1Department of Biotechnology, Naraina Vidyapeeth Engineering & Management Institute, Kanpur,
2Department of Biotechnology, IET, Lucknow,
3Department of Biochemical Engineering, HBTU, Kanpur
Corresponding author Email: drvinay@yahoo.com
Article Publishing History
Received: 05/07/2019
Accepted After Revision: 07/09/2019
Microbial fuels are the 3rd generation biofuel. These fuels are made by the conversion of microbial lipid into fuel. Rhodotorula glutinis, a pink oleaginous microbe has the capacity to produce microbial lipids from cuture medium containing carbon and nitrogen sources. These microbial lipids can further be converted in to fuel. In this work, the growth conditions for R. glutinis were optimized with different C:N ratios by having various concentrations of Carbon and Nitrogen sources. Glucose, Fructose, Sucrose, Galactose and Xylose were evaluated as carbon sources (70 g/L). (NH4H2PO4, NH4NO3, (NH4)2SO4, Urea, Aspargine were evaluated as Nitrogen source (20g/L). Subsequently, the influence of surfactants (Tween 20 and Tween 80 (0.5 ml/L), pH (3, 4,5) and incubation temperature 0C (25,30,35 and 40) were also analysed in intial media composition. Rhodotorula glutinis showed significant growth with maximum biomass and lipid production in the media containing sucrose as carbon source, NH4NO3 as nitrogenous source at pH 4 and temperature 30 °C. The surfactant has shown no effect in lipid production. Thus, the results of this study indicated that these optimized conditions could be used to produce maximum production of lipids as biodiesel feedstock.
Rhodotorula glutinis, Microbial lipids, Microbial fuels, Oleaginous microbe
Verma G, Anand P, Pandey S, Nagar S, Dwivedi V. Optimization of Cultivation Conditions for Microbial Lipid Production by Rhodotorula Glutinis, an Oleaginous Yeast. Biosc.Biotech.Res.Comm. 2019;12(3).
Verma G, Anand P, Pandey S, Nagar S, Dwivedi V. Optimization of Cultivation Conditions for Microbial Lipid Production by Rhodotorula Glutinis, an Oleaginous Yeast. Biosc.Biotech.Res.Comm. 2019;12(3). Available from: https://bit.ly/31WFp3R
Introduction
The price of fuel rises every year with a worrying concern of depletion and complete loss of the available natural resources. With the idea of sustainable development and cutting the economic values of the fuels other options are like usage of plants, animals, aqua life and microbes are acquitted for research and development. Biofuels, namely bioethanol, butanol produced from cellulose are therefore of considerable interest to various researchers as well as governments (Deman et al., 2005; Hill et al., 2006, Zhou et al., 2014; Choudhary et al., 2017).
Yeast is generally being employed on a large scale yielding vitamins, industrial enzymes and pharmaceutical polypeptides. The oleaginous attribute of yeast renders it advantage over its proponent viz. bacteria, molds, and alga, primarily due to higher rates of proliferation & also due to the propensity to greater lipid yields (Saenge et al., 2011). High degree of lipid accumulation is due to carbon surplus and a nitrogen or some other nutrient deficit in the growth medium. (Ratledge & Boulton, 1985; Ratledge, 1986). Hence fatty acid profile of the microbial oil like the oleaginous yeast makes it potential host for biodiesel industry due to the accumulation of polyunsaturated fatty acid triacylglycerol inside the cells, which is similar to vegetable oils (Dai et al., 2007; Kot et al., 2016).
Jiru et.al., (2017) optimized the biotechnological production of lipid by Rhodotorula kratochvilovae (syn, Rhodosporidium kratochvilovae) SY89 for biodiesel preparation .It could serve as a replacement for conventional oil sources like crude oil replenishing the necessities of the energy sector & also industries like food, pharmaceutical or cosmetic. Rhodotrula glutinis is aerobic yeast characterized by pink, smooth colonies with a moist appearance, sexually reproduce via basidiospores arising from a teliospore developed primarily due to the existence of mycelial clamp connection. Multipolar budding serve as reproductive avenue. The significance of Rhodotorula glutinis yeast is being acknowledged & published in different journals for the production of numerous useful metabolites such as lipid (Li, et al,2013), carotenoids having anticancerous and antioxidant properties ( Gupta et al., 2012: Yen et al., 2015) and many industrially useful enzymes. Rhodotorula glutinis can produce brewery effluent (Yen et al., 2012), crude glycerol (Schneider et al., 2013) ,microbial lipids, carotene, cellulase and carotenoids by using cheap energy sources like carbon, nitrogen, Sulphur, (Karamerou et al., 2016; Pi et al., 2018; Elfeky et al., 2019). In this research Optimization of Microbial lipid production by Rhodotrula glutinis was done by using various carbon and nitrogen substrates at different pH, Temperature and time of cellular growth.
Materials and Methods
Yeast Rhodotorula glutinis strain was obtained from IMTECH (Chandigarh, India). The lyophilized culture was hydrated in medium containing Glucose (70g/l), (NH4)2SO4 (20g/l), a slightly modified basal medium recommended by Bhosale and Gadre (2001).Then a culture was transferred to slant tubes containing medium with the same composition, including 15 g/l agar and incubated at 30o C for 60 hr. After growth, the slants were kept at 4o C and were subcultured each 2 months. The inoculum was prepared in Erlenmeyer flasks of 250-ml with 100 ml of medium.
Optimization of Growth Conditions
The growth conditions for R. glutinis were optimized with different C:N ratios by having various concentrations of Carbon and Nitrogen sources. . Glucose, Fructose, Sucrose, Galactose and Xylose were evaluated as carbon sources (70 g/l) with Ammonium Sulphate (20 g/l) as nitrogen source .NH4H2PO4, NH4NO3, (NH4)2SO4, Urea, Aspargine were evaluated as Nitrogen source (20g/l) with glucose (70g/l) as carbon source. Subsequently, the influence of surfactants (Tween 20 and Tween 80 (0.5 ml/l), pH (3, 4,5) and incubation temperature 0C (25,30,35 and 40) were also analysed in initial media composition.
glutinis were inoculated and incubated at 30°C for 60 Hr. The dry weight of R. glutiniswas measured after each 12Hr. Dry mass of the cell was taken by centrifugation of one ml culture medium and dried the pallet at 80 °C for 12 hr. then the weight of dried mass was taken and converted into g/l.
Isolation of lipid from R. glutinis:Extraction of lipids was done by Soxhlet method in which hexane was used as solvent for the extraction from R. glutinis. The dried yeast was used for the isolation of lipid. The moisture content of the yeast sample should not exceed 10%.Weighed exactly 1 g of yeast sample which was kept on Whatman filter. Covered the top of each thimble with glass wool to prevent floating. Weighed the pre-dried flat-bottom extraction flask with a few boiling chips or glass beads. Extract lipids with 150 to 200 ml of hexane at the boiling point for 7 to 12 h in a Soxhlet extractor using a heating mantle. The condensation rate for the solvent was set at about 2 to 6 drops per second, depending on the extraction period envisaged. For longer extraction periods, a lower condensation rate was selected and vice versa. Usually,an extraction period of 8 hr at a rate of 150 drops per min was considered adequate. The sample was made to cool. Finally solvent was removed from the sample extract using rotary evaporator at 40°C under reduced pressure. Further calculations were performed for the amount of lipid recovered and its percentage in the original sample using following equation: –
Mass of lipid = (weight of the flask + boiling chips + extracted oil) − (weight of the
Flask + boiling chips)
Results and Discussion
The Rhodotorula glutinis was grown on different carbon source (Glucose, Fructose, Sucrose and Xylose) for different incubation time (12, 24, 36, 48, 60 hr.) As we can see from the Table 1 & Figure 1, both carbon source and incubation time play an important role in the production of lipid. The maximum biomass (17.14 g/l) , lipid production (8.82 g/l) and % lipid yield ( 51.45% ) were found with Sucrose in the incubation period of 60 hrs followed by glucose, xylose and fructose.
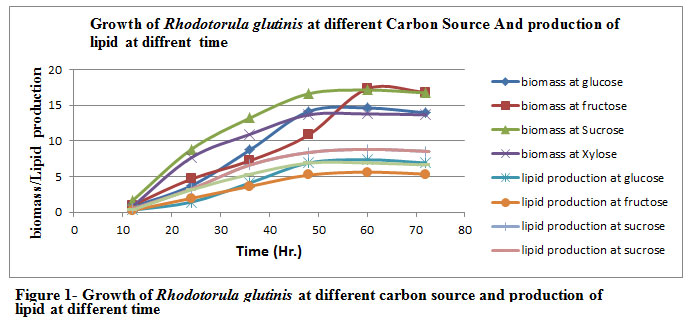 |
Figure 1: Figure 1- Growth of Rhodotorula glutinis at different carbon source and production of lipid at different time |
Table 1: Growth of Rhodotorula glutinis and production of lipid at different carbon sources.
| Carbon Source | Time (Hr.) | Biomass (g/l) | Lipid (g/l) | % Lipid yield |
| Glucose | 12 | 0.76 | 0.29 | 38.15 |
| 24 | 3.71 | 1.41 | 38.00 | |
| 36 | 8.73 | 4.11 | 47.07 | |
| 48 | 14.13 | 6.91 | 48.90 | |
| 60 | 14.63 | 7.36 | 50.30 | |
| 72 | 13.95 | 6.92 | 49.60 | |
| Fructose | 12 | 0.97 | 0.26 | 26.80 |
| 24 | 4.65 | 1.94 | 41.72 | |
| 36 | 7.23 | 3.63 | 50.20 | |
| 48 | 10.84 | 5.21 | 48.06 | |
| 60 | 17.32 | 5.62 | 32.44 | |
| 72 | 16.82 | 5.34 | 31.74 | |
| Sucrose | 12 | 1.64 | 0.47 | 28.65 |
| 24 | 8.81 | 3.25 | 36.88 | |
| 36 | 13.21 | 6.63 | 50.18 | |
| 48 | 16.62 | 8.38 | 50.42 | |
| 60 | 17.14 | 8.82 | 51.45 | |
| 72 | 16.74 | 8.55 | 51.07 | |
| Xylose | 12 | 0.83 | 0.31 | 37.34 |
| 24 | 7.65 | 3.13 | 40.91 | |
| 36 | 10.93 | 5.32 | 48.67 | |
| 48 | 13.66 | 6.89 | 50.43 | |
| 60 | 13.79 | 6.91 | 50.10 | |
| 72 | 13.67 | 6.63 | 48.50 |
Among the various nitrogen sources Table 2 & Figure 2, NH4NO3was the best nitrogen source for production of biomass (17.11 g/l) , lipid production (8.62 g/l) and % lipid yield ( 50.37% ) . Aspargine showed the poorest result.
Table 2: Growth of Rhodotorula glutinis and production of lipid at different nitrogen sources.
| Nitrogen Source | Time (Hr.) | Biomass (g/l) | Lipid Content(g/l) | Lipid Yield (%) |
| NH4H2PO4 | 12 | 1.21 | 0.31 | 25.61 |
| 24 | 5.62 | 2.19 | 38.96 | |
| 36 | 11.63 | 5.08 | 43.68 | |
| 48 | 14.25 | 7.11 | 49.89 | |
| 60 | 14.83 | 7.49 | 50.5 | |
| 72 | 14.52 | 7.29 | 50.2 | |
| NH4NO3 | 12 | 1.93 | 0.61 | 31.6 |
| 24 | 8.65 | 4.09 | 47.28 | |
| 36 | 12.82 | 6.32 | 49.29 | |
| 48 | 16.89 | 8.53 | 50.5 | |
| 60 | 17.11 | 8.62 | 50.37 | |
| 72 | 16.79 | 8.41 | 50.08 | |
| (NH4)2SO4 | 12 | 1.21 | 0.51 | 42.14 |
| 24 | 3.18 | 1.21 | 38.05 | |
| 36 | 5.62 | 2.31 | 41.1 | |
| 48 | 8.93 | 4.31 | 48.26 | |
| 60 | 9.32 | 4.19 | 44.95 | |
| 72 | 9.12 | 3.96 | 43.42 | |
| Urea | 12 | 0.919 | 0.27 | 29.37 |
| 24 | 8.82 | 3.89 | 44.1 | |
| 36 | 9.12 | 4.19 | 45.94 | |
| 48 | 9.16 | 4.41 | 48.14 | |
| 60 | 10.14 | 4.62 | 45.56 | |
| 72 | 9.83 | 4.3 | 43.74 | |
| Aspargine | 12 | 0.33 | 0.06 | 18.18 |
| 24 | 0.81 | 0.31 | 38.27 | |
| 36 | 1.96 | 0.68 | 34.69 | |
| 48 | 3.92 | 1.56 | 39.79 | |
| 60 | 5.72 | 2.22 | 38.81 | |
| 72 | 5.65 | 2.15 | 38.05 |
Various nitrogenous sources are taken for the optimization of cultivation conditions viz. ammonium dihydrogen phosphate, ammonium nitrate, ammonium sulphate, urea & aspargine in which the best possible nitrogenous substrate was found out to be ammonium nitrate ( NH4NO3 ) at an optimal temperature of 300 C at the 60th hour incubation as far the microbial proliferation with respect to lipid yield is concerned.
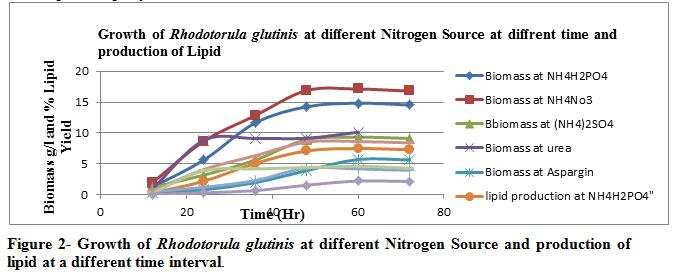 |
Figure 2: Figure 2- Growth of Rhodotorula glutinis at different Nitrogen Source and production of lipid at a different time interval. |
The cell dry weight increased gradually with an increase in the pH of the broth (at pH 3 & 4) later got decreased by increasing the pH ( pH 5). The optimum pH were found to be 4 & 3 as per Table 3 & Figure 3 for maximum yield of biomass, lipid production and % of lipid yield.
Table 3: Growth of Rhodotorula glutinis and production of Lipid at different pH and different time interval.
| S. No. | pH of the media | Time Duration
Hr. |
Biomass
g/l |
Lipid production g/l | Lipid Yield % |
| 1 | 3 | 12 | 0.61 | 0.23 | 37.70 |
| 24 | 2.93 | 1.15 | 39.24 | ||
| 36 | 7.63 | 3.43 | 44.95 | ||
| 48 | 14.22 | 7 | 49.22 | ||
| 60 | 15.61 | 7.97 | 51.05 | ||
| 72 | 15.34 | 7.4 | 48.23 | ||
| 2 | 4 | 12 | 1.92 | 0.72 | 37.50 |
| 24 | 4.32 | 2.17 | 50.23 | ||
| 36 | 12.33 | 6.12 | 49.63 | ||
| 48 | 17.61 | 8.92 | 50.65 | ||
| 60 | 18.98 | 9.68 | 51.00 | ||
| 72 | 18.56 | 9.32 | 50.21 | ||
| 3 | 5 | 12 | 1.62 | 0.41 | 25.30 |
| 24 | 9.82 | 4.13 | 42.05 | ||
| 36 | 11.31 | 5.67 | 50.13 | ||
| 48 | 16.13 | 8.12 | 50.34 | ||
| 60 | 16.92 | 8.42 | 49.76 | ||
| 72 | 16.82 | 8.32 | 49.46 |
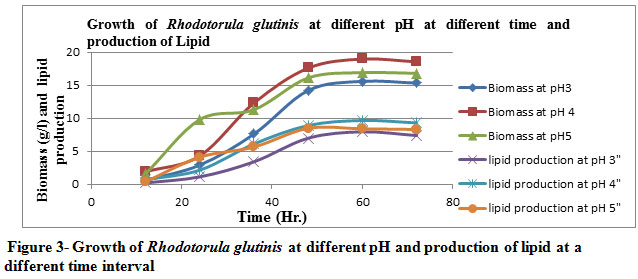 |
Figure 3: Growth of Rhodotorula glutinis at different pH and production of lipid at a different time interval |
R. glutinis was able to grow at all the temperatures examined 0C (25, 30, 35, 40). A considerably increased biomass (18.65 g/L) 30oC; whereas enhancement of temperature 350C to 40oC causes reduction in the biomass. The reduction in the biomass primarily is an indicator of microbial competency to withstand the temperature alterations (Table 4 and Figure 4).Biomass produced efficiently at 30oC suggests that the rate of replication has increased significantly during the or in other words the doubling time of oleaginous organism has decreased vis-à-vis to all the other incubation temperature. The sampling time of incubated organism in order to assess the biomass yield as well as the lipid content is strategically being conceived at a time interval of 12 hours depending upon the microbial growth curve of the organism.
Table 4: Growth of Rhodotorula glutinis and production of Lipid at different Temperature and different time interval.
| S. No. | Temp. (C) | Time Duration(Hr.) | Biomass (g/l) | Lipid Content(g/l) | Lipid Yield % |
| 1. | 25 | 12 | 1.01 | 0.28 | 27.72 |
| 24 | 13.13 | 3.72 | 28.33 | ||
| 36 | 14.99 | 4.43 | 29.55 | ||
| 48 | 16.43 | 5.43 | 33.04 | ||
| 60 | 17.34 | 6.32 | 36.44 | ||
| 72 | 16.98 | 6.12 | 36.04 | ||
| 2. | 30 | 12 | 1.21 | 0.35 | 28.92 |
| 24 | 15.08 | 7.18 | 47.61 | ||
| 36 | 15.98 | 8.17 | 51.12 | ||
| 48 | 18.32 | 9.24 | 50.43 | ||
| 60 | 18.65 | 9.41 | 50.45 | ||
| 72 | 18.43 | 9.21 | 49.97 | ||
| 3. | 35 | 12 | 1.09 | 0.31 | 28.44 |
| 24 | 2.93 | 1.29 | 44.02 | ||
| 36 | 13.17 | 6.48 | 49.20 | ||
| 48 | 14.68 | 7.32 | 49.86 | ||
| 60 | 15.73 | 7.95 | 50.54 | ||
| 72 | 15.21 | 7.12 | 46.81 | ||
| 4. | 40 | 12 | 0.66 | 0.21 | 31.81 |
| 24 | 4.32 | 2.22 | 51.38 | ||
| 36 | 8.11 | 3.94 | 48.58 | ||
| 48 | 12.13 | 5.93 | 48.88 | ||
| 60 | 12.56 | 6.45 | 51.35 | ||
| 72 | 12.09 | 6.38 | 52.77 |
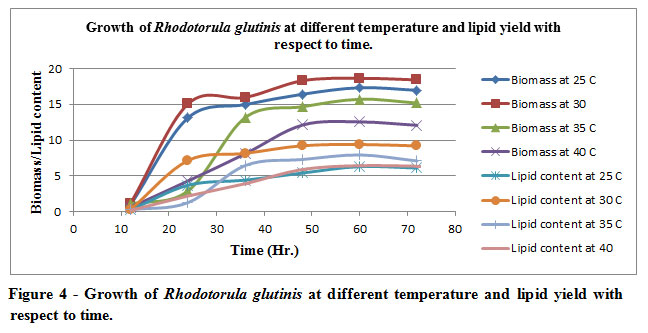 |
Figure 4: Growth of Rhodotorula glutinis at different temperature and lipid yield with respect to time. |
The illustration in the graphs primarily suggests the data which is being tabulated. The rate of production of the biomass is directly correlated lipid yielding efficiency of the biomass. As one can easily decipher from the depiction maximal biomass yield is being acquired at optimal mesophilic temperature i.e., 30oC, invariably also suggests the ratio of production of biomass vis-à-vis lipid content which is 1:2
The effect of surfactant (0.5ml/l) on the growth of R. glutinis was measured at different time intervals and observed that surfactants did not effected much. The production of Biomass and lipid were almost same for both the surfactants ( Table 5 & figure 5)
Table 5: Growth of Rhodotorula glutinisat different surfactant and production of lipid at different time interval
| S.No | Surfactant | Time (hr.) | Biomass (g/l) | Lipid production (g/l) | Lipid content (%) |
| 1 | Tween 20 | 12 | 0.95 | 0.21 | 22.10 |
| 24 | 6.52 | 2.91 | 44.63 | ||
| 36 | 11.62 | 5.24 | 45.09 | ||
| 48 | 13.73 | 6.36 | 46.32 | ||
| 60 | 14.81 | 7.23 | 48.81 | ||
| 72 | 14.65 | 7.09 | 48.39 | ||
| 2 | Tween 80 | 12 | 0.81 | 0.17 | 20.98 |
| 24 | 6.66 | 2.84 | 42.64 | ||
| 36 | 11.38 | 4.15 | 36.46 | ||
| 48 | 13.84 | 6.15 | 44.43 | ||
| 60 | 14.38 | 6.9 | 46.52 | ||
| 72 | 14.22 | 6.3 | 44.30 |
The table illustrates the implication of two surfactants being used viz. tween 20 & tween 80 which had no negative or positive effect on the biomass yield with respect to the lipid content. Hence one can imply the microbial culture or lipid yield remain unaffected with the aforementioned concentration (0.5ml/l) of surfactants being used
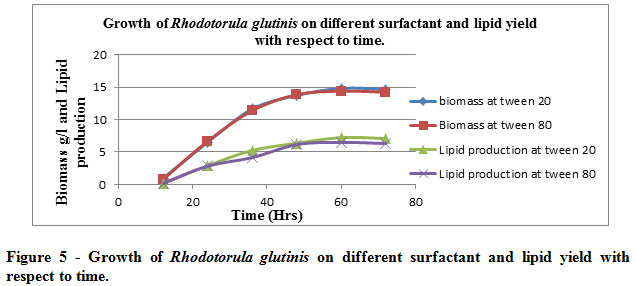 |
Figure 5: Growth of Rhodotorula glutinis on different surfactant and lipid yield with respect to time. |
The graphical illustrations of tabulated data is indicative of the fact aforesaid i.e., surfactant remains inert to the microbial proliferation & hence lipid yield unaffected, hence the indictment for the biomass production & lipid yield remains stable as for as the exposure to surfactant is concerned.
When R. glutinis was incubated with the medium containing sucrose as a carbon source (70 g/l), NH4NO3 as a nitrogen source(20 g/l) , yeast (15g/l) at 30̊0C with pH 4 . The yield of lipid was increased and reached 57.23% of total dry biomass with inoculums size 6%. (Table 6 and Figure 6)
Table 6: The growth of Rhodotorula glutinis at the optimized condition, and effect of inoculums size and production of lipid at a different time interval
| Media | pH | Temperature | Inoculum Size (%) | Biomass after 60 hrs of incubation (g/l) | Lipid production (g/l) | %Yield of lipid |
| 70 g/l Sucrose |
4 |
30 ̊C |
5
|
19.5 | 10.3 | 52.82 |
| 20 g/l NH4NO3 | 6 | 22.2 | 12.6 | 57.27 | ||
| 15 g/l Yeast Extract | ||||||
| 7 g/l KH2PO4 | ||||||
| 1 g/l MgSO4 | 7 | 20.1 | 10.7 | 53.23 | ||
| 15 g/l Agar |
Again the the table illustrates the significance of the optimized inoculum size with respect to optimal temperature & media components making growth maximal to its competency. The maximal biomass yield is achieved at pH 4 indicative of the operability of oleaginous organism to produce lipid under the influence of the acidic condition.Moving towards the alkaline scale the rate of microbial profillation decrese & withit the lipid content is invariably effected. The best possible microbial proliferation & lipid content is being percieved for the sucrose as the carbon substrate followed by glucose & fructose. Xylose is least catabolized substrate as far as microbial replication & lipid production is concerned
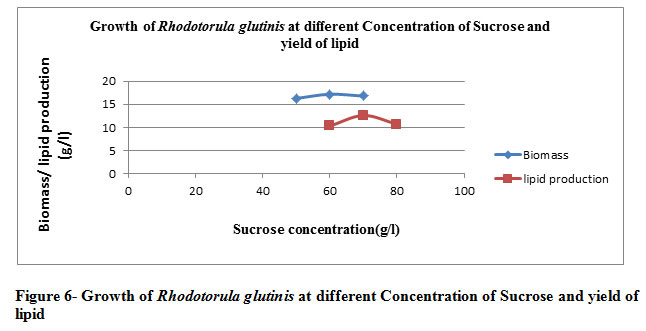 |
Figure 6: Growth of Rhodotorula glutinis at different Concentration of Sucrose and yield of lipid. |
Conclusion
In this present work, we optimize the growth conditions of Rhodotorula glutinis to get maximum production of Biomass and lipid content. Sucrose was found to be the best carbon source (70 g/l), NH4NO3 was found to be best nitrogen source (20g/l) with optimum pH 4, optimum temperature 300C and optimum time period 60 hrs. Surfactant does not have any effect on the growth and lipid production of Rhodotorula glutinis. This study reveals that cultivation conditions had an influence on lipid production. This study provides a valid and potential strategy for optimizing yeast cultivation conditions to improve the production of lipid with possibly broad biotechnological applications.
References
Bhosale, P. B. and Gadre, R.V. (2001). Production of ß-carotene by mutant of Rhodotorula glutinis. . Appl. Microbiol. Biotechnol., 55:423-427
Choudhary J, Singh S, Nain L.(2017). Bioprospecting thermotolerant ethanologenic yeasts for simultaneous saccharification and fermentation from diverse environments. J Biosci Bioeng. 123(3):342–346.
Dai, C-c.,Tao, J., Xie, F., Dai, Y.-j. & Zhao, M. (2007). Biodiesel generation from oleaginous yeast Rhodotorula glutinis with xylose assimilating capacity. African Journal of Biotechnology 6 (18), 2130-2134 .
Demain AL, Newcomb M, Wu JHD. (2005).Cellulase, Clostridia, and ethanol. Microbiol Mol Biol Rev.69(1):124–54.
Elfeky Nora, Elmahmoudy Mostafa, Zhang Yue, Guo JianLi, Bao Yongming (2019). Lipid and Carotenoid Production by Rhodotorula glutinis with a Combined Cultivation Mode of Nitrogen, Sulfur, and Aluminium Stress. Applied Sciences.9, 2444.
Gupta, A., Vongsvivut, J., Barrow, C. J. & Puri, M.(2012) .Molecular identification of marine yeast and its spectroscopic analysis establishes unsaturated fatty acid accumulation. Journal of Bioscience and Bioengineering 114, 411–417.
Hill J, Nelson E, Tilman D, Polasky S, Tiffany D.(2006). Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc Natl Acad Sci USA. 103(30):11206–10
Jiru Tamene Milkessa, Groenewald Marizeth, Pohl Carolina, Steyn Laurinda, Kiggundu Nicholas, Abate Dawit (2017). Optimization of cultivation conditions for biotechnological production of lipid by Rhodotorula kratochvilovae (syn, Rhodosporidium kratochvilovae) SY89 for biodiesel preparation. 3 Biotech.7(2): 145.
Karamerou, E. E., Theodoropoulos, C. & Webb, C. (2016)A biorefinery approach to microbial oil production from glycerol by Rhodotorula glutinis. Biomass and Bioenergy 89, 113–122.
Kot, A. M., Błażejak, S., Kurcz, A., Gientka, I. & Kieliszek, M. (2016). Rhodotorula glutinis—potential source of lipids, carotenoids, and enzymes for use in industries. Applied Microbiology and Biotechnology 100, 6103–6117
Li Z, Sun H, Mo X, Li X, Xu B, Tian P (2013).Overexpression of malic enzyme (ME) of Mucor circinelloides improved lipid accumulation in engineered Rhodotorula glutinis. Applied Microbiology and Biotechnology 97, 4927–4936
Pi Hong-Wei, Anandharaj Marimuthu, Kao Yi-Ying, Chang Jui-Jen, Li Wen-Hsiung (2018). Engineering the oleaginous red yeast Rhodotorula glutinis for simultaneous β-carotene and cellulase production. Scientific Reports. 8 (1), 10850
Raltedge, C.& Boulton, C. A. (1985). Fats and Oils. In Comprehensive Biotechnology,. 3, 983-1 003. Edited by S. Drew, D. I. C. Wang & H. Blanch. Oxford : Pergamon Press
Raltedge, C. (1986). The potential of micro-organisms for oil production – a review of recent publications. In Proceedings of the World Conference on Emerging Technologies in the Fats and Oils Industry, 318-330. Edited by A. R. Baldwin. Champaign, Illinois : American Oil Chemists’ Society
Saenge, C., Cheirsiep, B., Suksaroge, T. T. & Bourtoom, T. (2011). Potential use of oleagenous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 46: 210-218.
Schneider, T. & Graeff-Hönninger, S. & French, W.T. & Hernandez, R. & Merkt, N. & Claupein, W. & Hetrick, M. & Pham, P( 2013). Lipid and carotenoid production by oleaginous red yeast Rhodotorula glutinis cultivated on brewery effluents, Energy, 61(C), 34-43.
Yen, H.-W., Yang, Y.-C. & Yu, Y.-H.(2012). Using crude glycerol and thin stillage forthe production of microbial lipids through the cultivation of Rhodotorula glutinis. Journal of Bioscience and Bioengineering 114, 453–456
Yen, H.-W., Liao, Y.-T. & Liu, Y. X.(2015). The growth of oleaginous Rhodotorula glutinis in an airlift bioreactor on crude glycerol through a non-sterile fermentation process. Bioprocess and Biosystems Engineering 38, 1541–1546.
Zhou H, Cheng JS, Wang BL, Fink GR, Stephanopoulos G. (2012).Xylose isomerase overexpression along with engineering of the pentose phosphate pathway and evolutionary engineering enable rapid xylose utilization and ethanol production by Saccharomyces cerevisiae. Metab Eng.14(6):611–22.


