1Department of Biochemistry & Plant Physiology, Centurion University of Technology and Management, Odisha, India
2Department of Biotechnology, Centurion University of Technology and Management, Odisha, India
Corresponding author email: pradipta.banerjee@cutm.ac.in
Article Publishing History
Received: 10/07/2020
Accepted After Revision: 17/09/2020
Plant growth and development are dependent upon complex biochemical reactions where phyto-hormones are key players. Recent scientific developments in the research field of crop physiology have identified a class of novel plant hormones called strigolactones (SLs). Strigol, the first strigolactones was isolated in 1966. After twenty years of this discovery, the structure of strigol was completely elucidated. The significance of strigolactones lies in the fact that they act as rhizosphere signalling molecules, play important role in regulation of plant architecture, promote germination of root parasitic weeds which have fatal effects on plant growth. Strigolactones are play significant roles in plant biotic and abiotic stress responses. They have emerged as important biological targets to study different signalling pathways, stress responses and developmental stages of plant. Presently, two naturally occurring SL families have been reported. One of those is having stereochemical configuration of (+)-strigol and the other is having (−)-orobanchol.
The most prominent role of SLs has found to help in seed germination in the Orobanche and Striga, parasitic weeds. They proved to be important bioactive compounds in in-planta and ex-planta signalling pathways and molecular botany. The potential uses of SLs in controlling parasitic weeds seed in agriculture, amplification of the branching in arbuscular mycorrhizal (AM) fungi is discussed in this review along with the biosynthesis, mode of action and roles of synthetic SL mimics in sustainable agriculture are highlighted. The objective of this work is to harness the benefits of SLs for sustainable agriculture in the near future. There are about 285 free full text out which 98 review articles are archived in PubMed database in the last five years, i.e, 2015-20, with the keyword “strigolactone”.
Strigolactones (SLs), Plant Hormone, Stereochemistry, Parasitic Weeds.
Banerjee P, Bhadra P. Mini Review on Strigolactones: Newly Discovered Plant Hormones. Biosc.Biotech.Res.Comm. 2020;13(3).
Banerjee P, Bhadra P. Mini Review on Strigolactones: Newly Discovered Plant Hormones. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/2D9B2LG
Copyright © Banerjee and Bhadra This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
In plant kingdom, the physiological features of plants are greatly affected by five phytohormones, namely, cytokinins (CK), auxins (IAA), abscisic acid (ABA), gibberellins (GA), and the aging hormone ethylene. The found hormones regulate different physiological functions; often one hormone is involved in controlling of several mechanisms in plants. Auxins are known to be primarily involved in stimulation of plant growth; cytokinins play role in regulation of cell division, making new plant organs; gibberellins(GAs) regulates stem elongation; ABA’s major function is to regulate moisture; and ethylene plays important role in ripening of fruits and rotting. Phytohormones are interrelated in a complex manner and a number of their mechanisms are yet to be understood. A new class of phytohormones have been discovered recently, known as strigolactones (SLs). This class of phytohormones are involving in several biological processes that includes initiation of plant-fungal symbiosis, triggering of germination of parasitic plants that pose a major threat to farming (Bürger and Chory, 2020).
The rationale of writing this review article is as follows: Strigolactones (SLs) are most recently discovered plant hormones that has potential application in agriculture.SL biosynthesis takes place via carlactone (CL) intermediate and can serve as important target to study plant signalling pathways, stress responses and architectural development in molecular level. SL has been found to help in seed germination in the Orobanche and Striga, parasitic weeds, in regulation of the branching activity in AM fungi and also in control of plant architecture, which helps in sustainable agriculture.
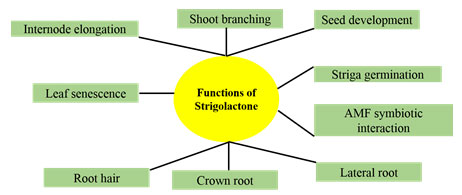
Of the various functions of strigolactones,most important ones are, that they can stimulate branching in plants, growth of symbiotic arbuscular mycorrhizal fungi (AMF) in the soil, impede shoot branching and trigger the germination of parasitic plant seeds as depicted in Figure 1.
Figure 1: Functions of Strigolactones
Strigolactones performs a pivotal role in controlling plant growth and the developmental phases. This hormone has stirred curiosity among the scientists that is reflected in a good number of research publication published so far. In this article, recent developments in SL research has been described. Strigolactone was first identified by Cook et al., 1966 as (+)-strigol has been isolated from cotton (root exudates). It took almost twenty years to elucidate its full structural information (Cook et al., 1972; Brooks et al., 1985). One of the most important function of strigol is to help in germination of the seeds of Orobanche and Striga spp., these are the most common parasitic weeds found in agricultural fields.
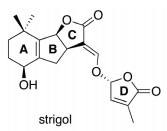
Before 1990, only one naturally occurring SL was known to the scientific community, that is, strigol. Novel SLs started to come in lime light from the year 1990. They were being isolated from various natural resources. For an example sorgolactone has been isolated from the root exudates of sorghum (Hauck et al., 1992), in red clover, orobanchol has found (Yokota et al., 1998) and in tobacco, solanacol has found (Xie et al., 2007). Strigolactones were found in negligible quantity in root exudates, thus, their structural elucidation was very difficult; especially determining their stereochemistry was troublesome (Zwanenburg and Pospíšil, 2013). All SLs have the basic structural features, containing 3 annular rings, an ABC scaffold that is connected to a butenolide ring via an enol ether unit forming ABC-D structure as shown in Figure 1 (Zwanenburg and Ania, 2018).
There are from the known SLs families, namely strigol and orobanchol. These two families differ in stereochemistry of B-C junction as in (+)-strigol and (−)-orobanchol. At C-2′ position, the spatial configuration of the butenolide D-ring in each naturally occurring SLs always remains the same (Figure 2).
Figure 2: Basic structure of Strigol
Biosynthesis of Strigolactones (SLs)
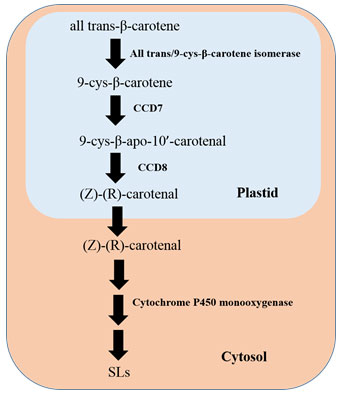
Strigolactones derive from carotenoids via two carotenoid cleavage dioxygenases CCD7 and CCD8 (Aly et al., 2014). Biosynthesis of SLs take place via a carlactone (CL) intermediate. Carlactone is formed from C40 all-trans-β-carotene by the enzymatic complex isomerization (β-carotene isomerase D27, chloroplastic) and oxidation reactions of the two carotenoid cleavage dioxygenases (Seto and Yamaguchi, 2014; Al-Babili and Bouwmeester, 2015; Flematti et al., 2016). Biochemical steps involving SL biosynthesis occurs in plastid of a plant cell and the product, i.e., CL is being exported to the cytosol as described in Figure 3 (Mishra et al., 2017). Carotenoid is the precursor of the central intermediate compound carlactone, and the stereospecific enzymes, viz., all-trans/9-cis-β-carotene isomerase (D27), the 9-cis-specific CAROTENOID CLEAVAGE DIOXYGENASE 7 (CCD7), CCD8 play are the major players (Jia et al., 2019).
Once the CL is exported in cytoplasm, it undergoes oxidation, closure of ring and functionalization of the involvement members from the family of CYP711 (Cytochrome P450 monooxygenase MAX1) (Zhang et al., 2014), and ultimately results in formation of SLs and SL-like compounds. It was reported that the enzyme cytochrome P450 monooxygenase or MAX1 in Arabidopsis thaliana was able to convert CL to carlactonoic acid, which in turn, again transform into a compound like SL called methyl carlactonoate (MeCLA) by an unknown enzymatic reaction (Abe et al., 2014). Once synthesized, these composites are transported through the plant and transferred into the rhizosphere. PhPDR1, a member of ABC family, is regarded as the key SL transporter (Kretzschmar et al., 2012; Sasse et al., 2015).
Biosynthesis of SL occurs mostly in roots (sometimes in stems) is highly synchronised (Al-Babili and Bouwmeester, 2015). Starvation of Phosphate is reported to be strongly inducing the biosynthesis of SL (López-Ráez et al., 2008). The mutation study of SL-deficient and SL-insensitive has found in different plant species show that there must be a feedback mechanism of biosynthesis of SL regulation (Hayward et al., 2009; Proust et al., 2011).
Figure 3: Biosynthetic Pathway of Strigolactones (SLs), Adapted from Mishra et al., 2017
Biochemical and genetic modulation of the SL pathway is identified as a promising approach to modify plant architecture, although whether and how the genes involved in the SL pathway play role in breeding still remain elusive (Wang et al., 2020).
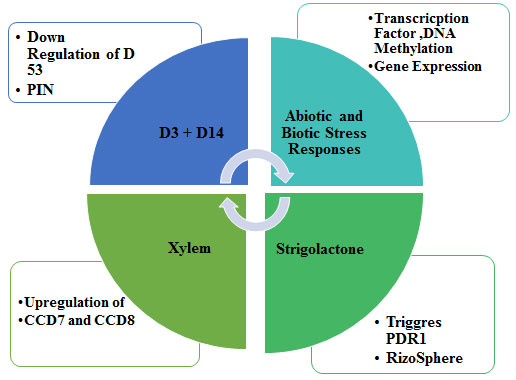
Mode of Action Strigolactones: The mechanism of strigolactones action depends on the detachment of the D-ring by an enzyme forming a hydroxyl butenolide structure which is capable of inducing a change in conformation in a receptor pocket which will be initiating a biochemical cascade of reactions, reflecting in a signal transduction pathway (Zwanenburg et al., 2016). The first step of SLs activity is its interaction with a protein receptor called strigolactone esterase or D14 protein ((Hamiaux et al., 2012; Kagiyama et al., 2013). An underlying molecular mechanism of germination process requires involvement of a part of active protein is called α/β-type hydrolase. This hydrolase contains a well-established catalytic chain of serine–histidine–aspartate having a big cone-shaped internal cavity that can encapsulate a SL molecule to accomplish the detachment of a hydroxy D-ring. It was reported that SLs change the phenotypic yield of PIN-FORMED (PIN) protein family of auxin transporters for calibrating of development and formative reactions (Hýlová et al., 2019; Lee et al., 2020; Zhang et al., 2020).
PIN genes are present in the genomes of multicellular plants exclusively and regulates asymmetric auxin distribution in multiple developmental processes that includes embryogenesis, organogenesis, tissue differentiation and tropic responses. Signalling pathway of SLs in rice is described in Figure 4. The signalling components in rice include a putative αβ hydrolase receptor (D14), F-box protein, a component of SCF complex (D3), and a ClpATPase (D53). This complex is known to regulate the gene expression by modulating the degradation of various transcription factors, which act as either repressors or activators of transcription. Strigolactone distribution is regulated via PDR1 transporter within the plants and outside into the rhizosphere. PDR1 encodes Pdr1p (Pleiotropic Drug Resistance), a transcription factor involved in general drug response.
Figure 4: Signaling pathway of strigolactone
Mimics of Strigolactones: A class of compounds which lacks ABC scaffold but are capable to stimulate germination are known as SL mimics. As the organic synthesis of natural SLs is strenuous, there is an acute need for easy-to-synthesize and efficient analogs of SLs. These mimics perform the same functional activity like naturally occurring SLs, but have not exhibited the typically featured structured SLs. One of this group of mimics has an aryloxy substituent at C-5 and are called debranones (branching furanones) and inhibits shoot branching (Fukui et al., 2013). Strigahermonthica seeds have high to medium response to debranones, whereas, parachlorophenoxy-debranone has the highest activity. Another finding says that there is a group of SL mimics are having an aroyloxy substituent at C-5 position of the D-ring (Zwanenburg et al., 2013) and they are modest germination stimulant for S. hermonthica seeds but are hyper active in Orobanchecernua and Pelipancheramosa seeds (Zwanenburg et al., 2016).
Recently, Jamil et al., (2019) have synthesized and studied the biological activity of the SL analogs MP13 and MP26. They reported that methylation at C-3′ position in D-Ring of Strigolactone analogs reduces biological activity in root parasitic plants and rice. There is a lot of scope of research in the area of SL mimics to study their mechanism of action more clearly.
Applications of Strigolactones in Agriculture: Strigolactones play important role in molecular mechanism of plants including regulation of protonema branching, quorum which acts like signalling of a sensor in the moss Physcomitrella patens (Protust et al., 2011), factors affecting the branching form AM fungi, controlling parasitic weeds as well as plant architecture, etc. Some of the important applications of SLs are described below.
a) Strigolactones, a factor, helping in branching for arbuscular mycorrhizal (AM) fungi: SLs are reported to be the branching factors for AM fungi (Parniske, 2008). Orobanchol exhibits the highest activity, next comes 5-deoxystrigol. Like strigol, strigolactone analog GR24 is remarkably active but the mirror image of GR24 is ten thousand times less active. Another strigolactone analog GR7 (lacking A-ring) was found to be one thousand times less active than GR24. From the previous information it can be deduced that to act as an active branching factor for fungi, SL should have all the rings of the ABC scaffold. The stereochemistry too must be at par with the strigol family. However, it was reported that that the B-ring is not mandatory for branching factors.
The parasitic plants have symbiotic relationship with AM fungi (Bonfante and Requena, 2011) and the fungi behaves as natural soil fertiliser by expediting the mineral (phosphates, nitrates) uptake from soil. Phytohormones, microRNAs, secreted peptides regulate the development of arbuscular mycorrhizal (AM) symbiosis with the phosphorous status of the plant (Müller and Harrison, 2019). Rhizophagus triggers strigolactone biosynthesis gene expression in Arabidopsis roots and in the early stages of interaction, Rhizophagus activates the strigolactone biosynthesis genes CCD 7 and CCD 8 (Fernandez et al., 2019).
In depth understanding of this type of symbiotic relationship may pave the path for newer experimental plans for controlling and managing the symbiosis between beneficial fungi and the parasitic weeds in agricultural lands.
b) Strigolactones are inhibitors of shoot branching and control plant architecture: Strigolactones (SLs) are butenolide molecules that play essential roles in plant growth and development (Jamil et al., 2019). SLs crosstalk with other plant hormones in a cascade of events, though details of these interaction are still not clearly understood. Like all other phytohormones, the biosynthesis and action of SLs are controlled by other hormones. For example, cytokinins antagonizes the function of Sls in the outgrowth of axillary bud regulation (Dun et al., 2011) and mesocotyl elongation in dark (Hu et al., 2014). Similarly, auxins are key regulators of biosynthesis of SL (Hayward et al., 2009; Al- Babili and Bouwmeester, 2015). Auxins also found to be an antagonists of enhancement of auxin transport by SLs (Cheng et al., 2013). Lopez-Raez et al. (2010) demonstrated that abscisic acid, one of the important hormones took part in plant abiotic stress, finds importance in SL biosynthesic pathway. Vice-versa, SLs also regulate the abscisic acid biosynthesis (Al-Babili and Bouwmeester, 2015). Moreover, it was also reported other than hormones, presence of phosphates is inversely related with SL biosynthesis (Koltai, 2015).
Plant architecture control studies have been carried out with increased branching mutants, mainly with ramosus (rms) in garden pea has found decreased apical dominance (dad) in Petunia hybrid, more axillary growth (max) in Arabidopsis, dwarf (d) and high tillering dwarf (htd) in rice. Treatment with exogenous SL analog GR24 resulted in:
- inhibiting branching of shoots (Dun et al., 2013)
- stimulating growth of internode (Germain et al., 2013)
- speeding up of leaf senescence (Yamada et al., 2014)
- upregulating root hair elongation and growth of primary roots (Kapulnik et al., 2011)
- inhibiting outgrowth of axillary buds (Minakuchi et al., 2010)
- inhibiting formation of adventitious and lateral roots (Rasmussen et al., 2013)
- inducing stem thickness and secondary growth (Agusti et al., 2011) and other morphological changes.
c) Strigolactones control parasitic weeds: Parasitic weeds are responsible for huge crop losses and potential threat to agricultural production in all developing countries like India, Africa, and Middle East. The parasitic weeds seeds can be in inactive condition inside the soil for long period of time (several years of dormancy has been reported). They are activated by strigolactones secreted by any plant in vicinity that acts as host for the parasitic weeds (Parker, 2013). It is difficult to get control over these parasitic weeds in an eco-friendly way. Manual weeding is a tough job and least effective if already the parasitic weed has been invaded the host root system, exploiting the necessary minerals and water. The crop rotation system and application of herbicides are sometimes not the best choice in under-developed or developing countries due to lack of modernized agricultural methods.
“Suicidal germination” is frequently used to combat with the situation. This approach relies on applying synthetic stimulant to the soil that can enhance germination in absence of host plant. Germination of parasitic weed will take place, but the seed will face untimely death due to the scarcity of nutrients. Now, the seedling of the crop are safely planted which can avoid the noxious parasitic weed. Suicidal germination approach was first tested in Hyderabad, India using GR5 to control the Strigahermonthica in sorghum (Johnson et al., 1976). It should be noted that the accumulation of synthetic analog of SLs in soil must be prohibited. A promising data of Orobanche ramose L. (hemp broomrape) in tobacco was obtained in field trials using Nijmegen-1 as a synthetic analog of SLs, along with a suitable emulsifying agent (Zwanenburg et al., 2016a).
Dissociation of the synthetic stimulant before commencing its action (Kannan et al., 2015) is an acceptable alternative to suicidal germination. Such type of decomposition is obtained in high alkalisoil by using borax (pH 9.2) or thiourea. Both borax and thioureaare nucleophiles that can separate the HO–D-ring. The parasitic weed seeds’ germination by tomato seedlings was prohibited by the eco-friendly chemicals thiourea or borax application in the soil. Besides being a nucleophile, thiourea is an excellent antioxidant and thus, can readily deactivate reactive oxygen species required for the attachment of radicle of the germinated seed to the host plant’s root system. Both the parasitic weed control methods, namely, suicidal germination and also decomposition of stimulant- suggest fascinating views for managing weed pests.
Future Scope: With the advancement of technology, it will be useful to identify and isolate new composites of SLs. Very recently, new compounds like heliolactone, avenaol and methyl zealactonoate had been reported, which may find important application in agriculture. Avenaol and heliolactone are referred to be a non- canonical SLs as they have incomplete ABC-D scaffold. The details of the structure and their stereochemistry will lead to pinpoint the mechanistic details of SL biosynthesis. The SL analogs will play an important role in other biochemical processes other than germination activity.
Thus, continuous evaluation of these SL like compounds as factors affecting the branching of AM fungi, regulators of growth and development – will be carried out. Recently, nitro phenlactone was proved to be effective as an agent for seed germinating of Striga and Phelipanche species. Research on SLs has developed as a fascinating field that has produced a variety of different signaling models that reflects a complex picture of hormonal perception (Bürger and Chory, 2020). Moreover, it was found that strigolactones exhibit a positive effect on hypodermal passage cells (HPC) density while administration of ABA, ethylene or auxin result in a strong reduction of HPCs and may play a role in shaping exodermal morphology (Liu et al., 2019). SLs and their synthetic analogs have found to be anti-carcinogenic. (Mayzlish-Gati et al., 2015). Hence, it can be stated that strigolactones seem to be a promising compound not only in agriculture, but also in medical sciences.
REFERENCES
Abe S, Sado A, Tanaka K, et al. (2014) Carlactone is converted to carlactonoic acid by MAX1 inArabidopsisand its methyl ester can directly interact with AtD14 in vitro. Proceedings of the National Academy of Sciences 111(50): 18084–18089.
Agusti J, Herold S, Schwarz M, et al. (2011) Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proceedings of the National Academy of Sciences 108(50): 20242–20247.
Al-Babili S and Bouwmeester HJ (2015) Strigolactones, a Novel Carotenoid-Derived Plant Hormone. Annual Review of Plant Biology 66(1): 161–186.
Aly R, Dubey NK, Yahyaa M, et al. (2014) Gene silencing of CCD7and CCD8 in Phelipanche aegyptiaca by tobacco rattle virus system retarded the parasite development on the host. Plant Signaling & Behavior 9(8).
Bonfante P and Requena N (2011) Dating in the dark: how roots respond to fungal signals to establish arbuscular mycorrhizal symbiosis. Current Opinion in Plant Biology 14(4): 451–457.
Brooks DW, Bevinakatti HS and Powell DR (1985) The absolute structure of (+)-strigol. The Journal of Organic Chemistry 50(20): 3779–3781.
Bürger M and Chory J (2020) The Many Models of Strigolactone Signaling. Trends in Plant Science 25(4): 395–405.
Cheng X, Ruyter-Spira C and Bouwmeester H (2013) The interaction between strigolactones and other plant hormones in the regulation of plant development. Frontiers in Plant Science 4.
Cook CE, Whichard LP, Turner B, et al. (1966) Germination of Witchweed (Striga lutea Lour.): Isolation and Properties of a Potent Stimulant. Science 154(3753): 1189–1190.
Cook CE, Whichard LP, Wall M, et al. (1972) Germination stimulants. II. Structure of strigol, a potent seed germination stimulant for witchweed (Striga lutea). Journal of the American Chemical Society 94(17): 6198–6199.
Dun EA, Germain ADS, Rameau C, et al. (2011) Antagonistic Action of Strigolactone and Cytokinin in Bud Outgrowth Control. Plant Physiology 158(1): 487–498.
Dun EA, Germain ADS, Rameau C, et al. (2013) Dynamics of Strigolactone Function and Shoot Branching Responses in Pisum sativum. Molecular Plant 6(1): 128–140.
Fernández I, Cosme M, Stringlis IA, et al. (2019) Molecular dialogue between arbuscular mycorrhizal fungi and the nonhost plant Arabidopsis thaliana switches from initial detection to antagonism. New Phytologist 223(2): 867–881.
Fukui K, Ito S and Asami T (2013) Selective Mimics of Strigolactone Actions and Their Potential Use for Controlling Damage Caused by Root Parasitic Weeds. Molecular Plant 6(1): 88–99.
Germain ADS, Ligerot Y, Dun EA, et al. (2013) Strigolactones Stimulate Internode Elongation Independently of Gibberellins. Plant Physiology 163(2): 1012–1025.
Hamiaux C, Drummond RS, Janssen BJ, et al. (2012) DAD2 is an α/β Hydrolase Likely to Be Involved in the Perception of the Plant Branching Hormone, Strigolactone. Current Biology 22(21): 2032–2036.
Hauck C, Müller S and Schildknecht H (1992) A Germination Stimulant for Parasitic Flowering Plants from Sorghum bicolor, a Genuine Host Plant. Journal of Plant Physiology 139(4): 474–478.
Hayward A, Stirnberg P, Beveridge C, et al. (2009) Interactions between Auxin and Strigolactone in Shoot Branching Control. Plant Physiology 151(1): 400–412.
Hu Z, Yamauchi T, Yang J, et al. (2013) Strigolactone and Cytokinin Act Antagonistically in Regulating Rice Mesocotyl Elongation in Darkness. Plant and Cell Physiology 55(1): 30–41.
Hýlová A, Pospíšil T, Spíchal L, et al. (2019) New hybrid type strigolactone mimics derived from plant growth regulator auxin. New Biotechnology 48: 76–82.
Jia K-P, Li C, Bouwmeester HJ, et al. (2019) Strigolactone Biosynthesis and Signal Transduction. Strigolactones – Biology and Applications: 1–45.
Johnson AW, Rosebery G and Parker C (1976) A novel approach to Striga and Orobanche control using synthetic germination stimulants. Weed Research 16(4): 223–227.
Kagiyama M, Hirano Y, Mori T, et al. (2013) Structures of D14 and D14L in the strigolactone and karrikin signaling pathways. Genes to Cells 18(2): 147–160.
Kannan C, Aditi P and Zwanenburg B (2015) Quenching the action of germination stimulants using borax and thiourea, a new method for controlling parasitic weeds: A proof of concept. Crop Protection 70: 92–98.
Kapulnik Y, Resnick N, Mayzlish-Gati E, et al. (2011) Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. Journal of Experimental Botany 62(8): 2915–2924.
Koltai H (2015) Cellular events of strigolactone signalling and their crosstalk with auxin in roots: Fig. 1. Journal of Experimental Botany 66(16): 4855–4861.
Kretzschmar T, Kohlen W, Sasse J, et al. (2012) A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 483(7389): 341–344.
Lee HW, Sharma P, Janssen BJ, et al. (2020) Flexibility of the petunia strigolactone receptor DAD2 promotes its interaction with signaling partners. Journal of Biological Chemistry 295(13): 4181–4193.
Liu G, Stirnemann M, Gübeli C, et al. (2019) Strigolactones Play an Important Role in Shaping Exodermal Morphology via a KAI2-Dependent Pathway. iScience 17: 144–154.
López-Ráez JA, Charnikhova T, Gómez-Roldán V, et al. (2008) Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytologist 178(4): 863–874.
López-Ráez JA, Kohlen W, Charnikhova T, et al. (2010) Does abscisic acid affect strigolactone biosynthesis? New Phytologist 187(2): 343–354.
Mayzlish-Gati E, Laufer D, Grivas CF, et al. (2015) Strigolactone analogs act as new anti-cancer agents in inhibition of breast cancer in xenograft model. Cancer Biology & Therapy 16(11): 1682–1688.
Minakuchi K, Kameoka H, Yasuno N, et al. (2010) FINE CULM1 (FC1) Works Downstream of Strigolactones to Inhibit the Outgrowth of Axillary Buds in Rice. Plant and Cell Physiology 51(7): 1127–1135.
Mishra S, Upadhyay S and Shukla R K (2017) The Role of Strigolactones and Their Potential Cross-talk under Hostile Ecological Conditions in Plants. Frontiers in Physiology, 7. doi:10.3389/fphys.2016.00691
Mishra S, Upadhyay S and Shukla RK (2017) The Role of Strigolactones and Their Potential Cross-talk under Hostile Ecological Conditions in Plants. Frontiers in Physiology 7.
Müller LM and Harrison MJ (2019) Phytohormones, miRNAs, and peptide signals integrate plant phosphorus status with arbuscular mycorrhizal symbiosis. Current Opinion in Plant Biology 50: 132–139.
Pandey A, Sharma M and Pandey GK (2016) Emerging Roles of Strigolactones in Plant Responses to Stress and Development. Frontiers in Plant Science 7: 1-17
Parker C (2013) The Parasitic Weeds of the Orobanchaceae. Parasitic Orobanchaceae: 313–344.
Parniske M (2008) Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nature Reviews Microbiology 6(10): 763–775.
Proust H, Hoffmann B, Xie X, et al. (2011) Strigolactones regulate protonema branching and act as a quorum sensing-like signal in the moss Physcomitrella patens. Development 138(8): 1531–1539.
Rasmussen A, Heugebaert T, Matthys C, et al. (2013) A Fluorescent Alternative to the Synthetic Strigolactone GR24. Molecular Plant 6(1): 100–112.
Sasse J, Simon S, Gübeli C, et al. (2015) Asymmetric Localizations of the ABC Transporter PaPDR1 Trace Paths of Directional Strigolactone Transport. Current Biology 25(5): 647–655.
Wang Y, Shang L, Yu H, et al. (2020) A Strigolactone Biosynthesis Gene Contributed to the Green Revolution in Rice. Molecular Plant 13(6): 923–932.
Xie X, Kusumoto D, Takeuchi Y, et al. (2007) 2′-Epi-orobanchol and Solanacol, Two Unique Strigolactones, Germination Stimulants for Root Parasitic Weeds, Produced by Tobacco. Journal of Agricultural and Food Chemistry 55(20): 8067–8072.
Yamada Y, Furusawa S, Nagasaka S, et al. (2014) Strigolactone signaling regulates rice leaf senescence in response to a phosphate deficiency. Planta 240(2): 399–408.
Yokota T, Sakai H, Okuno K, et al. (1998) Alectrol and orobanchol, germination stimulants for Orobanche minor, from its host red clover. Phytochemistry 49(7): 1967–1973.
Zhang J, Mazur E, Balla J, et al. (2020) Strigolactones inhibit auxin feedback on PIN-dependent auxin transport canalization. Nature Communications 11(1):1-10.
Zhang Y, Dijk ADJV, Scaffidi A, et al. (2014) Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nature Chemical Biology 10(12): 1028–1033.
Zwanenburg B and Blanco-Ania D (2018) Strigolactones: new plant hormones in the spotlight. Journal of Experimental Botany 69(9): 2205–2218.
Zwanenburg B and Pospíšil T (2013) Structure and Activity of Strigolactones: New Plant Hormones with a Rich Future. Molecular Plant 6(1): 38–62.
Zwanenburg B, Mwakaboko AS and Kannan C (2016) Suicidal germination for parasitic weed control. Pest Management Science 72(11): 2016–2025.
Zwanenburg B, Nayak SK, Charnikhova TV, et al. (2013) New strigolactone mimics: Structure–activity relationship and mode of action as germinating stimulants for parasitic weeds. Bioorganic & Medicinal Chemistry Letters 23(18): 5182–5186.
Zwanenburg B, Pospíšil T and Zeljković SĆ (2016a) Strigolactones: new plant hormones in action. Planta 243(6): 1311–1326.