Parasitology and Microbiology Research Laboratory, Department of Zoology, Burdwan University, Purba Bardhaman 713104, West Bengal, India
Corresponding author Email: soumen.microbiology@gmail.com
Article Publishing History
Received: 02/07/2019
Accepted After Revision: 07/09/2019
The tape worm, Raillietina tetragona (Molin 1858) is a common parasite of domestic fowl, Gallus domesticus in West Bengal. This cestode parasite plays a significant role in growth and reproduction of fowl. In the current study, Light microscopic (LM) examination showed
that the present cestode has a minute pin like scolex or head (18.98µm – 19.76 µm) and a long flat body divided into immature, mature and gravid proglottids. Head bears a rostellum (2.94 -3.11µm), four acetabula (6.79µm – 7.4µm) and followed by a neck (15.1µm – 16.27µm).Seasonal variation of R. tetragona from Gallus domesticus in Purba Bardhaman, West Bengal, India has been studied through two years span (January, 2016 to December, 2017). Monthly variations of infection of Raillietina tetragona showed comparatively higher prevalence in hot summer months (58.33 – 70 %) and humid rainy season (80-95.24%) and lower prevalence during winter season (0 – 10%). Mean intensity of Raillietina tetragonawas also higher in summer (1-2.5) and rainy season (3.36-10.2). The present study on the morphology and seasonal prevalence of Raillietina tetragona in Purba Bardhaman region of West Bengal showed that more awareness should be focused towards upgrading and maintenance of the local free ranging chicken breeds.
Raillietina tetragona, Gallus domesticus, Light microscopic observations, prevalence, Purba Bardhaman, India.
Ghosh S, Nandi A. P, Chatterjee S. Morphology and Seasonal Variation of Fowl Tape Worm, Raillietina Tetragona (Molin, 1858) in Purba Bardhaman, West Bengal, India. Biosc.Biotech.Res.Comm. 2019;12(3).
Ghosh S, Nandi A. P, Chatterjee S. Morphology and Seasonal Variation of Fowl Tape Worm, Raillietina Tetragona (Molin, 1858) in Purba Bardhaman, West Bengal, India. Biosc.Biotech.Res.Comm. 2019;12(3). Available from: https://bit.ly/2z4Ym88
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
Introduction
Domestic fowl, Gallus domesticus (Linnaeus 1758) (Galiformes: Phasianidae), is supposed to have derived from the South East Asian and wild Indian red forest chicken (Permin and Ranvig, 2001). These fowls are with high nutritional value. Sometimes these are domesticated traditionally under free-range management systems in villages where little or no supplementary foodstuff is provided and the poultry farmers are unaware of any veterinary care, which finally leads to the parasitic infections to the chicken (Gary and Richard 2012). According to Rohde (1994), there are some other factors like temperature, humidity, rainfall and parasite maturation which influence the parasitic infection in domestic fowl. Thus environment plays a significant function in the seasonal variability of these cestode parasites. During the present study G. domesticus were collected from Purba Bardhaman and surrounding rural areas while some other gastrointestinal cestode parasites were also recovered from the domestic fowl gut and R. tetragona was found to be the most prevalent species among all. This is also supported by the findings of Khan et al. (2016).In the present survey morphological observations were made using light microscope (LM) and measurements of its cephalic regions were taken. Other morphological characters like shape of the scolex; rostellum and suckers, number of eggs in the egg capsules and shape of the eggs were also made.
However, reports on seasonal prevalence of Raillietina tetragona in domestic fowl of Purba Bardhaman region of West Bengal could not be noticed in the accessible reports. The two year survey was carried out to determine the prevalence of this cestode parasite inG. domesticusoccurring in and around Purba Bardhaman, West Bengal, India.
Material and Methods
Freshly dissected out guts of the domestic fowl, Gallus domesticus were purchased from the local markets of Bardhaman, West Bengal, brought to the laboratory, as soon as possible, and examined for the presence of Raillietina tetragona in each week of every month for a period of consecutive two year study period from January 2016 to December 2017. Live worms were washed in normal saline (0.85% NaCl) and freed from the adhering host materials, fixed and preserved in 70% alcohol. For identification and morphological observations, the adult cestodes were washed in normal saline, dried and flattened on filter paper, fixed in 70% alcohol and observed with the help of compound light microscope. The prevalence of infection and mean intensity were calculated from the recorded data with the help of the following formulas (In percentage).
Results
General morphology of the cestode
Light microscopic (LM) study showed that the body of R. tetragona was typically divided into scolex, followed by a short unsegmented region, the neck, succeeded by a chain of proglottids consisting of immature, mature and gravid segments. Scolex is small pin like and provided with four well-formed hemispherical acetabula, armed with hooks and an armed disc shaped rostellum (Plate 1). In the mature proglottids the genital pores are unilateral (Plate 3) and in the gravid proglottids (Plate 4) eggs are found in egg capsules, each containing six to twelve eggs.
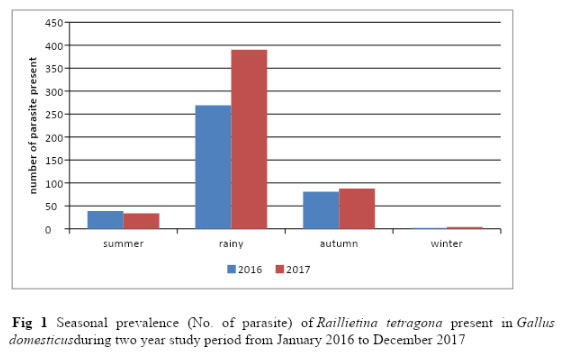 |
Figure 1: Seasonal prevalence (No. of parasite) of Raillietina tetragona present in Gallus domesticus during two year study period from January 2016 to December 2017 |
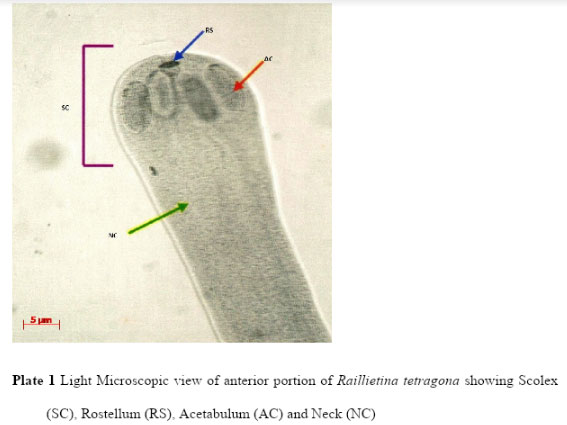 |
Plate 1: Light Microscopic view of anterior portion of Raillietina tetragona showing Scolex (SC), Rostellum (RS), Acetabulum (AC) and Neck (NC) |
The current LM observation showed that the scolex of Raillietina tetragona was about 18.98µm – 19.76µmin width, neck was about 15.1µm- 16.27µ mandrostellum was 2.94µm – 3.11µmin width and armed with one row of hooks. Four acetabula were measured about 7.08 µm, 6.95µm, 6.79µm and 7.4µm in length and 4.12µm, 4.03µm, 3.17µm and 3.71µm in width respectively. Mature proglottids (Plate 2) were rectangular in shape (65.68µm – 68.96µm) and gravid proglottids (60.85µm- 61.44µm) were more or less square containing numerous egg capsules (Plate 5) which were more or less round or oval in shape and measured about 0.85µm- 1.09µm.
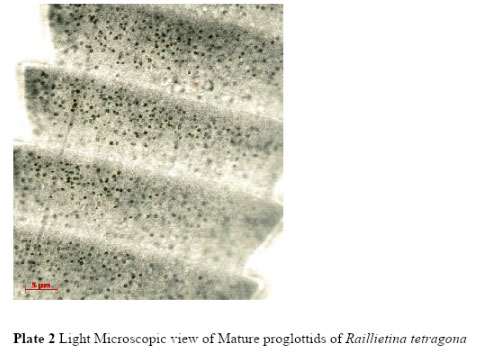 |
Plate 2: Light Microscopic view of Mature proglottids of Raillietina tetragona |
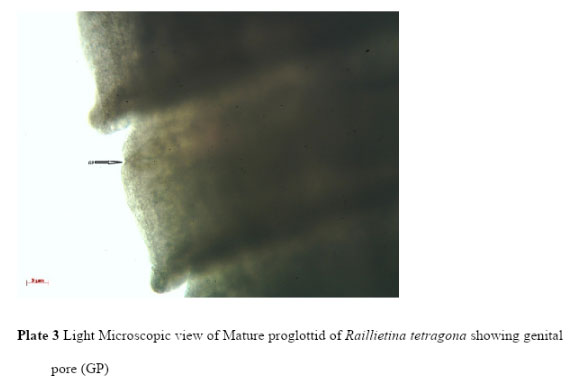 |
Plate 3: Light Microscopic view of Mature proglottid of Raillietina tetragona showing genital pore (GP) |
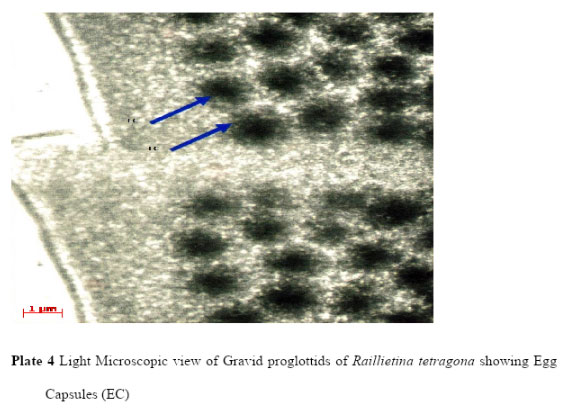 |
Plate 4: Light Microscopic view of Gravid proglottids of Raillietina tetragona showing Egg Capsules (EC) |
During the two year study period from January 2016 to December 2017, out of 382 fowl guts examined, 230 were found infested with R. tetragona and totally 907 parasites were collected. As R. tetragona was isolated all through the year from Gallus domesticus, characterization and seasonal prevalence were done for this species only. Monthly variations in respect to percentage of prevalence and the mean intensity of R. tetragona infection have been presented in Table 1 and total number of parasite present in Gallus domesticus in each season for the two year study period has been shown in Figure 1 .The result showed that average prevalence percentage was 51.91%. The study revealed that the higher percentage of prevalence of R. tetragona occurred in summer season showing higher peak in May (61.58% -70%) and it showed its highest values during rainy season and the maximum value of percentage of prevalence was recorded in the month of July (93.33% – 95.24%). From September to November it tends to lower down and the lowest prevalence occurred in winter (0 -10%). The mean intensity of infection also declined in the winter season (0-1)during the two year study period.
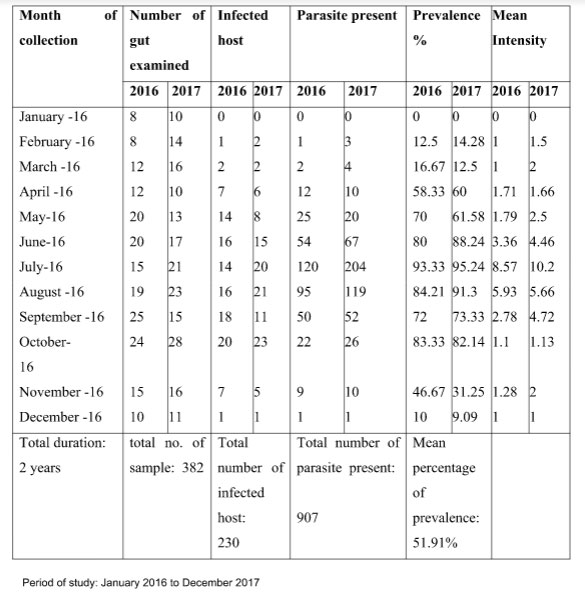 |
Table 1: Monthly variation in % of prevalence and Mean intensity of infection of Raillietina tetragona infecting Gallus domesticus |
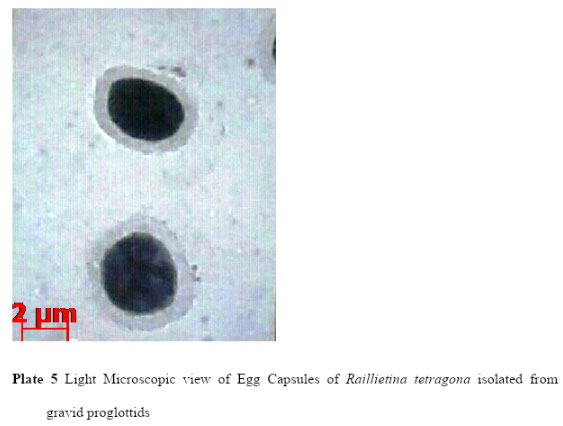 |
Plate 5: Light Microscopic view of Egg Capsules of Raillietina tetragona isolated from gravid proglottids |
Discussion
Raillietina tetragona has a cosmopolitan distribution occurring in pigeon, chicken and guinea fowl. This tapeworm completes its development in two hosts. Birds are the definitive hosts and ants, mostly of the species Tetramorium, and housefly of the species Pheidole and Musca act as the intermediate hosts (Horsfall 1938;Soulsby 1982; Su 1986;). Light Microscopic observations revealed that Raillietina tetragona was different from the other Raillietina spp. in respect to the shape of scolex, rostellum and acetabula. The hooks of the rostellum were placed in one row (Butboonchoo et al. (2016). This type of study was also carried by some scientists earlier (Hofstad et al. 1984; Sawada 1964 and 1965) but scanty literature is available. Lalchhandama (2009) observed the morphological structures of R. echinobothridafrom fowl gutand Mu et al. (2009) worked on R. echinobothrida and R. tetragonal from the gut of domestic chicken.In the recent year, Butboonchoo et al. (2016)distinctly differentiated four different species of Raillietina spp. includingRaillietina tetragona on the basis of Light Microscopic and Scanning Electron Microscopic observations. The present study showed similar findings.
Seasonal variability of R. tetragona in domestic fowl, Gallus domesticus, was checked throughout the year and comparatively higher percentage of prevalence and mean intensity of this parasite were observed in the summer and rainy season. Seasonal variability of cestodes were showed that changes in prevalence and infection rates could depend upon host, parasite and also upon the geographical location (Biswal et al. 2013).Oniye et al. (2001) in Nigeria reported a high prevalence of Raillietina tetragona in the month of June and August. Fakae et al. (1991) from Eastern Nigeria reported 72.5 % prevalence of R. tetragona in Gallus gallus during dry season (November to April). These finding were supported by the previous results as reported byFrontovo, (2000), Fakae et al. (1991) and Oniye, et al. (2001).
Seasonal prevalence of R. tetragona also varied with geographical location and other climatic conditions. According to Adang et al. (2008) R. tetragona was found to be the most common cestode parasite in domestic pigeon, occurring in 11 months of the annual cycle, showing highest prevalence in rainy season in Zaria, Northern Nigeria. Salam et al. (2010) and Sheikh et al. (2016) reported that the prevalence of R.
cesticillus from Gallus gallus domesticus in Kashmir is highest in summer followed by autumn, spring and winter months respectively. Butt et al. (2014) reported that the average percentage of prevalence of Raillietina cesticillus in G. domesticus was 83.5% during July to November, 2013 in Hyderabad, Sindh, Pakistan. According to the observations of Sheikh et al. (2016) the high prevalence of Raillietina sp. infection was seen during warm summer followed by autumn, spring and winter months. Except winter, the high prevalence of cestode infection throughout the year is a strong evidence of unscientific management and control in domestic fowls. As a result an infection creating environment originates continuously.
However, Shukla et al. (2012) reported that the maximum prevalence of Raillietina sp. in G. domesticus from Ahmednagar district, Maharashtra, India was found in winter followed by rainy season and minimum prevalence was noted in summer.According to Patil and Bhamare (2018), a high prevalence of Raillietina sp. in G. domesticus from Nashik district of Maharashtra, India occurred in winter season followed by summer season and low in rainy season. A possible explanation of this report is the availability of the intermediate host and oncosphere stages of Raillietina sp. increases during cold climatic condition. The oncospherestages are taken by the intermediate hosts and grow into cysticercoids which become adult in the definitive host during summer season.
In our survey greater number of mature cestodes were obtained during summer and rainy seasons whereas in autumn mostly immature stages were obtained. High rate in the percentage of prevalence and mean intensity of R. tetragona in the summer and rainy season may be a result of the abundance of infective stage and the stage bearing intermediate hosts in the survey zone during these seasons. It seems that moisture and temperature favour the development of intermediate hosts, the development of eggs in soil as well as proliferate the reproductive rate of parasites (Møller, 2010). Low prevalence of R. tetragona in winter season may be the result of elimination of multiple infections.This condition may occur due to intraspecific antagonistic reaction.
In fact, reports from other researchers around the world in different times showed that Raillietina spp. has a very high prevalence of infection in birds especially in indigenous chickens. ( Ahmed and Sinha, 1993; Puttalakshmamma et. al. 2008; Nnadi et al. 2010; Sreedevi et al. 2016; da Silva et. al. 2016). According to a recent report of Jajere et al. (2018) who found the guinea fowls have been heavily infected with Raillietina spp. and among the various species, Raillietina tetragona was most abundant having 72.8% prevalence of infection. When the infective stages of intestinal parasites pass out to the surroundings, they have to get over the environmental hazards before they reach their suitable hosts for further development (Gillett, 1974). So, favourable environmental conditions are also necessary for the effective transmission of the parasites(Smith, 1990)and seasonal variations in the social conduct of the host and their accumulation can also describe for seasonal dynamics of parasitic infections (Hosseini et. al. 2004).
The time period for obtaining table size, in case of indigenous breed, is longer than exotic breeds which are usually feed on artificial supplementary diets. So another reason of higher infection is may be due to improper management, the inadequacy of food grains for the local breeds and they have to feed on insects, mites and worms which may be the intermediate hosts for the cestode parasite and has been shown to improve susceptibility to parasitism in the system (Shukla and Mishra, 2013).
Moreover, earlier studies have shown that the immune systems of humans, rodents and birds got weaker by rough climatic conditions, under-nutrition or investment in reproduction (Lloyd 1995; Nelson et al. 2002) and due to low immunity level, hosts become more vulnerable to infections (Hillgarth and Wingfield 1997). A longer breeding period of the host bird provides a selective advantage to the parasites (Dunn and Winkler 2010; Møller et. al. 2010). Reports have shown that during the breeding season of host birds, antibody production and cell mediated immunity rate is getting weaker which is the reason for higher rates of parasitic infection (Hillgarth and Wing field, 1997; Moreno et. al. 2001). According to Sheikh et al. (2016) the host birds have shown a moderate resistance against the cestode parasites with the advancement of age because of the improved immune status in grown-ups than in young ones. Chicks hatch out during late spring. During summer and autumn they are in their young age when they get exposed to parasite infection. The young and the juvenile forms are susceptible to the parasite because they are immunologically weak. This may also affect the seasonal variability of Raillietina tetragona. Till now there are many surveys on Raillietina spp reported from differents parts of the world but it is still needs to execute more studies to follow the changing dynamics of helminth infection in domestic poultry management.
Acknowledgement
Thankful acknowledgement is due to the Department of Zoology, University of Burdwan, for giving the laboratory facilities
Author contribution
Sreenita Ghosh has carried out the present work under the guidance of Dr. A. P. Nandi and Dr. Soumendranath Chatterjee (Professor of Zoology).
Compliance with ethical standards
Though our Institution does not have such ethics committee, domestic fowl has been slaughtered for the present work following the guiding principle of CPCSEA (The Committee for the Purpose of Control and Supervision of Experiments on Animals) established by the Act of the Indian Parliament.
Conflict of interest
All the authors have declared that in the present work there is no conflict of interest.
References
Adang K.L., Oniye S.J., Ajanusi O.J., Ezealor A.U., Abdu P.A. (2008) Gastrointestinal helminths of the domestic pigeons (Columba livia domestica Gmelin, 1789 Aves: Columbidae) in Zaria, Northern Nigeria. Sci World J Vol. 3 No 1: Pp 33–37
Ahmed M.I., Sinha P.K. (1993) Prevalence of poultry helminthiasis in an arid zone in Nigeria. Indian Vet J Vol. 70: Pp 703–704
Biswal D., Nandi A.P., and Chatterjee S. (2013) Temporal variation of the cestode, Cotugniacuneata (Meggit, 1924) in their host, domestic pigeons, Columba liviadomestica (Gmelin, 1789). J Parasit Dis Vol. 39 No 2: Pp 194–199
Butboonchoo P., Wongsawad C., Rojanapaibul A., Chai J.Y. (2016) Morphology and Molecular phylogeny of Raillietina spp. (Cestoda: Cyclophyllidea: Davaineidae) from domestic chickens in Thailand. Korean J Parasitol Vol. 54 No 6: Pp 777- 786
Butt Z., Shaikh A.A., Memon S.A., Mal B. (2014) Prevalence of Cestode parasites in the intestine of local chicken (Gallus Domesticus) from Hyderabad, Sindh, Pakistan. Journal of Entomology and Zoology Studies Vol. 2 No 6: Pp 301-303
Da Silva G.S., Romera D.M., Fonseca L.E.C., Meireles M.V. (2016) Helminthic parasites of chickens (Gallus domesticus) in different regions of São Paulo State, Brazil. Braz J PoultSci Vol. 18: Pp 163–168.
Dunn P.O., Winkler D.W. (2010) Effects of climate change on timing of breeding and reproductive success in birds. In: Effects of climate change on birds. Pp 113–128 (Eds) Møller A.P., Fiedler W., Berthold P., Oxford University Press, Oxford.
Fakae B.B., Umeorizu J.M., Orajaka L.J.E. (1991) Gastrointestinal helminth infection of the domestic fowl (Gallus gallus) during the dry season in eastern Nigeria. J Zool Vol. 105: Pp 503–508.
Frantovo D. (2000) Some parasitic nematodes (Nematoda) of birds (Aves) in the Czech Republic. Acta Societatis Zoological Bohemicae
Gary D.B. and Richard D.M. (2012) Intestinal parasites in backyard chicken flock 1 In: Series of Veterinary Medicine- Large animal clinical sciences. Vol 76, University of Florida. http://edis.ifas.ufi.edu
Gillett J. (1974) Direct and indirect influences of temperature on the transmission of parasites from insects to man. In: The effects of meteorological factors upon parasites. Pp 79–95 (Ed) Taylor A.E.R., Muller R., Blackwell Scientific Publications, London.
Hillgarth N., Wingfield J.C. (1997) Testosterone and immunosupression in vertebrates: implications for parasite-mediated sexual selection. In: Parasites and pathogens: effects on host hormones and behaviour. Pp 143–155 (Ed) Beckage N.E., Chapman and Hall, New York.
Hofstad M.S., Calnek B.W., Helmboldt C.F., Reid W.M., Yoder H.W. (1984) Diseases of Poultry. Iowa State University Press
Horsfall M.W. (1938) Observations on the life history of Raillietina echinobothrida and of R. tetragona (cestoda).The Journal of Parasitology Vol. 24 No 5: Pp 409-421
Hosseini P.R., Dhondt A.A., Dobson A. (2004) Seasonality and wildlife disease: how seasonal birth, aggregation and variation in immunity affect the dynamics of Mycoplasma gallisepticum in house finches. Proc R SocLond B Vol. 271: Pp 2569–2577.
Jajere S.M., Lawal J.R., Atsanda N.N., Hamisu T.M., Goni M.D. (2018) Prevalence and burden of gastrointestinal helminthes among grey-breasted helmet guinea fowls (Numidameleagrisgaleata) encountered in Gombe state, Nigeria. International Journal of Veterinary Science and Medicine Vol. 6 No 1: Pp 73-79
Khan A., Bhutto B., Shoaib M., Fahad S., Ahmed A., Khetran B.I., Nizamani A.R., Zeb A., Rahman M.U., Khan S. (2016) Prevalence of gastrointestinal helminths in Banaraja fowls reared in semi-intensive system of management in Mayurbhanj district of Odisha, journal of Animal Health and Production Vol. 4 No 1: Pp 26-30.
Lalchhandama K. (2009) On the structure of Raillietina echinobothrida, the tapeworm of domestic fowl. Sci Vis Vol. 9 No 4: Pp 174-182
Linnaeus C. (1758) System Nat. ed. 10:58
Lloyd S. (1995) Environmental influences on host immunity. In: Ecology of infectious diseases in natural populations. Pp 327–361 (Ed) Grenfell B.T., Dobson A.P., Cambridge University Press, Cambridge.
Møller A.P. (2010) Host-parasite interactions and vectors in the barn swallow in relation to climate change. Global Change Biol Vol. 16 :Pp 1158–1170
Møller A.P., Flensted-Jensen E., Klarborg K., Mardal W., Nielsen J.T. (2010) Climate change affects the duration of the reproductive season in birds. J AnimEcol Vol. 79: Pp 777–784
Moreno J., Sanz J.J., Merino S., Arriero E. (2001) Daily energy expenditure and cell-mediated immunity in pied flycatchers while feeding nestlings: interaction with moult. Oecologia Vol. 129: Pp 492–497
Mu L., Li H.Y., Yan B.Z. (2009) Comparative study on morphology and development of two species of Raillietina from chicken. Chinese Journal of Parasitology & Parasitic Diseases Vol. 27 No 3: Pp 232-236
Nelson R.J., Demas G.E., Klein S.L., Kriegsfeld L.J. (2002) Seasonal patterns of stress, immune function, and disease. Cambridge University Press, New York.
Nnadi P.A., George S.O. (2010) A cross-sectional survey on parasites of chickens in selected villages in the subhumid zones of south-eastern Nigeria. J Parasitol Res Vol. 2010: Pp 141824
Oniye S.J., Audu P.A., Adebote D.A., Kwaghe B.B., Ajanusi O.J., Nfor M.B.(2001) Survey of helminth parasites of Laughing Dove (Streptopeliasegalensis) in Zaria, Nigeria. African Journal of Natural Sciences Vol. 4: Pp 65–66
Patil S.D. and Bhamare A.V. (2018) Seasonal variation of cestode parasite Raillietina in an edible bird Gallus domesticus(L.).Environment Conservation Journal Vol. 19 No 3: Pp 77-80
Permin A. and Ranvig H. (2001) Genetic resistance in relation to Ascaridiagalli in chickens. Veterinary Parasitology Vol. 102 No 12: Pp 101-111.
Puttalakshmamma G.C., Ananda K.J., Prathiush P.R., Mamatha G.S., Rao S. (2008) Prevalence of gastrointestinal parasites of poultry in and around Bangalore. Vet World Vol. 1: Pp 201–202.
Rohde K. (1994) Niche restriction in parasites: proximate and ultimate causes. Parasitology Vol. 109(suppl.): Pp S69–S84.
Salam S.T., Mir M.S., Khan A.R. (2010) The prevalence and pathology of Raillietina cesticillus in indigenous chicken (Gallus gallus domesticus) in the temperate Himalayan region of Kashmir. VeterinarskiArhiv Vol. 80: Pp 323–328.
Sawada I. (1964) On the genus Raillietina Fuhrmann, 1920 (I). J Nara GakugeiUniv (Natural Science) Vol. 12: Pp 19–36.
Sawada I. (1965) On the genus RaillietinaFuhrmann 1920 (II). J Nara GakugeiUniv (Natural Science) Vol. 13:Pp 5–38
Sheikh B.A., Ahmad F., Sofi T.A. (2016) Morphology and Prevalence of Some Helminth Parasites in Gallus domesticus from Gurez Valley of Jammu and Kashmir, India. Journal of Fisheries and Livestock Production Vol. 4: Pp 159
Shukla S. and Mishra P. (2013) Gastro Intestinal Helminths Parasites of Local Chickens Samples from Tribal Areas of Madhya Pradesh, India. Int. J. of Life Sciences Vol. 1 No 4: Pp 284-287
Shukla S.J., Borde S.N., Humbe A., Bhavare V.V. (2012) Seasonal variation of intestinal Tapeworms in Gallus gallus domesticus at Ahmednagar region. IntMultidiscip Res J Vol. 2 No 4: Pp 01–03
Smith G. (1990) The population biology of the free-living phase of Haemonchuscontortus. Parasitology Vol. 101: Pp 309–316.
Soulsby E.J.L. (1982) Helminths, arthropods and protozoa of domestic animals, 7th edn. FLBS Barrierve Tindal. London, Pp- 235-244
Sreedevi C., Jyothisree C.H., Rama Devi V., Annapurna P., Jeyabal L. (2016) Seasonal prevalence of gastrointestinal parasites in desi fowl (Gallus gallus domesticus) in and around Gannavaram, Andhra Pradesh. J Parasit Dis Vol. 40: Pp 656–661.
Su X.L.Y. (1986) Studies on the life history of Raillietina tetragona Molin and its natural intermediate host in Xiamen. Wuyi Science Journal 06 (epub)


