1Department of Botany, University of Delhi, Delhi 110007
2School of Life Sciences, Jawaharlal Nehru University, New Delhi 110067, India
Corresponding author Email baishnabtripathy@yahoo.com
Article Publishing History
Received: 19/07/2019
Accepted After Revision: 20/09/2019
Algal biodiesel is a sustainable and a renewable biofuel. Therefore, it is important to optimize the lipid production in microalgae. To enhance bio-diesel production the unicellular alga Chlorella vulgaris was grown in ambient air and high CO2 (5% CO2, rest air) in the absence or presence of NaHCO3 (12 mM) that provides additional carbon source for algal photosynthesis and biomass production. In ambient air the algal biomass increased 2.5 fold in the presence of NaHCO3. High CO2 purging of the algal growth medium in the presence of NaHCO3 partially increased the biomass by 10%. In the same vein the chlorophyll content of algal culture increased (100% to 139%) due to the abundant availability of the carbon source. Addition of NaHCO3 increased biodiesel amount by 12%. These demonstrate that addition of extra carbon source i.e., NaHCO3 that is readily photosynthetically fixed by the coordinated action of carbonic anhydrase and rubisco increases the carbon skeleton to be used to optimize biodiesel production by microalgae. Further, the nitrogen deficiency decreased the algal biomass due to reduced N assimilation and amino acid synthesis. The carbon skeletons in N-deficient culture were diverted towards fatty acid synthesis that resulted in 50% increase in lipid content i.e., from 40% to 60% per algal dry cell weight. This approach could be further optimised for large scale biodiesel production.
Biodiesel, Chlorella vulgaris, NahCo3 Supplementation,Nitrogen Deficiency
Kumari K, Sahoo D, Tripathy B. C. Modulation of Biodiesel Production by Sodium Bicarbonate and Nitrogen Deficiency In the Microalga Chlorella vulgaris. Biosc.Biotech.Res.Comm. 2019;12(3).
Kumari K, Sahoo D, Tripathy B. C. Modulation of Biodiesel Production by Sodium Bicarbonate and Nitrogen Deficiency In the Microalga Chlorella vulgaris. Biosc.Biotech.Res.Comm. 2019;12(3). Available from: https://bit.ly/2kMuJ8d
Copyright © Kumari et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Microalgae are fast-growing photosynthetic organisms that fix CO2 to generate millions of tons of algal biomass in nature. In addition to photosynthetic production of carbohydrates, several microalgae synthesize lipids by using carbon skeletons and reducing equivalents generated from carbohydrate metabolism. Lipids extracted from green algae can be utilized for biodiesel generation, and whatever is left of the biomass can be changed over into ethanol that may be used as biofuel. Biodiesel generation from microalgae is a desirable option. However, their lipid content is required to be high to be an economically viable option. The green alga Chlorella vulgaris is widely studied for biodiesel production (Chiu et al., 2008). It has higher productivities than land plants and generates a lot of fatty acids the precursors of biodiesel. To grow algae crop land is not required. It can be grown in ponds or lakes. However, a few challenges need to be overcome to use algae as a sustainable and economically viable option for commercial biodiesel production from Chlorella.Microalgae use bicarbonate as the exogenous carbon source for photosynthesis. NaHCO3 is converted to CO2 via carbonic anhydrase (Dixon et al., 1987; Nimer et al.,1997). Algae could use purged high CO2 in the growth medium for photosynthesis via same mechanism (Baldisserotto et al., 2014). Nitrogen is a main element controlling species component, diversity, and primary productivity of species (Mandal et al. 2018). Limiting or starving the availability of essential nutrients such as nitrogen (N) can induce triacylglycerol’s (TAGs) accumulation (Janssen et al. 2019).
In the present study, we have optimized biomass generation and lipid production of Chlorella by providing NaHCO3 as an inorganic carbon source. In addition we have purged the growth medium with ambient air or high CO2 (5% CO2, rest air) in the absence or presence of NaHCO3 as additional carbon source for photosynthetic carbon assimilation. These cells were further grown in the N-deficient conditions to increase their lipid content. We have shown than addition of NaHCO3 doubled the algal growth and increase biomass production when purged with either air or 5% CO2. Their lipid content increase by 12% by NaHCO3 and it further increased in N-deficient growth condition by 50%.
Material and Methods
Photobioreactor and cultivation conditions
Chlorella vulgaris (an isolate of river Ganges, courtesy: Prof. Nirupama Mallick, Indian Institute of Technology, Kharagpur, India), was used in this research. Pure culture of C. vulgaris were grown in N11 medium (Soeder and Bolze, 1981) at pH 6.8 and maintained in a culture room at 25 ± 2οC under a photoperiod of 14:10 h at a light intensity of 150 μmol photon m–2 s–1. The photobioreactor was setup in a temperature-controlled environment fitted with nine LED fluorescent lamps (Philips, 36W) containing 6 photobioreactor units. Photobioreactors were 1.5 L borosilicate glass tubes with a working- volume of 1.3 L, fitted with sealed stoppers and autoclaved as complete units. Airflow into the photobioreactor was supplied via filtered hydrocarbon-free building air and 5% CO2 through teflon tubing at a rate of 200 mL min-1 (i.e., 0.25 vvm, volume gas per volume media per min). The airflow was adjusted using a stainless steel micrometering valve (JTM LPA Air, Japsin Rotameter). Experimental replication was achieved by repeating each treatment 4 times. The initial biomass concentration for all cultures was 0.11 g L-1 and all runs studied for 14 days. When required 12 mM NaHCO3 was added to the growth medium to increase the carbon source for biomass and biodiesel production. After addition of NaHCO3 the pH was adjusted to 6.8.
To cause 90% N deficiency (N10), 90% of KNO3 was substituted by KCl.
Studies on nitrogen deprivation with and without NaHCO3
To study the effects of nitrate, starvation, algal cultures pre-grown in N 11 medium were harvested by centrifugation, washed with N 11 medium without the specific nutrient for 2-3 times and transferred to the 90% N-deficient medium (N10). C. vulgaris was grown in ambient or 5% CO2 without or with NaHCO3 (12 mM) as additional carbon source to support photosynthesis. Cultures were grown under bicarbonate supplementation into early stationary growth phase (10–12 days) and samples were taken for analysis at desired time point to monitor biomass production, chlorophyll content and cellular lipid content.
Analytic determinations
Dry cell weight (dcw) was measured gravimetrically (Rai et al., 1991). To determine the quantity of total chlorophyll, extraction was done according to Sartory and Grobbelaar (1984) with small modifications. To 1 mL of pellet and beaded culture, 2 mL of methanol was added and heated for 10 min at 70 °C in a water bath in a sealed tube. After cooling, the samples were centrifuged for 10 min (4 °C; 6,000g). For chlorophyll a, chlorophyll b quantification, absorbance was recorded for the supernatants at 666 nm, 653 nm and estimation was done using the equations proposed by Wellburn (1994).Extraction of lipid from the dried biomass was done following the protocol of binary solvents using chloroform and methanol (Bligh and Dyer, 1959).
Results and Discussion
In the present study, the NaHCO3 was given as an inorganic carbon source for algal growth. Simultaneously, it was bubbled with air or 5% CO2 to increase the carbon source.As shown in figure- 1, the algal growth, determined as dry biomass accumulation, saturated after 10 days of growth. The algal cells grown in the photo-bioreactor purged with ambient air accumulated 0.57 g of dry biomass l-1 on10th day of growth Upon purging the culture medium with 5% CO2, the growth and biomass accumulation increased by 38%.
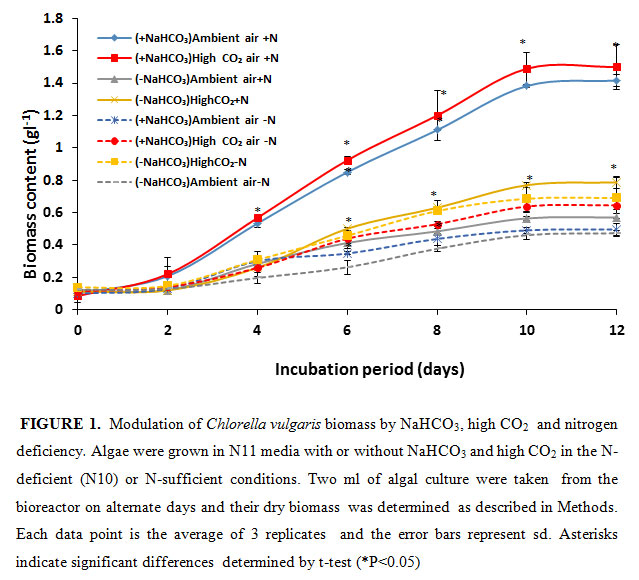 |
Figure 1 |
The biomass accumulation substantially increased in the presence of additional inorganic carbon source NaHCO3. When the growth medium was supplemented with 12 mM NaHCO3 and bubbled with air the biomass accumulation substantially increased i.e., by 2.5 fold to 1.41 g l-1. Upon purging the NaHCO3 supplemented growth medium with 5% CO2 the biomass accumulation further increased to 1.5 g l-1. This accumulation was 100% higher than biomass produced in high CO2 grown minus HCO3 samples. This demonstrates that by providing additional inorganic carbon source the algal growth could almost be doubled. Bubbling high CO2 alone does not achieve the desired result. Our results further demonstrate that high CO2 purging of algal culture could partially (8%) increase algal growth in the presence of 12 mM NaHCO3. Increase of carbon source as NaHCO3 could provide high CO2 needed by Rubisco to efficiently fix carbon by the photosynthetic Calvin-Benson cycle. NaHCO3 is not the substrate of Rubisco, rather it is CO2. The NaHCO3 would have converted to CO2 by intracellular carbonic anhydrase (Raven, 1995) before its fixation by Rubisco.
Nitrogen deficiency is known to cause reduction of algal growth (Wen et al. 2003; Hejazi et al. 2004; Aro 2016). To probe the role of nitrogen deficiency on algal growth supplemented with additional sources of inorganic carbon, we monitored the biomass of C vulgaris in the absence or presence of NaHCO3 + 5% CO2 in 90% N-deficient media (Fig. 1). Due to N deficiency, i.e., in 90% N deficient state (N10), the biomass of algal culture declined by 17%, 11%, 62% and 57% in -NaHCO3 ambient air, -NaHCO3 high CO2, +NaHCO3 ambient air and +NaHCO3 5% CO2 growth condition respectively. The highest percentage (61%) of reduction of biomass production in N -deficient condition was observed in high CO2-grown and NaHCO3-supplemented algal cells. These clearly suggest that N is a highly limiting factor for algal growth in the presence of high inorganic carbon sources. The nitrogen deficiency decreased the algal biomass due to reduced N assimilation and amino acid and protein synthesis. The reduction in biomass was predominantly due to reduced cell division in N-limited growth. This is in agreement with previous studies with Chlamydomonas reinhardtii and Scenedesmus subspicatus where nitrogen deficiency significantly inhibited their cell division (Dean et al., 2010).
As shown in figure 2, the chlorophyll content of algal cells after 10 days of grown in high CO2 was higher than ambient. In the presence of NaHCO3 the chlorophyll content of algal cells substantially increased (100% – 139%). This was probably owing to increased number of cells due to higher cell division in high CO2 environment and higher Chl content per cell.
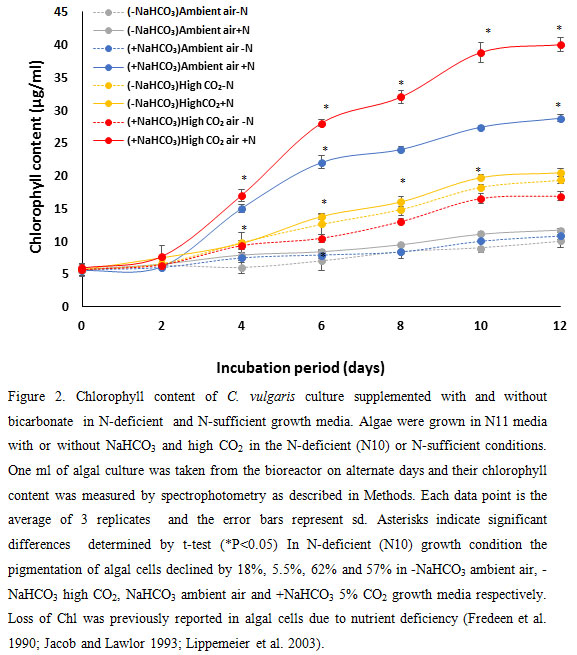 |
Figure 2 |
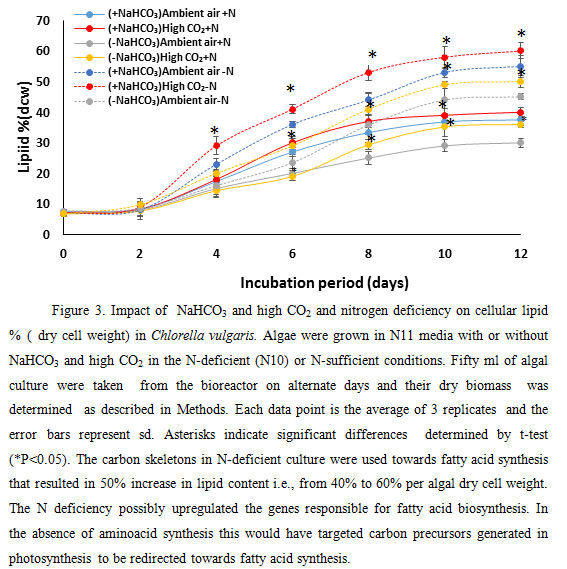 |
Figure 3 |
Addition of NaHCO3 increased the lipid amount by 12%. These demonstrate that addition of extra carbon source i.e., NaHCO3 that is fixed photosynthetically by the coordinated action of carbonic anhydrase and Rubisco to generate carbohydrates and carbon backbones could be used for lipid production by microalgae. Limitations of nitrate, under ambient and high (5%) CO2 stimulated lipid accumulation (Fig. 3) in the microalga C. vulgaris. Lipid percent of dry cell weight increased up to 10th day of culture both in ambient and high CO2. N deficiency is known to increase lipid content i.e., triacylglycerol in algal cells (Illman et al., 2000) (Janssen et al. 2019). On 10th day of culture, the lipid percent of dry cell weight (dcw) of algal culture in N-deficient (N10) growth condition increased by 51.7 %, 40%, 47% and 48% in -NaHCO3 ambient air, -NaHCO3 high CO2, NaHCO3 ambient air and +NaHCO3 5% CO2 growth media respectively.
Conclusion
Chlorella vulgaris grown photo-autotrophically is an excellent organism to generate biodiesel. The growth and biomass of the algal culture nearly doubled by adding NaHCO3 (12 mM) in high CO2 (5%) growth condition. The lipid production increased by 50% due to N deficiency. The growth of Chlorella vulgaris in high CO2, NaHCO3 in N-deficient conditions could be further optimized for large scale production of biodiesel in an industrial scale.
Acknowledgement
This work is supported by the IBSD Department of biotechnology grant (2662017_IBSD(A/C) Govt. of India.
Conflict of interest
Authors have declared no conflict of interest.
References
Aro EM (2016) From first generation biofuels to advanced solar biofuels Ambio 45(1): 24-31
Bligh E G and Dyer WJ (1959) A rapid method of total lipid extraction and purification Canadian journal of biochemistry and physiology 37(8): 911-917
Dean AP Sigee DC Estrada B Pittman JK (2010) Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae Bioresource Technol 101(12): 4499-4507
Dixon GK Patel BN Merrett MJ (1987) Role of intracellular carbonic anhydrase in inorganic-carbon assimilation by Porphyridium purpureum Planta 172(4): 508-513
Dokmanic I Sikic M Tomic S (2008) Metals in proteins correlation between the metal ion type coordination number and the amino acid residues involved in the coordination Acta Crystallographica Section D Biological Crystallography 64(3): 257-263
Fredeen AL Raab TK Rao IM Terry N (1990) Effects of phosphorus nutrition on photosynthesis in Glycine max L Merr Planta 181(3): 399-405
Gardner RD Lohman E Robin Gerlach R Cooksey KE Peyton BM (2013) Comparison of CO2 and bicarbonate as inorganic carbon sources for triacylglycerol and starch accumulation in Chlamydomonas reinhardtii Biotechnology and bioengineering 110(1): 87-96
Hejazi MA and Wijffels RH (2004) Milking of microalgae Trends in biotechnology 22(4): 189-194
Illman AM Scragg AH Shales SW (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium Enzyme and microbial technology 27(8): 631-635
Jacob J and Lawlor D (1993) In vivo photosynthetic electron transport does not limit photosynthetic capacity in phosphate deficient sunflower and maize leaves Plant Cell & Environment 16(7): 785-795
Janssen JH Wijffels RH Barbosa MJ (2019) Lipid production in Nannochloropsis gaditana during nitrogen starvation Biology 8(1): 1-5
Lippemeier S Frampton DMF Blackburn SI Geier SC Negri AP (2003) Influence of phosphorus limitation on toxicity and photosynthesis of Alexandrrium minutum (Dinophyceae) monitored by in line detection of variable chlorophyll fluorescence Journal of phycology 39(2): 320-331
Mandal S Shurin JB Efroymson RA Mathew TJ (2018) Functional divergence in nitrogen uptake rates explains diversity productivity relationship in microalgal communities Ecosphere 9(5): 1-14
Nimer NA Rodriguez MDI Merrett MJ (1997) Bicarbonate utilization by marine phytoplankton species Journal of Phycology 33(4): 625-631
Parodi TV Cunha MA Becker AG et al (2014) Anesthetic activity of the essential oil of Aloysia triphylla and effectiveness in reducing stress during transport of albino and gray strains of silver catfish Rhamdia quelen Fish Physiology and Biochemistry 40(2): 323-334
Peng X Liu S Zhang W Zhao et al (2014) Triacylglycerol accumulation of Phaeodactylum tricornutum with different supply of inorganic carbon Journal of applied phycology 26(1): 131-139
Rai LC Mallick N Singh JB Kumar HD (1991) Physiological and biochemical characteristics of a copper tolerant and a wild type strain of Anabaena doliolum under copper stress Journal of plant physiology 138(1): 68-74
Raven JA (1995) Photosynthetic and non-photosynthetic roles of carbonic anhydrase in algae and cyanobacteria Phycologia 34(2): 93-101
Sartory D and Grobbelaar J (1984) Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis Hydrobiologia 114(3): 177-187
Soeder CJ and Bolze A (1981) Sulfate deficiency stimulates release of dissolved organic matter in synchronous cultures of Scenedesmus obliquus Physiologia Plantarum 52(2): 233-238
Wellburn AR (1994) The spectral determination of chlorophylls a and b as well as total carotenoids using various solvents with spectrophotometers of different resolution Journal of plant physiology 144(3): 307-313
Wen ZY and Chen F (2003) Heterotrophic production of eicosapentaenoic acid by microalgae Biotechnology Advances 21(4): 273-294
White DA Pagarette A Rooks P Ali ST (2013) The effect of sodium bicarbonate supplementation on growth and biochemical composition of marine microalgae cultures Journal of applied phycology 25(1): 153-165
Zhao Y Yu Z Song Cao X (2009) Biochemical compositions of two dominant bloom-forming species isolated from the Yangtze River Estuary in response to different nutrient conditions Journal of Experimental Marine Biology and Ecology 368(1): 30-36


