindoline from Catharanthus roseus & Rosiglitazone which showed good results on this parameter. Skin Permeability of Vindoline among all the Phyto chemicals and Rosiglitazone was highest with log Kp cm/h of -3.088 but all the bioactive such as Petunidin, Hirsutidin, Catharanthine, and Vindoline from Catharanthus roseus & Rosiglitazone showed violation and are greater than -2.5 log Kp cm/h.
P-glycoprotein substrate was formed by all the bio compounds whereas Rosiglitazone have not formed P-glycoprotein substrate and P-glycoprotein II inhibitor. Only Vindoline had all the P-glycoprotein substrate , P-glycoprotein I inhibitor and P-glycoprotein II inhibitor during PkCSM assessment. VDss (human) was low for Hirsutidin and Rosiglitazone with -0.215 L/kg and – 183 L/kg. Fraction unbound (human) for all the phyto chemical and synthetic drug was considered safe while BBB permeability and CNS permeability was only satisfactory for Catharanthine during the analysis . CYP3A4 substrate was not formed by Petunidin whereas CYP2C9 inhibitior was not produced by Catharanthine and CYP2C9 inhibitior by Hirsutidin. CYP3A4 inhibitior none of the natural or synthetic compound forms CYP3A4 inhibitior at the metabolism parameters of pharmacokinetic . On excretion parameters Total Clearance and Renal OCT2 substrate Catharanthine performs poorly . AMES toxicity was also positive for only Catharanthine while Hepatotoxicity was positive for Rosiglitazone only.
Table 3. ADMET properties of the bio actives of Catharanthus roseus & Rosiglitazone
| ABSORPTION | |||||
| PROPERTY | Petunidin
|
Hirsutidin
|
Catharanthine
|
Vindoline
|
Rosiglitazone
|
| Water solubility | -2.943 | -3.583 | -3.399 | -3.414 | -3.762 |
| Caco2 permeability | -0.47 | 0.041 | 1.114 | 0.222 | 0.964 |
| Intestinal absorption (human) | 84.429 | 84.297 | 93.597 | 96.576 | 93.757 |
| Skin Permeability | -2.735 | -2.735 | -2.932 | -3.088 | -2.844 |
| P-glycoprotein substrate | Yes | Yes | Yes | Yes | No |
| P-glycoprotein I inhibitor | No | No | Yes | Yes | Yes |
| P-glycoprotein II inhibitor | No | Yes | No | Yes | No |
| DISTRIBUTION | |||||
| VDss (human) | 0.837 | -0.215 | 1.485 | 0.542 | -0.183 |
| Fraction unbound (human) | 0.182 | 0.054 | 0.312 | 0.265 | 0.078 |
| BBB permeability | -1.415 | -1.292 | 0.287 | -0.261 | -0.727 |
| CNS permeability | -3.48 | -3.018 | -1.939 | -3.375 | -2.785 |
| METABOLISM | |||||
| CYP2D6 substrate | No | No | Yes | No | No |
| CYP3A4 substrate | No | Yes | Yes | Yes | Yes |
| CYP1A2 inhibitior | Yes | Yes | No | No | No |
| CYP2C19 inhibitior | No | Yes | No | No | Yes |
| CYP2C9 inhibitior | No | Yes | No | No | No |
| CYP2D6 inhibitior | No | No | Yes | No | No |
| CYP3A4 inhibitior | No | No | No | No | No |
| EXCRETION | |||||
| Total Clearance | 0.647 | 0.746 | 1.167 | 0.511 | 0.107 |
| Renal OCT2 substrate | No | No | Yes | No | No |
| TOXICITY | |||||
| AMES toxicity | No | No | Yes | No | No |
| Max. tolerated dose (human) | 0.534 | 0.657 | -0.572 | -0.661 | 0.066 |
| hERG I inhibitor | No | No | No | No | No |
| hERG II inhibitor | No | No | Yes | Yes | No |
| Oral Rat Acute Toxicity (LD50) | 2.459 | 2.32 | 3.139 | 3.225 | 2.692 |
| Oral Rat Chronic Toxicity (LOAEL) | 2.45 | 1.68 | 1.488 | 1.599 | 1.415 |
| Hepatotoxicity | No | No | No | No | Yes |
| Skin Sensitisation | No | No | No | No | No |
| T.Pyriformis toxicity | 0.294 | 0.341 | 0.445 | 0.295 | 1.038 |
| Minnow toxicity | 2.463 | 1.626 | -0.727 | 1.365 | 1.63 |
Molecular docking analysis: Binding energy (kcal/mol) and inhibition constant (µM) values of the docking complexes of proteins Tyrosine phosphatase PTP1B(2BGD) with Hirsutidin calculated by molecular docking study was -7.62 kcal/mol and 2.61 µM suggestive of its preference over the synthetic drug Rosiglitazone whose Binding energy was -7.12 kcal/mol with inhibition constant at 6.02 µM . All the bio actives selected from Catharanthus roseus performs good in comparison to the Rosiglitazone at parameters like Binding energy (kcal/mol) and inhibition constant (µM) values during Molecular docking analysis of the docking complexes of proteins Tyrosine phosphatase PTP1B(2BGD) as shown in table no 4.
All the bio actives selected from Catharanthus roseus performs good in comparison to the Rosiglitazone at the parameters like Binding energy (kcal/mol) and inhibition constant (µM) values during Molecular docking analysis of the docking complexes of proteins Thioredoxin interacting protein TXNIP (4LL1) as shown in table no 4. Binding energy (kcal/mol) and inhibition constant (µM) values of the docking complexes of proteins Thioredoxin interacting protein TXNIP (4LL1) with Catharanthine calculated by molecular docking study was -5.8 kcal/mol and 56.12 µM suggestive of its preference over the synthetic drug Rosiglitazone whose Binding energy was -7.12 kcal/mol with inhibition constant at 6.02 µM.
Table 4. Binding energy (kcal/mol) and inhibition constant (µM) values of the docking
complexes of proteins with ligands calculated by molecular docking study.
| Protein | Petunidin | Hirsutidin | Catharanthine | Vindoline | Rosiglitazone
|
|||||
| (kcal/
mol) |
µM | (kcal/
mol) |
µM | (kcal/
mol) |
µM | (kcal/
mol) |
µM | (kcal/
mol) |
µM | |
| TXNIP (4LL1) | -4.04 | 1100 | -4.67 | 378.3 | -5.8 | 56.12 | -3.51 | 2650 | -4.75 | 327.53 |
| PTP1B(2BGD) | -6.81 | 10.11 | -7.62 | 2.61 | -5.73 | 63.18 | -4.79 | 308.1 | -7.12 | 6.02 |
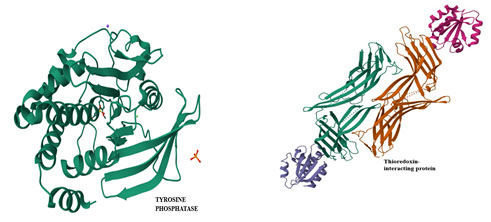
Figure 3: The docking complexes of proteins Tyrosine phosphatase PTP1B (2BGD) with
Catharanthine calculated by molecular docking study.
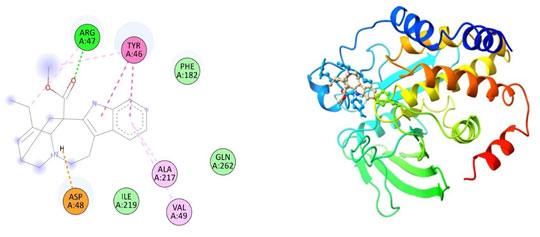
Figure 4: The docking complexes of proteins Tyrosine phosphatase PTP1B(2BGD)
with Hirsutidin calculated by molecular docking study.
Figure 5: The docking complexes of proteins Tyrosine phosphatase PTP1B(2BGD)
with Petunidin calculated by molecular docking study.
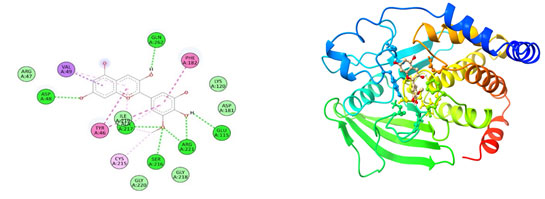
Figure 6: The docking complexes of proteins Tyrosine phosphatase PTP1B(2BGD)
with Vindoline calculated by molecular docking study.
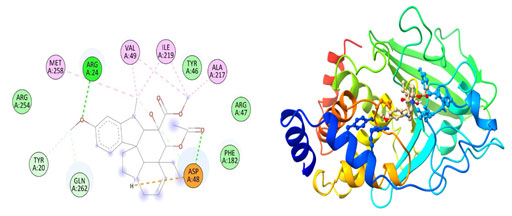
Figure 7: The docking complexes of proteins Tyrosine phosphatase PTP1B(2BGD)
with Rosiglitazone calculated by molecular docking study.
The docking complexes of proteins Tyrosine phosphatase PTP1B(2BGD) with Catharanthine has three van der waals bond at PHE 182, ILE 219 and GLN 262 , two Alkyl bonds at ALA 217 and VAL 49 with a single Pi-Pi Bond at TYR 46, Hydrogen bond at AGR 47 and a salt bridge at ASP 48. The docking complexes of proteins Tyrosine phosphatase PTP1B(2BGD) with Hirsutidin had five conventional hydrogen bond at ASP 48, GLN 262, SER 216 , ARG 221 and GLU 115 followed by Van der waals active forces at ARG 47, GLY 218, ILE 219 and GLY 220. The complex also do have carbon hydrogen bond at ASP 181 and LYS 120, Pi- Pi bond at PHE 182 and TYR 46 , Alkyl bond at ALA 217 & CYS 215 followed by Pi sigma bond at VAL 49.Tyrosine phosphatase PTP1B(2BGD) on docking with Petunidin produces 6 conventional hydrogen bond at ASP 48, SER 216, AGR 221, GLU 115, GLN 262 and ALA 217 followed by Pi sigma bond at VAL 49. The docking complex also have Pi-Pi bonds at TYR 46 and PHE 182.
The complex between Tyrosine phosphatase PTP1B(2BGD) and Petunidin also have five Van Der Waals forces site at GLY 220, GLY 128, ASP181, ARG 47 and LYS 120 with an alkyl bond at CYS 215.Tyrosine phosphatase PTP1B(2BGD) on interaction with Vindoline produces Pi- alkyl bonds at MET 258, ALA 217, VAL 49 AND ILE 219 and a salt bridge at ASP 48. Only one conventional hydrogen bond at ARG 24 with four Van der waals site at ARG 46, PHE 182, ARG 254 and TYR 46. The complex also do have two Carbon Hydrogen bonds at TYR 20 and GLN 262.The docking complexes of proteins Tyrosine phosphatase PTP1B(2BGD) with Rosiglitazone had a Pi sulphur bond at PHE 182 and Pi-Pi bonds at PHE 182 and TYR 46 . The Interaction studies also reveals that there is also an unfavourable donor at AGR 221 with nine Van Der Waals site of interactions at SER 222, ASP 181, AGR 47, ASP 48, VAL 49, LYS 120, SER 216 and GLN 262. The docking complexes of proteins Tyrosine phosphatase PTP1B(2BGD) with Rosiglitazone had seven conventional hydrogen bonds positioned at GLY 218, GLY 220, ALA 217, ILE 219, CYS 215, GLN 266 and PHE 182.
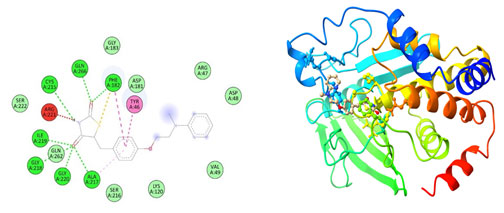
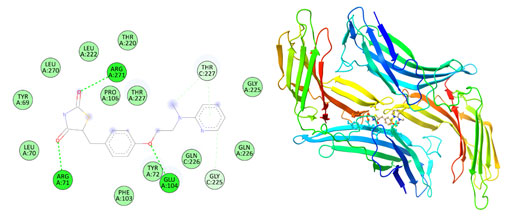
Figure 8: The docking complexes of proteins Thioredoxin interacting protein TXNIP (4LL1)
with Catharanthine calculated by molecular docking study.
Van Der Waals interactions were active at TYR 72, GLN 226, GLY 225, THR 227, PRO 106, GLN 107, GLN 226, THR 227, LEU 222, PHE 103, LEU 270, THR 69 and ARG 71 in the docking complexes of proteins Thioredoxin interacting protein TXNIP (4LL1) with Catharanthine. The docking complexes of proteins Thioredoxin interacting protein TXNIP (4LL1) with Catharanthine had conventional hydrogen bond at GLU 104 and GLY 225 with Alkyl bond at PRO 106 and Leu 70. Hirsutidin on interaction with TXNIP produces conventional hydrogen bond at GLY 266 , THR 66 and Lys 64 and carbon hydrogen bond at SER 298 and ILE 296. PRO 262, ILE 260, ILE 269, THR 274 was the site for Pi- alkyl bond in the docking complexes of proteins Thioredoxin interacting protein TXNIP (4LL1) with Hirsutidin.
Figure 9 : The docking complexes of proteins Thioredoxin interacting protein TXNIP (4LL1)
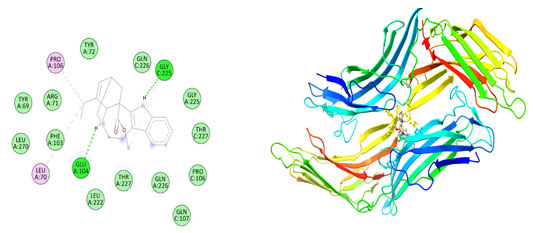
with Hirsutidin calculated by molecular docking study.
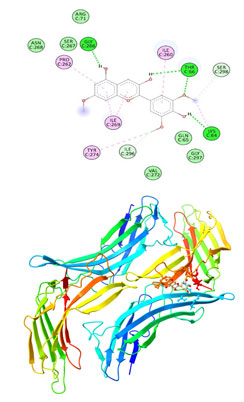
Figure 10: The docking complexes of proteins Thioredoxin interacting protein TXNIP (4LL1)
with Petunidin calculated by molecular docking study.
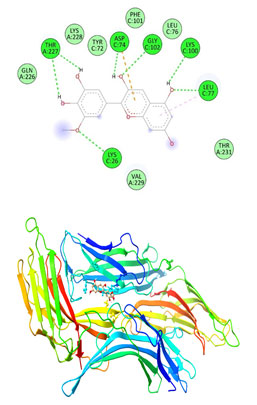
Figure 11: The docking complexes of proteins Thioredoxin interacting protein TXNIP (4LL1)
with Vindoline calculated by molecular docking study.
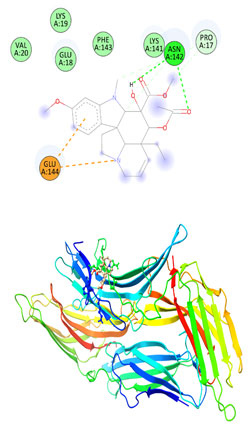
Figure 12: The docking complexes of proteins Thioredoxin interacting protein TXNIP (4LL1)
with Rosiglitazone calculated by molecular docking study
Van Der Waals interactions in the docking complexes of protein TXNIP (4LL1) with Hirsutidin were at GLN 65, GLY 297, VAL 272 , ASN 268, SER 267 and AGR 71.Thioredoxin interacting protein ,TXNIP (4LL1) on interactions with Petunidin had Pi Anion bond at ASP 74 with Pi alkyl bond at LEU 77. Conventional hydrogen bonds were represented by THR 227, ASP 74, GLY 102, LYS 100 and LEU 77.Van Der Waals interactions were active at GLN 226, LYS 228, THR 72, PHE 101, LEU 76, THR 231 and VAL 229 in the docking complexes of proteins Thioredoxin interacting protein TXNIP (4LL1) with Petunidin.
The docking complexes of proteins Thioredoxin interacting protein TXNIP (4LL1) with Vindoline calculated by molecular docking study showed Pi Anion or active charge at GLU 144 with conventional hydrogen bond at ASN 142. There was also Van Der Waals interactions at VAL 20, GLU 18, LYS 19 , PHE 143 and a carbon hydrogen bond at PRO 17. Thioredoxin interacting protein TXNIP (4LL1) on interaction with Rosiglitazone had three conventional hydrogen bonds at ARG 71, GLU 104 and AGR 271 with two Carbon hydrogen bond at THR 227 AND GLY 225. Van Der Waals forces were active at LEU 70, PHE 103, TYR 72, GLN 226, GLY 225, THR 220, LEU 222, LEU 270 and TYR 69.
The docking complexes of proteins Thioredoxin interacting protein TXNIP (4LL1) with Vindoline calculated by molecular docking study showed Pi Anion or active charge at GLU 144 with conventional hydrogen bond at ASN 142. There was also Van Der Waals interactions at VAL 20, GLU 18, LYS 19 , PHE 143 and a carbon hydrogen bond at PRO 17. Thioredoxin interacting protein TXNIP (4LL1) on interaction with Rosiglitazone had three conventional hydrogen bonds at ARG 71, GLU 104 and AGR 271 with two Carbon hydrogen bond at THR 227 AND GLY 225. Van Der Waals forces were active at LEU 70, PHE 103, TYR 72, GLN 226, GLY 225, THR 220, LEU 222, LEU 270 and TYR 69.
CONCLUSION
The molecular docking revealed that Hirsutidin had a lower binding energy (-7.62 Kcal/mol) on the TP1B receptor than Catharanthine on the TXNIP receptor (-5.8 Kcal/mol) when compared with synthetic medicines Rosiglitazone which had binding energy (-7.12 Kcal/mol) & (- 4.75 Kcal/mol) on the TP1B and TXNIP receptors. When evaluated using different parameters, the ADMET qualities of bioactive from Catharanthus roseus, such as Vindoline, Hirsutidin, Petunidin, and Catharanthine, as well as Rosiglitazone, demonstrated satisfactory findings. ADMET properties like a AMES toxicity was positive for only Catharanthine whereas on Hepatotoxicity parameters Rosiglitazone was found to be positive .Finally, the predictions indicated that Hirsutidin or Catharanthine might represent a promising lead option for T2D prevention. It is proposed that the current prediction be validated with experimental toxicology & pharmacological assays in the future.
Conflict of interest: The authors have no conflict of interest.
Funding: Authors did not receive any funding for this work.
Data Availability: Data will be available on request.
REFERENCES
Sanyaolu A, Marinkovic A, Prakash S, Williams M, Dixon Y, Okorie C, Orish VN, Izurieta R. Diabetes mellitus: (2023) An overview of the types, prevalence, comorbidity, complication, genetics, economic implication, and treatment. World J Meta-Anal 2023; 11(5): 134-143 [DOI: 10.13105/wjma.v11.i5.134]
Alhawiti NM, S Al-Mahri, MA Aziz, SS Malik and S. Mohammad. TXNIP in metabolic regulation: Physiological role and therapeutic outlook. Curr. Drug Targets 2017; 18, 1095-103.
Boyle NMO, M Banck, CA James, C Morley, T Vandermeersch and GR Hutchison. Open babel: An open chemical toolbox. J. Cheminformatics 2011; 3, 33.
Design L Pharmacophore and ligand-based design with Biovia Discovery Studio®. BIOVIA. California, 2014.
Forli, S., Huey, R., Pique, M. E., Sanner, M. F., Goodsell, D. S., & Olson,A. J. (2016). Computational protein–ligand docking and virtual drug screening with the AutoDock suite. Nature Protocols, 11(5), 905–919. https://doi.org/10.1038/nprot.2016.051
Ganie MA and S Kotwal. Recent advances in management of diabetes mellitus. J. Int. Med. Sci. Acad. 2012; 25, 171-5.
International Diabetes Federation. Diabetes atlas. International Diabetes Federation. Brussels, Belgium, 2013.
Jarrahpour A J Fathi, M Mimouni, MH Youssoufi, M Mimouni, ZH Chohan and TB Hadda. Petra, osiris and molinspiration (POM) together as a successful support in drug design: Antibacterial activity and biopharmaceutical characterization of some azo Schiff bases. Med. Chem. Res. 2012; 21, 1984-90.
Jayanthi, M N Sowbala, G Rajalakshmi, U Kanagavalli and V Sivakumar. Study of anti hyperglycemic effect of Catharanthus roseus in alloxan induced diabetic rats. Int. J. Pharm. Pharmaceut. Sci. 2010; 2, 114-6.
Lipinski, CA F Lombardo, BW Dominy and PJ Feeney. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001; 46, 3-26.
Liu, CY YN Hao, F Yin, YL Zhang and J Liu. Geniposide accelerates proteasome degradation of Txnip to inhibit insulin secretion in pancreatic β-cells. J. Endocrinol. Investig. 2017; 40, 505-12.
Morris, G. M., Huey, R., Lindstrom, W., Sanner, M. F., Belew, R. K., Goodsell, D. S., & Olson, A. J. (2009). AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. Journal of Computational Chemistry, 30(16), 2785–2791. https://doi.org/10.1002/ jcc.21256.
Morris, GM DS Goodsell, RS Halliday, R Huey, WE Hart, RK Belew and AJ Olson. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998; 19, 1639-62.
Pires, DE TL Blundell and DB Ascher. pkCSM: Predicting small-molecule pharmacokinetic properties using graph-based signatures. J. Med. Chem. 2015; 58, 4066-72.
Radhika R and Sudarsanam D (2012). Docking of Rheum Emodi compounds against Protein Tyrosine Phosphatase
Rao GS1, Ramachandran MV, Bajaj JS(2006). In silico structure-based design of a potent and selective small peptide inhibitor of protein tyrosine phosphatase 1B, a novel therapeutic target for obesity and type 2 diabetes mellitus: a computer modeling approach. J Biomol Struct Dyn.23(4):377-84.
Rohan V. Bamane, Trupti S. Chitre & Vijay K. Rakholiya(2011) Molecular docking studies of quinoline-3-carbohydrazide as novel PTP1B inhibitors as potential antihyperglycemic agents, Der Pharma Chemica. 3(4):227-237.
Sanyaolu A, Marinkovic A, Prakash S, Williams M, Dixon Y, Okorie C, Orish VN, Izurieta R. Diabetes mellitus: (2023) An overview of the types, prevalence, comorbidity, complication, genetics, economic implication, and treatment. World J Meta-Anal 2023; 11(5): 134-143 [DOI: 10.13105/wjma.v11.i5.134]
Saul N, K Pietsch, R Menzel, SR Stürzenbaum and CE Steinberg. Catechin induced longevity in C. elegans: From key regulator genes to disposable soma. Mech. Ageing Dev. 2009; 130, 477-86.
Sun C, C Zhao, E Capanoglu, P Paoli, J Simal-Gandara, K Ramkumar, W Shengpeng, B Florina, P Ana, T Vladiana, D Georgiana, D Simona, T Merve, K Washim, W Mingfu, D Dominique, PP Maria, D Parsa, C Lei and J Xiao. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front. 2020; 1, 18-44.
Wadood, A. N Ahmed, L Shah, A Ahmad, H Hassan, S Shams (2013). In-silico drug design: An approach which revolutionised the drug discovery process. OA.Drug Design & Delivery,1(1): 3.
Wondafrash DZ , AT Nire’a, GG Tafere, DM Desta, DA Berhe and KA Zewdie. Thioredoxin-interacting protein as a novel potential therapeutic target in diabetes mellitus and its underlying complications. Diabetes Metab. Syndrome Obes. 2020; 13, 43-51.
Yoshihara E, S Masaki, Y Matsuo, Z Chen, H Tian and J. Yodoi. Thioredoxin/TXNIP: Redoxisome, as a redox switch for the pathogenesis of diseases. Front. Immunol. 2014; 4, 514.


