Department of Microbiology, Kakatiya University, Warangal -506009, Telangana (TS), India.
Corresponding author email: munjam17@gmail.com
Article Publishing History
Received: 24/12/2019
Accepted After Revision: 29/03/2020
Pectinases are major constituents in many fundamental life processes and have copious relevance in biotechnology and industry. Pectinase have immense perspective in food, textile and pharmaceuticals industries. With this regard, there has been a great increase in industrial applications of pectinase owing to their significant biotechnological uses. This study was undertaken with main objectives of meeting the growing industrial demands of pectinase, by improving the yield without increasing the cost of production. The present investigations were aimed at to study the occurrence and distribution and later on to isolate and characterize pectinolytic fungi from different habitats in and around the Warangal district of Telangana state, India. Further the study includes the factors influencing pectinase production by selected isolates, optimization of parameters for over production of pectinase. In the present investigation thirty soils known to harbor the pectinolytic fungi were selected for sampling. About 30 isolates of fungi showing pectinase production were isolated. Colonies exhibiting more than 2.0 mm pectinolytic zone was picked and further screened for pectinolyic activity on pectin screening agar medium. Out of these studies 2 efficient strains producing pectinolytic zone, were selected and an attempt was made to characterize and identify them tentatively by following the guidelines of Bergeys manual. The selected strains were identified as Aspergillus niger and Aspergillus flavus based on analysis profile of 18S rRNA sequence. These 2 isolates were showing promising pectinolysis were chosen for further studies. The production of pectinase was improved in submerged fermentation. Both the organisms produced all the pectic enzymes (Exo-pectinase, Exo-PGase, Endo-PG, Endo-PL and PME). However, the optimal pH and temperature varied with the species. Maximum enzyme production by A. niger and A. flavus was recorded at 8th and 12th days of incubation with optimum temperature 30oC and 35oC, optimum pH 6.0 and 5.0 respectively at agitation rate 140 rpm.
A. Niger, A. Flavus, Pectinases, Optimization, Submerged Fermentation
Begum G, Munjam S. Optimization of Cultural Conditions Temperature and pH for Production of Pectinases by Two Species of Aspergillus. Biosc.Biotech.Res.Comm. 2020;13(1).
Begum G, Munjam S. Optimization of Cultural Conditions Temperature and pH for Production of Pectinases by Two Species of Aspergillus. Biosc.Biotech.Res.Comm. 2020;13(1). Available from: https://bit.ly/2WYCkRG
Copyright © Begum and Munjam This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Pectinases are enzymes that catalyze break down of the glycosidic bonds in the galacturonic acid chains of the pectin rich materials. Because of their wide range of applications in food and feed industry, pectinases make up almost 25% of the global food enzyme market. Pectinases are major enzymes involved in pectin degradation process in the cell wall of plants and also act as major cell wall components in plants. Microbes are well known source for the production of extracellular enzymes and industrially important secondary metabolites. Pectic substances are a class of complex glycosidic polysaccharide compounds with a high molecular weight. Pectin an important component of plant cell wall is a polymeric material having carbohydrate group esterifies with methanol. It is present in high concentrations in the middle lamella, where it acts as a cementing material between adjacent cells, (El Enshasy et al. 2018, Kamalambigeswari et al. 2018 and Abd El Tawab et al. 2019).
Three major pectic polyssacharide groups viz, HG, RGI ansd RGII are recognized. Homogalacturonan (HG) is a linear polymer formed by D-galacturonic acid which can be acetylated and/or methyl esterified. It can also be called as smooth regions of pectin, (Jayani et al. 2005). Rhamnogalacturonan I (RGI) is composed of the repeating disaccharide rhamnose galacturonic acids and Rhamnogalacturonan II (RGII) is a homogalacturonan chain with complex side chains attached to the galacturonic residues, (Willats et al. 2001). The occurrence of pectinolytic enzymes has been reported in a large number of bacteria and fungi. However, most commercial preparations of pectic enzymes are obtained from fungal sources. This is due to a wide range of pH optima of enzymes produced by fungal strains. These enzymes not only provide an economically viable alternative, but are also ecofriendly, (Vikari et al. 2001). The microbial pectinase accounts approximately for 25% of the total worldwide enzyme sale, (Voragen et al. 2004 El Garhy, et al. 2020).
These groups of enzymes harbor a very huge commercial potential as their biotechnological applications span broad spectra in diverse industries such as biofuels, pulp-paper, food, animal feed, textile, fiber, etc. Out of these, biofuel industries demand these xylanolytic and pectinolytic enzymes play their major role to the enzymes for improving plant biomass saccharification. Whereas, animal feed industries require combination of cellulase, xylanase and pectinase for improving the nutrition quality of grain and feed (Thite et al. 2020).
The important constraint for commercialization of new sources of enzymes is higher cost of the production. It defines the optimal microbial cultivation conditions for capable microbial strains and cheap raw substrate may reduce the cost of enzymes production. A large number of bacteria, yeasts and many filamentous fungi are potential pectinase producers. Fungi like T. viride, A. flavus, A. niger, F. oxysporum, A. terreus, and P. chrysogenum have attracted the most attention as enzymes producers because of the prolific yield and their long history in fermentation industries (El Garhy, et al. 2020).
Enzyme breakdown of the biomolecules depends up on the type of enzyme, application, temperature, incubation time, agitation, concentration, pH and use of different enzyme preparations (Dominguez et al. 1994 and Chadha et al. 2003). Owing to the vast potential applications of pectinase in various sectors of industries it is pertinent to undertake research on screening of microorganisms for pectinases and determine optimal conditions for production of microbial pectinase. Generally for the production of high-priced materials and for the study of biochemical and physiological aspects of the microbial metabolites, submerged fermentation system is very useful ( Pereira et al. 1993). The usage of submerged fermentation is technically easier than solid state fermentation (Pedrolli et al. 2009). The purpose of this research was to evaluate pectinase production by the selected fungi using various vegetable waste dump yard soils as substitutes of pectin to make its production cost effective under submerged state fermentation.
MATERIAL AND METHODS
Isolation and Screening of pectinolytic fungi: Soil samples were collected from the site where the vegetable wastes were dumped and laced in sterile polythene bags and transferred to the laboratory. One gram of soil sample from each collection site was mixed in 100 ml of sterilized distilled water and 10-fold serial dilutions were prepared. One ml of each dilution was spread on potato dextrose agar (PDA) plates and incubated in an inverted position at 28˚C for 7 days (Kaur et al. 2004). Fungal colonies developing from the plates were picked up and purified and sub cultured onto slants and maintained for identification and enzyme studies. Promising producers of pectinase enzymes were screened by plating on modified pectin agar medium. (Pectin-10 g, K2HPO4-0.05%, MgSO4-0.05% KCl-0.05%, FeS04–0.01% Sucrose-1%, ZnSO4-0.001%, CuSO4–0.001%, Agar-20g, Distilled water-1000ml, pH-5.5) supplemented with streptomycin.
Screening and Identification of Fungal Isolates for Pectinolytic Activity: Pectin agar medium was used for the screening of isolated fungal strains. They were incubated for a week at room temperature. After incubation, the plates were flooded with iodine-potassium iodide solution (Iodine-1.0g, potassium-iodide-5.0g in 330ml distilled water) observed for zone of hydrolysis around the colonies. Positive cultures were selected from these isolates after screening and identified as A. niger and A. flavus by 18S rRNA sequencing.
Production of Pectinase Enzyme Cultures were grown in 250 ml Erlenmeyer flask containing 100 ml of broth [pH 7.0, contains 1% of rice bran 0.2%, NaNO3, 0.1%, K2HPO4; 0.05%, MgSO4.7H2O; 0.05%, KCl; 0.01%, FeSO4.7H2O; 0.001%, ZnSO4; 0.001%, CuSO4] for production of pectinases and exo-polygalacturonases. After sterilization of the Erlenmeyer flasks containing fermentation medium, young fungal mycelium of 3 days old cultures at the growing edges were used to inoculate aseptically. Inoculated culture flasks were incubated in the incubator shaker operating at 120-180 rpm at 28±1ºC for 16 days. 10 ml of culture broth was withdrawn from the flasks at different time intervals of incubation. The supernatants obtained from the centrifugations of the same were used as enzyme sources for enzyme assay.
Assay of enzymes: Exo-pectinase: Supernatants from the incubated shake culture flasks at intervals of 8th and 12th days were used as enzyme source. Activity was assayed by DNS method (Miller 1959). The Exo-pectinase activity was determined using 1% pectin as substrate. Reaction mixture containing equal amounts of 1% pectin (1.0 mL ) prepared in citrate buffer (0.05 M; pH 5) and partially purified enzyme (1.0 mL).The mixture was incubated at 50oC in water bath for 30 min .The reaction was terminated by addition of 3ml of 3,5-dinitrosalicilic acid DNS reagent and the contents were boiled for 15 minutes. After cooling the color developed was read at 540nm. The amount of reducing sugar released was quantified using galactouronic acid as standard. Standard galacturonic was prepared by taking 100mg galacturonic acid in 100ml standard flask and made up the volume to 100ml. A standard curve of D-Galactouronic (1mg/mL) was prepared under identical conditions to determine the reducing sugars formed. The enzymatic activity was expressed as Unit per ml (U/ml), which is defined as the amount of enzyme that liberates 1μmole of reducing sugar per mL per minute under assay conditions.
Assay of Exo-Polygalacturonase (Exo-PGase): Supernatants from the incubated shake culture flasks at intervals of 8th and 12th days were used as enzyme source of exo-polygalacturonases, activity was assayed by quantifying reducing sugars using DNS (3,5-dinitrosalicylic acid) method (Miller,1959). The exo-PGase activity was determined using 1% polygalacturonic acid (PGA) as substrate, prepared in sodium acetate buffer (0.1M; pH 4.5). The reaction mixture (2mL) containing equal amounts of enzyme (1.0 mL) and substrate (1.0 mL) and incubating at 50º C for 30 min in a water bath. The reaction was stopped by addition of 3ml of 3,5- dinitrosalicilic acid DNS reagent and the contents were boiled for 15 minutes. The color developed was read at 540nm. A standard curve of D-Galactouronic acid (1mg/mL) was developed under identical conditions to determine the reducing sugars formed. The enzymatic activity of filtrate was expressed as Unit per ml (U/ml), which is defined as the amount of enzyme, which liberates 1μmole of galacturonic acid (reducing sugar) per mL per minute under assay conditions.
Pectin methyl esterase activity (PME): Pectin methyl esterase activity was estimated by the method suggested by Kertesz (1955). Pectin esterase activity was measured by increase in free carboxyl group by titrating against NaOH in the presence of a pH indicator like phenolphthalein. For assaying pectinesterase activity 20ml of 1% pectin was dissolved in 0.15M NaCl (pH-7.0) and 4ml of enzyme extract was taken in a beaker and incubated for 1 hour. After incubation, the solution was titrated against 0.02N NaOH to reach pH 7.0 using phenolphthalein as indicator (colour change from colourless to pink) and the heat killed enzyme extract was used as control.
Pectin esterase activity = Vs – V b (Normality of NaOH ×100/Vt
Where, Vs-volume of NaOH used to titer sample (ml), V b-volume of NaOH used to titer blank (ml), V-volume of incubation mixture (ml), t-reaction time (min). Pectin esterase activity was expressed as milli equivalents of NaOH consumed min-1 ml-1 of enzyme extract under the assay conditions
Assay of Endo Poly galacturonase (Endo-PG): Wood’s viscometric method (1955) was followed to estimate the endo-PG. Polygalacturonic acid (0.5%) was prepared by dissolving 0.5g of polygalacturonic acid in 100 ml citrate (buffer (pH 5.5). The reaction mixture for the estimation of endo-PG was with polygalacturonic acid (0.5%) substrate, citrate buffer (pH 5.5) and enzyme source in 4:1:2 ratios. The reaction mixture consisting of 12ml of substrate, 4ml of enzyme and 1ml of citrate buffer. The loss of viscosity was measured for every 10 minutes over a period of 30 minutes. The reaction mixture with heat killed (inactivated) enzyme and distilled water served as control. The percentage loss of viscosity was calculated by using the formula.
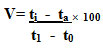
Where,
V = percentage of loss of viscosity ti = flow time of reaction mixture + inactive enzyme.
ta = flow time of reaction mixture + active enzyme t0 = flow time of distilled water+ active enzyme at ‘‘O’’ time.The Relative Enzyme Activity (REA) of endo-PG was calculated by dividing 1000 with time required for 50% loss of viscosity (t50) and expressed the enzyme activity in relative viscometric units (RVU). REA = 1000 / tv50
Where tv50 = time required in minutes taken for 50% loss of initial viscosity
Endo-pectin lyase (Endo-PL): Endo-pectin lyase activity was assayed viscometrically as suggested by Wood (1955). 1% pectin was used as substrate in this assay. 4ml of culture supernatant and 0.8 ml of tris HCl buffer pH (8.0) were added to 12ml of pectin solution. Viscosity of supernatant was determined by using Ostwald viscometer. Initial reading time was noted and incubated for 30 minutes and take all the final reading time was recorded.The loss of viscosity was measured for every 10 minutes over a period of 30 minutes. The reaction mixture with heat killed (inactivated) enzyme and distilled water served as control. Enzyme activity measured in RVU units (relative viscometric units).
Statistical analysis: The enzyme activities are presented as Mean± SE of all values. Results obtained in this study were subjected to analysis of variance using one way ANOVA and difference between means were separated by Duncan Multiple Range Test using SPSS software 17.0 version.
The effect of temperature on enzyme activity: Production media were prepared in, 50ml/100ml Erlenmeyer flasks, and inoculated by fungal inoculums. Effect of different temperatures on pectinase production was observed by incubating the active culture broth at various temperatures 25oC, 30oC, 35oC, 40oC and 45oC for 16 days. The cell suspension was collected on 8th and 12th day and the pectinase production was estimated with the procedure mentioned earlier.
The effect of pH on enzyme activity: Production media were prepared in 50ml/100ml Erlenmeyer flasks. Effect of various pH levels on pectinase production was observed by incubating the culture broth at pH levels ranging from 2.0 to 8.0. The different pH levels were adjusted using 2N NaOH (sodium hydroxide) to the 50 ml production medium taken in 250ml conical flask, inoculated with active fungal culture and incubated at 35oC for 16 days. The pectinase production was estimated on 8th and 12th day using the procedure mentioned earlier. The results are presented in tables and figures.
RESULTS AND DISCUSSION
Effect of incubation temperature on exo-pectinase production: Temperature is directly related to the metabolic activities of the microorganism and it affects the proper growth and product formation by the organism (Lonsane et al. 1985). Enzyme organism has its own optimal temperature at which it grows its maximum and produces the desired products maximally. Hence maintenance of optimal temperature is a must.Many research workers have reported at different temperature for maximum pectinase production in SmF studies, suggesting that the optimal temperature for pectinase production depends on the strain variation of the microorganisms. Temperature of medium have very good influence on enzyme production. To assess the effect of temperature on pectinase production, isolates were grown in production medium at their optimized pH and at different temperatures from 20˚C to 45˚C for pectinase production under submerged fermentation in an incubator shaker with agitation of 140 rpm for 12 days.
Temperature play a great role in the enzyme production as it is especially significant in microorganism’s growth regulation and physiological activity and microbial product formation and it varies from each microorganism. Optimal temperature is defined as that temperature which results in maximum velocity of the enzymatic reaction above which, the rate of reaction decreases due to thermal inactivation. The very slight changes in the growth temperature, may affect pectinase production (Sandri et al. 2011). Temperature is directly related to the metabolic activities of the microorganism and it affects the growth and product formation of the organism (Sandhya and Kurup 2013).
From the results presented in Table-1 it is evident that A. niger showed optimum exo-pectinase activity at 30oC (0.516 U/ml) while the least activity of enzyme at 45oC (0.071U/ml). Exo-pectinase produced by A. flavus was optimum at 35ºC (0.460 U/ml). A. flavus produced more exo-pectinase at all temperatures under investigation except at 45oC. Similar results were observed in case of A. niger which showed best performance at a temperature of 45°C (Bhardwaj and Garg 2014). Temperature of 30°C was observed to yield optimum pectinase production in case of Bacillus sphaericus (MTCC 7542), Aspergillus niger, S. cerevisiae, Aspergillus foetidus and Kluyveromyces wickerhamii (Moyo et al. 2003; Kumar et al. 2012 and Ahmed et al. 2015). Very few species of fungi have the ability to grow vigorously at temperatures between 45°C and 55°C. A temperature of 30°C was reported to be the optimum growth temperature which was similar to the findings of Mathew et al. (2008); Gupta and Kalpana (2011).
Table 1. Effect of temperature on exo-pectinase production by A. niger and A. flavus on 12th day of incubation.
| Temperature | A. niger | A. flavus |
| 25˚C | 0.360b±0.005 | 0.356c±0.003 |
| 30˚C | 0.516a±0.008 | 0.423b±0.003 |
| 35˚C | 0.110d±0.005 | 0.460a±0.005 |
| 40˚C | 0.130c±0.005 | 0.320d±0.010 |
| 45˚C | 0.071e±0.004 | 0.300e±0.000 |
| Control | 0.130c±0.005 | 0.143f±0.003 |
Values are significant at P< 0.005
Effect of incubation temperature on exo-PG production: Activity of enzymes extracted from the isolates was determined to check out the optimum range of temperature for exo-polygalacturonase enzyme (Table 2). Among all temperatures A. niger showed optimum exo-PG activity at 30˚C with 0.880 U/ml and A. flavus showed optimum exo-PG activity at 35˚C with 0.786U/ml respectively. When temperature is altered below or above the optimum, the activity decreased. The maximum production of exo-PG enzyme was obtained at 30°C by the A. niger. Further increase in the temperature resulted in the decrease of pectinolytic activity by both the organisms under investigation. Ahmed and Sohail (2020) studied on the activity of pectinase by G. candidum AA15 revealed that the enzyme shows its maximum activity at 35°C and any further increase in temperature reduces the activity drastically
Table 2. Effect of temperature on exo-PG production by A. niger and A. flavus on 12th day of incubation.
| Temperature | A. niger | A. flavus |
| 25˚C | 0.580b±0.005 | 0.123c±0.003 |
| 30˚C | 0.880a±0.005 | 0.550b±0.005 |
| 35˚C | 0.170c±0.005 | 0.786a±0.003 |
| 40˚C | 0.090d±0.005 | 0.106d±0.006 |
| 45˚C | 0.070e±0.005 | 0.060e±0.000 |
| Control | 0.070e±0.005 | 0.110cd±0.005 |
Values are significant at P< 0.005
Effect of incubation temperature on endo-PG production: Effect of incubation temperature on endo-PG production was studied in both the organisms in the range of 25˚C to 45˚C. In both organisms, optimum production was recorded at 30˚C and 35˚C. A. niger produced 43.56RVU at 30˚C whereas A. flavus produce 38.66RVU at 35˚C (Table 3). Production was started at 25˚C recorded optimum at 35˚C and gradually decreased with subsequent incubation temperature. Maximum enzyme activity at optimum temperature may be due to the faster metabolic activity and increase in protein content and extracellular enzyme production in culture supernatant.
Ketipally et al. (2019) reported that at various temperatures between 25°C and 45°C, 35°C was the most suitable temperature for the growth and production of polygalacturonase activity and 30°C was the favourable temperature for the growth and production of pectinase by A. nomius MR 103. At very low temperatures, membranes solidify and high temperatures damage microorganisms by denaturing enzymes, transport carriers and other proteins thus lowering enzyme activity.
Table 3. Effect of temperature on Endo-PG production by A. niger and A. flavus on 8th day of incubation.
| Temperature | A. niger | A. flavus |
| 25˚C | 40.0b±0.577 | 11.53d±0.290 |
| 30˚C | 43.56a±0.881 | 27.40b±0.305 |
| 35˚C | 37.26c±0.881 | 38.66a±0.333 |
| 40˚C | 37.26c±0.881 | 26.66b±0.333 |
| 45˚C | 23.33d±0.666 | 12.70c±0.351 |
| Control | 23.33d±0.666 | 11.53d±0.290 |
Values are significant at P< 0.005
Effect of temperature on endo-pectin lyase production: Incubation temperature has been found to be a significant controlling factor for enzyme production. Table 4, revealed that the temperature has great influence for the production pectinase. A. niger produced maximum endo-pectin lyase activity at 30oC 91.32RVU whereas A. flavus produced enzyme at 35˚C (58.73RVU) and lower activity 13.03RVU was showed at control by A. flavus. Kamalambigeswari et al. (2018) found the influence of temperature at 30°C, the enzyme activity at high titer (209.04). Celestina et al. (2006) reported the effect of temperature on PG production by Monascus sp. N8 and Aspergillus sp. N12 and the optimum temperature were found to be 45°C. Bailey and Pessa (1990) studied lower temperature slows down the hydrolysis of pectin. From above findings it is evident that lower and higher temperature conditions inhibit growth of the microorganisms
Table 4. Effect of temperature on endo-pectin lyase production by A. niger and A. flavus on 8th day of incubation.
| Temperature | A. niger | A. flavus |
| 25˚C | 58.06b±0.635 | 45.16c±0.166 |
| 30˚C | 91.32a±0.695 | 47.66b±0.333 |
| 35˚C | 48.0c±0.000 | 58.73a±0.371 |
| 40˚C | 37.0d±0.577 | 42.83d±0.440 |
| 45˚C | 37.0d±0.577 | 41.80e±0.200 |
| Control | 37.0d±0.577 | 13.03f±0.333 |
Values are significant at P< 0.005
Effect of temperature on PME production: Incubation temperature is the most important physical factor which affects enzyme production dramatically and their stability. Maximum PME activity was found at 30oC (0.043 meq. of NaOH consumed/min/ ml) by A. niger followed by A. flavus produced PME at 35oC (0.035 meq. of NaoH consumed/min/ml ). Least enzyme production was recorded at 45oC (0.020 meq. of NaOH consumed/min/ml) by A. niger (Table 5). Gummadi et al. (2007) reported that an optimum temperature of 30°C for pectin lyase production with A. niger NCIM548. Our strain of A. niger is a mesophilic fungus and therefore it can grow in the range of temperature between 25°C to 40°C. There was no detectable growth of the microorganism above 45°C. Optimum temperature for PG of A. fumigatus by Phutela et al. (2005) was reported to be 50ºC. Thakur et al. (2010) reported the same for Mucor circinelloides and the optimum temperature was found to be 42ºC. Variations of these results are also studied by Patil and Chaudhari (2012), where the optimum temperature for PG production was found to be 35ºC.
Table 5. Effect of temperature on PME production by A. niger and A. flavus on 8th day of incubation.
| Temperature | A. niger | A. flavus |
| 25˚C | 0.031b±0.001 | 0.020cd±0.000 |
| 30˚C | 0.043a±0.001 | 0.031ab±0.001 |
| 35˚C | 0.028b±0.001 | 0.035a±0.002 |
| 40˚C | 0.022c±0.002 | 0.025bc±0.002 |
| 45˚C | 0.020c±0.000 | 0.025bc±0.002 |
| Control | 0.020c±0.000 | 0.020cd±0.000 |
Values are significant at P< 0.005
Effect of pH on exo-pectinase production: Some of the various parameters, the foremost one studied were the pH. Every organism has its own pH. Furthermore to this the production of enzymes also depends on the pH of the medium. Hence pH is a critical factor in the production of microbial enzymes. In the present study hydroxide salts and hydrochloric acids were used for control of pH in the fermentation medium for the production of polygalacturonase. pH of medium have great influence on enzyme production to assess the effect of pH on pectinase production medium was adjusted at 2.0 to 8.0 and incubated at an incubator shaker under submerged fermentation. After incubation fermented medium was centrifuged and supernatant was analyzed for pectinase production.
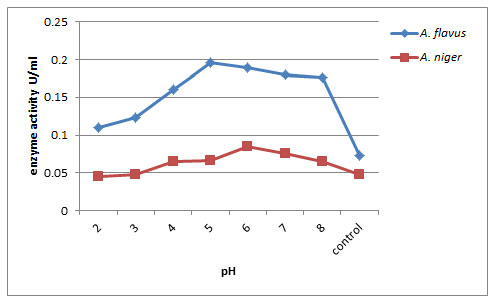
Exo-pectinase production was tested when the organism was cultured at different pH ranging from 2.0 – 8.0. As shown in fig. 1, Production began at pH 2.0, increased gradually and showed optimum at pH 5.0 and pH 6.0 and decreased subsequently. The optimum pectinase production by A. flavus was observed at pH 5 (0.196U/ml) whereas A. niger produced exo-pectinase at pH 6.0 (0.085U/ml). This can be an indication that the isolate requires acidic conditions for its optimum enzyme production. A. flavus was a good producer in exo-pectinase comparatively than A. niger. El Garhy, and Azzaz et al. (2020) reported that the pectinase production by Aspergillus terreus grown on different pH of beet pulp powder medium (BPPM) showed its highest values at pH 4.0. Medium initial pH has great effect on the microbial growth, cell osmotic pressure, nutrient uptake and enzymes production and secretion and concluded that optimum pectinase activity has been given from different fungus within the acidic pH range (Kholif et al. 2018).
Figure 1: Effect of pH on exo-pectinase production by A. flavus and A. niger on 12th day of incubation.
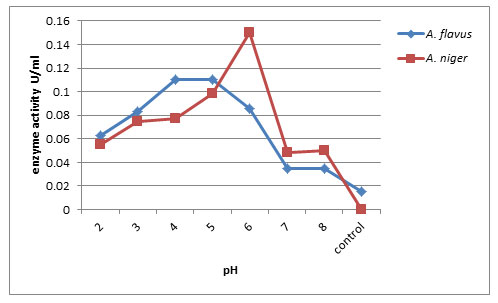
Effect of pH on exo-PG production: The exo-polygalacturonase production was optimized by supplementation using different pH range medium from 2.0 to 8.0 (Fig. 2). In submerged fermentation, maximum pectinase production by A. niger was noticed at pH 6.0 (0.150U/ml) followed by A. flavus at pH 5.0 (0.110U/ml) on 12th day of incubation respectively. Least enzyme production was recorded at pH 8.0 (0.035U/ml) and control (0.015U/ml) by A. flavus. Ahmed et al. (2020) found that the pectinase activity was drastically decreased when enzymatic reaction was carried out at a pH higher than 5 and towards alkaline side, although, a slight increment in the activity was observed at pH 7.5. The present result is similar with the results of (Panda et al. 2012) who observed high pectinolytic activity (0.195U/ml) by Aspergillus flavus at pH 6.0. The pectinase activity of 23.9EU/ml was exhibited by FW5 isolate (Mehta et al. 2013) and by Penicillium chrysogenum (Laha et al. 2014) at a pH of 6.0, which is similar to the present work.
Figure 2: Effect of pH on Exo-PG production by A.flavus and A. niger on 12th day of incubation.
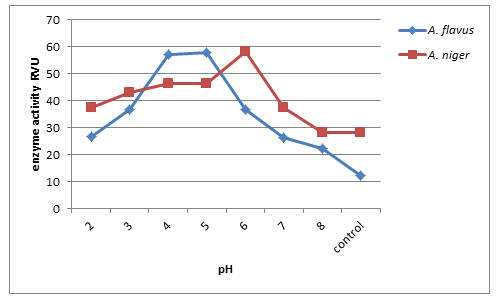
Effect of pH on endo-PG production: Endo-pectinase activity of A. niger and A. flavus was checked at different pH (2.0 – 8.0). Maximum activity of enzyme produced by A. niger was observed at pH 6.0 (58.03 RVU) followed by A. flavus at pH 5.0 (58.10 RVU) and minimum endo-pectinase production at control (12.20RVU). Thus pH 5.0 and pH 6.0 are considered as optimum pH for enzyme production (Fig. 3). Kamalambigeswari et al. (2018) reported the effect of pH on the production of pectinase. The optimum pH revealed that the enzyme was highly active at pH 5.5. The reduction of pectinase enzyme activity after increasing medium pH level is may be due to occurring partial or irreversible denaturation in the enzyme protein (Khattab et al., 2019)
Figure 3: Effect of pH on Endo-PG production by A. flavus and A. niger on 8th day of incubation.
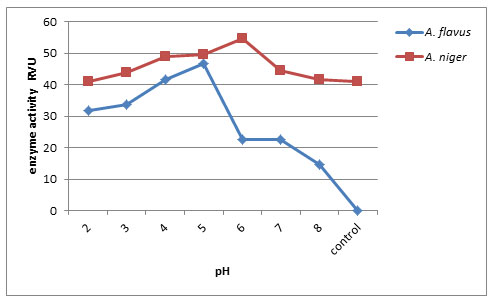
Effect of pH on endo-pectin lyase production: Fig. 4, shows that initial pH of 6.0 yielded maximum endo-pectin lyase activity of about (54.86RVU) by A. niger whereas A. flavus produced at pH 5.0 (46.66RVU) enzyme activity. Minimum enzyme activity produced at pH 8 by A. flavus (14.66RVU). Furthermore, optimal pH is important for microbial growth and their metabolic activities. Since the metabolic activities of the microorganisms are quite sensitive to changes in pH, pectinase production by A.flavus was affected by varying pH values of the medium. Ketipally and Ram (2018) found that the optimum pH was studied by using varied pH conditions in different flasks ranging from pH 4-9 for the production of pectinase by Aspergillus oryzae RR103. The enzyme activity of Aspergillus oryzae RR103 begins at pH 4.0 and slightly increased at pH 5.0, the maximum enzyme production 2.071U/ml at pH 6.0 finally stable at pH 6.5.
In Thermoascus aurantiacus maximum activity was reported at pH 5.0 by Martin et. al (2004). (Muthuprakash and Abraham 2011) found pH 5 was the optimal culture condition for enzyme production by fungal strains including Aspergillus sp. under solid state fermentation.
Figure 4: Effect of pH on endo-pectin lyase production by A.flavus and A. niger on 8th day of incubation.
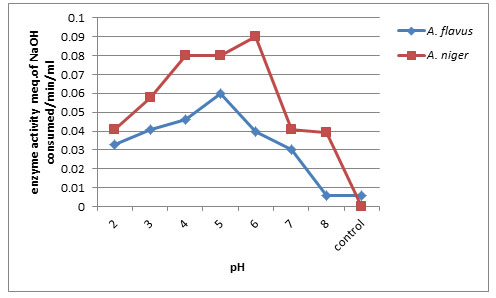
Effect of pH on PME production : Data from the Fig. 5, reveals that pH of 6.0 yielded maximum PME activity of about (0.090 Meq. of NaOH consumed/min/ml) by A. niger followed by A. flavus at pH 5.0 (0.060 Meq. of NaOH consumed/min/ml) and least enzyme production at pH 8.0 (0.006 Meq. of NaOH consumed/min/ml) by A. flavus. A. niger could fail to produce PME in control. The enzyme activity decreased with increase in pH of the substrate. El Enshasy et al. (2018) reported that the pH in the uncontrolled cultivation dropped from 5.5 to about 3.6 after 18h, and remained more or less constant until the end of the cultivation. In Mucor circinelloides ITCC 6025 maximum pectinases activity was observed at pH 4.0 (Thakur et al. 2010). Our findings are in similar with the works of Ellaiah et. al. (2002) who found pH 5.0 was the optimal culture condition for enzyme production by fungal strains including Aspergillus sp.
Figure 5: Effect of pH on PME production by A.flavus and A. niger on 8th day of incubation.
The maximum enzyme activity was observed with an initial pH of 6.5. pH range of 5.5-6.5 has been reported for maximum polygalacturonase production from A. niger (Acuna Arguelles et al. 1995), pH 3.5-5.5 for polygalacturonase production by Mucor and A. flavus and pH 4.5-6.0 for polygalacturonase production by A. awamori (Abbasi and Mortazavipur et. al. 2011).Kunte and Shastri (1980) reported similar results where maximum PG activity was observed at pH 4.4 and 8.6 for Alternaria alternata. It may be due to the presence of two isoenzymes of PG. Variations of these results were studied in Penicillium frequentans (Said et al. 1991) where maximum pectinase production was at initial pH 2.5 and A. niger CH-Y-1043. Aguilar et al. (1991) showed maximum production at pH 2.5.
CONCLUSION
In conclusion, it can be concluded that A. niger isolate is a good source of pectinase which is active at pH 6.0 and at temperature 30˚C whereas A. flavus showed maximum enzyme production at pH 5.0 and at temperature 35˚C. As there is need of bulk production of enzymes at a cost effective rate and in order to meet this goal, such strategies should be explored by which cost-efficient and ecofriendly method for bulk production can be achieved. In this study, a very assiduous and all-embracing optimization steps are carried out. The production of pectinase was enhanced good fold in submerged fermentation. The potential of agricultural wastes for the production of pectinase using submerged fermentation is highlighted in this study for the highest productivity of pectinase from A. niger and A. flavus on submerged fermentations. This result conveys the much economized production of pectinase. Attempts should also be made to adapt the enzyme to conditions which make it more useful in terms of commercial applicability in industries such as juice industry, paper, textiles and tea. This study discovers one of economic ways for pectinase production by A. niger and A. flavus under the optimum fermentation conditions using wheat bran as cheap substrate. The newly produced pectinase enzyme may give feed factories highly effective product with low cost. Application of the newly produced pectinase enzyme in enrichment of the feeding value of diary animal’s diet can be useful for animal’s breeders who suffering high prices of traditional feed stuff.
ACKNOWLEDGEMENTS
The authors would like to thank the Head, Department of Microbiology, Kakatiya University, Warangal for facilities and encouragement.
REFERENCES
Abbasi, H., Mortazavipour, S. R., & Setudeh, M. (2011). Polygalacturonase (PG) production by fungal strains using agro-industrial bioproduct in solid state fermentation. Chemical Engineering Research Bulletin, 15(1), 1-5.
Abd El Tawab, A.M., Murad, H.A., Khattab, M.S.A., & Azzaz. H.H. (2019). Optimizing production of tannase and in vitro evaluation on ruminal fermentation, degradability and gas production. Int. J. Dairy Sci., 14: 53-60.
Acuña-Argüelles, M. E., Gutierrez-Rojas, M., Viniegra-González, G., & Favela-Torres, E. (1995). Production and properties of three pectinolytic activities produced by Aspergillus niger in submerged and solid-state fermentation. Applied microbiology and biotechnology, 43(5), 808-814.
Aguilar, G., Trejo, B. A., García, J. M., & Huitrón, C. (1991). Influence of pH on endo-and exo-pectinase production by Aspergillus sp. CH-Y-1043. Canadian journal of microbiology, 37(12), 912-917.
Ahmed, A., & Sohail, M. (2020). Characterization of pectinase from Geotrichum candidum AA15 and its potential application in orange juice clarification. Journal of King Saud University-Science, 32(1), 955-961.
Ahmed, I., Zia, M.A., Hussain, M.A., Akram, Z., Naveed, M.T. & Nowrouzi, A. (2015). Bioprocessing of citrus waste peel for induced pectinase production by Aspergillus niger; its purification and characterization. J. Radiat. Res. Appl. Sci. 9, 1-7.
Bailey, M. J., & Pessa, E. (1990). Strain and process for production of polygalacturonase. Enzyme and microbial technology, 12(4), 266-271.
Bhardwaj, V. & Garg, N. (2014). Production, Purification of Pectinase from Bacillus sp. MBRL576 Isolate and its Application in Extraction of Juice. Int. J. of Sci. Res. 3(6), 648-652.
Celestino, S. M. C., De Freitas, S. M., Medrano, F. J., De Sousa, M. V., & Ferreira Filho, E. X. (2006). Purification and characterization of a novel pectinase from Acrophialophora nainiana with emphasis on its physicochemical properties. Journal of biotechnology, 123(1), 33-42.
Chadha, R., Kumbhar, B.K. & Sarkar, B.C. (2003). Enzymatic hydrolysis of carrot for increased juice recovery. J. Food Sci. Technol. 40, 35-39.
Dominguez, H., Nunez, M.J. & Lema, J.M. (1994). Enzymatic pretreatment to enhance oil extraction from fruits and oil seeds: A review. Food Chem. 49, 271-286.
El Enshasy, H. A., Elsayed, E. A., Suhaimi, N., Malek, R. A., & Esawy, M. (2018). Bioprocess optimization for pectinase production using Aspergillus niger in a submerged cultivation system. BMC biotechnology, 18(1), 71.
El Garhy, G. M., Azzaz, H. H., Abd El Mola, A. M., & Mousa, G. A. (2020). Fungal Pectinase Production Optimization and its Application in Buffaloe’s Diets Degradation.
Ellaiah P, Prabhakar T, Ramakrishna B, Taleb A.T., & Adinarayana K (2002). Strain improvement of Aspergillus niger for the production of lipase. Indian J. Microbiol. 42: 151-153.
Gummadi, S. N., Kumar, S., & Aneesh, C. N. A. (2007). Effect of salts on growth and pectinase production by halotolerant yeast, Debaryomyces nepalensis NCYC 3413. Current microbiology, 54(6), 472-476.
Gupta, R., & Kalpana. (2011). Optimization of production and reaction conditions of polygalacturonase from Byssochlamys fulva. Acta microbiologica et immunologica Hungarica, 58(4), 339-349.
Jayani, R.S., Saxena, S., & Gupta, R. (2005). Microbial pectinolytic enzymes: A review. Process Biochem. 40 (9), 2931-2944.
Kamalambigeswari, R., Yadav, S. A., Sivaswamy, N., & Ushani, U. (2018). Isolation, identification, screening and optimisation of pectinase producing soil fungi (Aspergillus niger). International Journal of Research in Pharmaceutical Sciences, 9(3).
Kaur, G., Kumar, S., & Satyanarayana, T. (2004). Production, characterization and application of a thermostable polygalacturonase of a thermophilic mould Sporotrichum thermophile Apinis. Bioresource Technology, 94(3), 239-243.
Kertesz , (1955). Z.I; In methods in enzymology/K. Colowick, Academic Press, New York, Vol, I,p.159.
Ketipally, R., & Ram, M. R. (2018). Optimization of pectinase production by Aspergillus oryzae RR 103. Current agriculture research journal, 6(1), 37-44.
Ketipally, R., Kumar, G. K., & Ram, M. R. (2019). Polygalacturonase production by Aspergillus nomius MR103 in solid state fermentation using Agro-industrial wastes. Journal of Applied and Natural Science, 11(2), 305-310.
Khattab, M.S.A., Azzaz, H.H., Abd El Tawab, A. M., & Murad, H.A., (2019). Production optimization of fungal cellulase and its impact on ruminal degradability and fermentation of diet. Int. J. Dairy Sci., 14: 61-68
Kholif, A.E., Kassab, A.Y., Azzaz, H.H., Matloup, O.H., Hamdon, H.A., Olafadehan, O.A., & Morsy, T.A. (2018). Essential oils blend with a newly developed enzyme cocktail works synergistically to enhance feed utilization and milk production of Farafra ewes in the subtropics. Small Rumin. Res., 161: 43-50.
Kumar, Y.S., Vijayakumar, P., & Reddy, O.V.S. (2012). Pectinase production from mango peel using Aspergillus foetidus and its application in processing of mangojuice. Food Biotechnol. 26, 107-123.
Kunte, S., & Shastri, N. V. (1980). Studies on extracellular production of pectolytic enzymes by a strain of Alternaria alternata. Indian Journal of Microbiology, 20(3), 211-215.
Laha, S., D. Sarkar & Chaki, S. (2014). Optimization of production and molecular characterization of pectinase enzyme produced from Penicillium chrysogenum. Scholar Academic Journal of Biosciences, 2(5): 326-335.
Lonsane, B. K., Ghildyal, N. P., Budiatman, S., & Ramakrishna, S. V. (1985). Engineering aspects of solid state fermentation. Enzyme and Microbial Technology, 7(6), 258-265.
Martin, N., Souza, S. R. D., Silva, R. D., & Gomes, E. (2004). Pectinase production by fungal strains in solid-state fermentation using agro-industrial bioproduct. Brazilian Archives of Biology and Technology, 47(5), 813-819.
Mathew, A., Eldo, A. N., & Molly, A. G. (2008). Optimization of culture conditions for the production of thermostable polygalacturonase by Penicillium SPC-F 20. Journal of industrial microbiology & biotechnology, 35(9), 1001-1005.
Mehta, S. A., Mitali, R., Nilofer, S., & Nimisha, P. (2013). Optimization of physiological parameters for pectinase production from soil isolates and its applications in fruit juice clarification. Journal of Environmental Research and Development, 7(4A), 1539-1546.
Miller, G.L. (1959). International Journal of Science and Nature. Use of di-nitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem. 31, 426-428.
Moyo, S., Gashe, B.A., Collison, E.K. & Mpuchane, S. (2003). Optimising growth conditions for the pectinolytic activity of Kluyveromyces wickerhamii by using response surface methodology. Int. J. Food Microbiol. 85, 87-100.
Muthuprakash, K.M.S. & Abraham, J. (2011). A comparative analysis of protease producing microbes isolated from tannery effluent. 2(1), 110-113.
Panda, S. S., Sahoo, K., Das, R., & Dhal, N. K. (2012). Pectinolytic and cellulolytic activity of soil fungal isolates from Similipal bioreserve forest. World Environment, 2(2), 1-3.
Patil, V., & Chaudari, R. Y. (2012). Spectrophotometric method for estimation of cefpodoxime proxetil and ofloxacin in tablet dosage form by simultaneous equation method. Int J Pharm Life Sci, 3, 1982-1984.
Pedrolli, D.B., Monteiro, A.C., Gomes, E. & Carmona, E.C. (2009). Pectin and pectinases: production, characterization and industrial application of microbial pectinolytic enzymes, The Open Biotechnology Journal. 3, 9-18.
Phutela, U., Dhuna, V., Sandhu, S., & Chadha, B. S. (2005). Pectinase and polygalacturonase production by a thermophilic Aspergillus fumigatus isolated from decomposting orange peels. Brazilian Journal of Microbiology, 36(1), 63-69.
Said, S., Fonseca, M. J. V., & Siessere, V. (1991). Pectinase production by Penicillium frequentans. World Journal of Microbiology and Biotechnology, 7(6), 607-608.
Sandhya, R., & Kurup, G. (2013). Screening and isolation of pectinase from fruit and vegetable wastes and the use of orange waste as a substrate for pectinase production. Int. Res. J. Biol. Sci, 2(9), 34-39.
Sandri, I.G., Fontana, R.C., Barfknecht D.M. & Silveira, M.M. da. (2011). Clarification of fruit juices by fungal pectinases. LWT-Food Science and Technology. 44(10), 2217-2222.
Thakur, A., Pahwa, R., Singh, S., & Gupta, R. (2010). Production, purification, and characterization of polygalacturonase from Mucor circinelloides ITCC 6025. Enzyme Research, 2010.
Thite, V. S., Nerurkar, A. S., & Baxi, N. N. (2020). Optimization of concurrent production of xylanolytic and pectinolytic enzymes by Bacillus safensis M35 and Bacillus altitudinis J208 using agro-industrial biomass through Response Surface Methodology. Scientific Reports, 10(1), 1-12.
Vikari, L. M., Tenkanen, A. & Suuranakki (2001). Biotechnology. In Biotechnology in the pulp and paper industry, Eds., Rehm H-J and G. Reed.VCH-Wiley, pp: 523-546.
Voragen, F.H. & Schols Visser, R. (2004). Advances in pectin and pectinase research. Kluwer Academic Publishers, p: 497.
Willats, W.G.T., Mc Cartney, L., Mackie & Knox W. J. P. (2001). Pectin: cell biology for functional analysis. Plant Mol. Biol. 47, 9-27.
Wood, R.K.S. (1955). Pectic enzymes secreted bypathogens and their role in plant infection. In Mechanism of microbial pathogenicity/J.W. Howle and A.J.O. Hea, University Press, Cambridge. p263-293.