1Department of Chemistry, Lakhimpur Girls’ College, North Lakhimpur, Assam, India
2Department of Agronomy and Horticulture, University of Nebraska – Lincoln, Lincoln, USA.
Corresponding author email: pegu.2010@gmaul.com
Article Publishing History
Received: 08/07/2020
Accepted After Revision: 10/09/2020
From the earliest detection of arsenic in the Brahmaputra basin back in 2004, several districts of Assam especially in the flood plains have joined the list of arsenic contamination. Lakhimpur district in Assam India, has been recorded with arsenic concentration several folds higher than the WHO and BIS recommendations. Scientific reports emphasize how native microorganisms can modulate the biogeochemical cycle of arsenic and their possible application in bioremediation. With the aim, this study was designed to isolate and carry out taxonomic characterization of arsenic resistant bacteria from potable water sources of the district. Based on the minimum inhibitory concentration test, two isolates LB6 and NB14 showing the highest resistance were characterized both by biochemical and molecular methods. Morphogenetic characterization identified the strains like Escherichia coli –LB6 and Acinetobacter baumannii –NB14. Taxonomic identification was further validated by fatty acid methyl ester analysis. This data of the entire study can conclude that two potential strains E.coli-LB6 and Acinetobacter baumannii-NB14 can resistant arsenic As V (100 to 200mM) and As III (10 to 50mM) concentration in the medium. The results further suggest that strains, LB6, and NB14 can survive under the arsenic stress and has been identified as a potential candidate for application in bioremediation field.
Arsenic, Bacteria, Assam, Brahmaputra
Pegu B. K, Buragohain M, Kakoti N, Sarmah P, Das S. Isolation and Molecular Characterization of Arsenic Resistant Bacteria from Brahmaputra River Basin of Assam, India. Biosc.Biotech.Res.Comm. 2020;13(3).
Pegu B. K, Buragohain M, Kakoti N, Sarmah P, Das S. Isolation and Molecular Characterization of Arsenic Resistant Bacteria from Brahmaputra River Basin of Assam, India. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/3bVtKZn
Copyright © Pegu et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Arsenic is a potent human carcinogen. Wide distribution in potable water sources and the negative health impacts of arsenic has raised concerns among scientific societies. Arsenic exists in four valency states in environment viz. arsine (-III), elemental arsenic (0), arsenite (III), and arsenate (V) (Smith et al., 2002). Anthropogenic and natural activities impact its distribution in the aquifer systems. Among the four valency states, arsenite and arsenate are most dominant in a natural environment. Incessant consumption of arsenic-contaminated water can cause skin, lungs, bladder, and kidney cancers (Wang et al., 2018). The maximum contaminant level (MCL) and permissible limit set by the US Environmental Protection Agency (US EPA) and World Health Organization (WHO) is 0.010 mg/L (Hughes., 2002; Shi et al., 2004). This is equivalent to 0.010 parts per million (ppm), 10 micrograms/liter (µg/L), or 10 parts per billion (ppb). Accumulation of arsenic in potable water sources is a serious problem in many parts of the world including India. States of northeastern India were detected with arsenic in several fold higher than the standard permissible level. Districts like Jorhat, Dhemaji, and Lakhimpur were recorded with arsenic in the range of 300 – 600 µg/L ( Singh et al., 2004 Das et al., 2015, 2017 Das and Barooah 2018.).
An estimated over 7 million people are using arsenic-contaminated water as a potable source. This alarming fact underlines the importance of this study. Microorganisms are ubiquitous in the environment. They are adapted to environmental extremes. In the evolutionary process, microorganisms had developed an array of metabolic processes to adapt to stressful environments. Microorganisms can actively control the biogeochemical cycles of nutrients and minerals. They are detected in several toxic environments like aluminum (Piña & Cervantes, 1996), cadmium (Ron et al., 1992), uranium (Nevin et al., 2003), cobalt (Sai Ram et al., 2000), mercury (Poulain et al., 2007), lead (Jarosławiecka & Piotrowska-Seget, 2014) and arsenic (Das et al., 2017a). Bacteria can use arsenic in the electron transport chain for energy generation or can oxidize and reduce in a process of homeostasis (Das & Barooah, 2018b, Jihang et al., 2019).
The flexibility of using different elemental compounds in metabolic pathways makes microorganisms as a potential candidate for bioremediation. The innocuous applicability of bioremediation is well documented (Kumar et al., 2018). The involvement of microorganisms in arsenic geocycle is well studied and reported by many researchers (Das & Barooah, 2018; Gnanaprakasam et al., 2017). There is a diverse group of microorganisms that are associated with arsenic biogeochemical cycles (Das et al., 2017a). Microorganisms can oxidize, reduce, and methylate arsenic compounds (Ehrlich.1976; Ahmann et al., 1994; Shariatpanahi et al., 1983). The active association of microorganisms controls the biogeochemical pathway of arsenic. Thus, characterization and identification of microorganisms in the arsenic environment will help us in understanding the inherent community and their association with geocycle of arsenic in the groundwater system. In the present study, two arsenic resistant bacteria were chemotaxonomically characterized and their efficiency of resistance was evaluated. This study will provide a snapshot of the inherent bacterial species of contaminated aquifers of the Brahmaputra river basin and highlight their involvement in arsenic mobilization and speciation.
MATERIAL AND METHODS
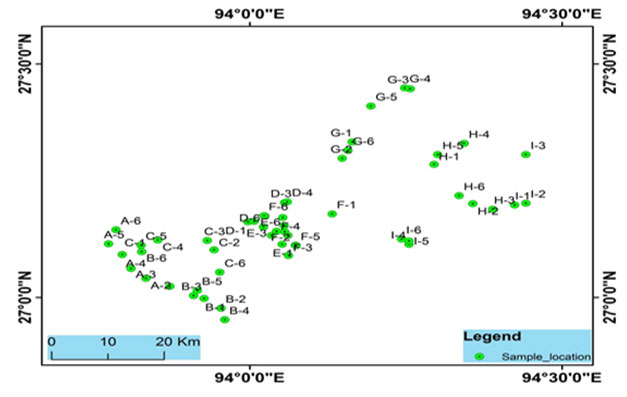
Study sites: For the present study, fifty-four water samples were collected from 9 blocks of Lakhimpur district. Samples were collected from tubes, ring wells, and rivers, which are mostly used as potable water sources in the district. Samplings were done in the post-monsoon season (July 2018). Samples were collected in sterilized Nalgene water bottles in replicates. For arsenic analysis, water samples were collected in water bottles pretreated with nitric acid. Collected samples were stored at 40C prior analysis. The locations of the sampling points were obtained with a handheld GPS device (Model: Garmin GPS 72) (Fig. 1).
Figure 1: A cross sectional view of the study area of the Brahmaputra river basin, Lakhimpur district, Assam, India.
Bacterial isolation: For bacterial isolation, 1 ml of water sample was serially diluted and cultured over nutrient agar (NA) (Hi-Media, India) plates containing arsenic at a concentration of As (V) – 1 mg/L and As (III) – 0.5 mg/L. Plates were incubated at 37 0C for 48 h and observed for bacterial growth. Morphologically distinct colonies were selected for further analysis.
Figure 2: Sampling sites location of the Brahmaputra river basin, Lakhimpur district, Assam, India.
Minimum Inhibitory Concentration:The minimum inhibitory concentration (MIC) of arsenate As (V) and arsenite As (III) was used to determine the arsenic resistance efficiency of the isolates. Bacterial isolates were cultured in freshly prepared NA broth at 30 0C for 48 h and then 100 μl of the freshly cultured bacterial suspension was added to nutrient broth supplemented with different concentration of As(III) (0 – 50 mM) and As(V) ( 0– 200 mM). Tubes are incubated for 72 h at 30 0C and 150 rpm. Microbial growth was recorded with a UV-Visible spectrophotometer (Himduzu, Japan) at 600 nm.
Morphological and Biochemical characterization: Morphological and biochemical characterization of potential isolates was be done as per Bergey’s Manual of Determinative Bacteriology (9th Edn.), Bergey’s Manual of Systematic Bacteriology (2nd Edn. 2005).
Genomic DNA extraction: Bacterial DNA was extracted from LB6 and NB14 (selected bacteria based on MIC) using QIAGEN (CA, USA) DNA extraction kit. The concentration of DNA was determined using NanoDrop. Fragments of 16S rRNA were amplified using universal primers 27f (16SF-AGAGTTTGATCCTGGCTCAG) and 1492R (16SR-TACGGTTACCTTGTTACGACTT). The PCR product was purified using QIAGEN PCR purification kit and sequenced using ABI 3730 capillary sequencer (16 capillary) with a big-dye terminator reaction.
Sequence analysis and phylogeny: Sequence files were analyzed and quality filtered prior assembly. Sequences were assembled using Codon-Code Aligner (ver 4.0). The assembled sequence was identified using the nucleotide Blast program against NCBI-nr/nt database and taxonomic profiling was done based on the identity percentage (> 97%). Sequence Alignment has been performed using ClustalW. The evolutionary relationship was determined by a neighbor-joining phylogenetic tree. The base substitution was calculated based on Juke-cantor, one parameter model with 1000 bootstrap values in MEGA 6.0 (Kumar 2013). Identified sequences were compared with the reference sequence from the NCBI nucleotide database.
Fatty acids profiling: The extraction and analysis of the fatty acid methyl ester (FAME) profiles of arsenic resistance bacteria were performed according to the method described by Buyer J. S., (2002). Statistical analysis: All the experiments were done in triplicates and results were presented in mean value with a standard deviation
RESULTS AND DISCUSSION
A total of 20 arsenic resistant bacteria were isolated and selected based on morphological distinctness. Arsenic resistant activity and bacterial screening were done by MIC test of different concentration of As (III) and As (V) [Table 1]. It was observed that arsenic affected the growth physiology of all the isolates. Results showed the highest tolerance and growth in isolates LB6 and NB14 in arsenic amended medium compared to control [Table 1]. Other isolates less growth as compared to control and other isolates. Based on MIC value isolate LB6 and NB14 were selected for further study.
Table 1. Minimum inhibitory concentration of different isolates
| Sr. No. | Isolate code | Control | As(V) concentration
(mM) |
As (III) concentration
(mM) |
||
| 0 | 100 | 200 | 10 | 50 | ||
| 1 | LB1 | 0.3±0.04 | 0.12±0.02 | 0.09±0.02 | 0.15±0.02 | 0.05±0.01 |
| 2 | LB3 | 0.4±0.02 | 0.23±0.01 | 0.15±0.04 | 0.21±0.05 | 0.08±0.01 |
| 3 | LB6 | 0.81±0.04 | 0.99±0.02 | 0.66±0.02 | 0.87±0.02 | 0.45±0.01 |
| 4 | NB-2 | 0.4±0.03 | 0.12±0.05 | 0.14±0.01 | 0.21±0.04 | 0.167±0.02 |
| 5 | NB10 | 0.54±0.07 | 0.13±0.01 | 0.167±0.04 | 0.31±0.02 | 0.1±0.05 |
| 6 | NB-14 | 0.68±0.03 | 0.85±0.05 | 0.84±0.01 | 0.9±0.04 | 0.71±0.02 |
| 7 | NB16 | 0.64±0.01 | 0.56±0.03 | 0.51±0.04 | 0.34±0.08 | 0.21±0.8 |
| 8 | GB3 | 0.34±0.01 | 0.21±0.03 | 0.2±0.04 | 0.08±0.04 | 0.06±0.01 |
| 9 | GB4 | 0.3±0.05 | 0.21±0.01 | 0.11±0.02 | 0.32±0.07 | 0.23±0.03 |
| 10 | BS1 | 0.53±0.04 | 0.51±0.02 | 0.43±0.07 | 0.21±0.05 | 0.18±0.05 |
| 11 | BS2 | 0.44±0.01 | 0.4±0.03 | 0.054±0.08 | 0.25±0.06 | 0.20±0.07 |
| 12 | BS3 | 0.57±0.03 | 0.48±0.04 | 0.43±0.03 | 0.15±0.08 | 0.12±0.04 |
| 13 | DS2 | 0.43±0.04 | 0.5±0.04 | 0.41±0.02 | 0.21±0.05 | 0.19±0.04 |
| 14 | DS4 | 0.32±0.05 | 0.26±0.05 | 0.14±0.07 | 0.31±0.08 | 0.27±0.02 |
| 15 | DS8 | 0.56±0.03 | 0.41±0.04 | 0.36±0.02 | 0.21±0.03 | 0.19±0.04 |
| 16 | HS1 | 0.5±0.02 | 0.49±0.03 | 0.38±0.2 | 0.34±0.04 | 0.25±0.03 |
| 17 | HS6 | 0.6±0.01 | 0.34±0.02 | 0.53±0.08 | 0.21±0.03 | 0.11±0.04 |
| 18 | NS8 | 0.44±0.01 | 0.25±0.02 | 0.18±0.07 | 0.17±0.08 | 0.15±0.02 |
| 19 | NS11 | 0.52±0.02 | 0.39±0.03 | 0.26±0.04 | 0.35±0.07 | 0.12±0.08 |
| 20 | NS19 | 0.45±0.03 | 0.22±0.01 | 0.15±0.01 | 0.27±0.31 | 0.14±0.03 |
| # Value indicates the mean ± SD of three independent replicates. | ||||||
Selected isolates LB6 and NB14 showed distinct morphological characteristics [Table 2]. Biochemical characterization showed that isolate LB6 was gram-negative rod-shaped bacteria with positive results catalase, Indole acetate, and H2S production. Isolate NB14 was gram-negative, non-motile, rod-shaped bacteria with positive tests for catalase, H2S production, methyl red, and Voges-Proskauer test. All the biochemical tests and morphological features are presented in [Tables 2 and 3] respectively.
Table 2. Microscopic observation of the arsenic resistance, bacterial strains LB6 and NB14
| Morphological | LB6 | NB14 |
| Colony shaped | Rods | Rods |
| Color | White | Creamy |
| Spore | Non-spore forming | Non-spore forming |
| Gram stains | Negative | Negative |
| Motility | Motile | Non motile |
| Surface | Smooth | Smooth |
| Elevation | Circular | Irregular |
Table 3. Biochemical test results for the arsenic resistant bacterial strains LB6 and NB14
| Biochemical Test | LB6 | NB14 |
| Catalase | Positive | Positive |
| Oxidation | Negative | Negative |
| Hydrogen Sulfide Production | Positive | Positive |
| Indole acetate | Positive | Negative |
| Methyl red | Positive | Positive |
| Voges-Proskauer | Negative | Positive |
| Citrate utilization | Negative | Negative |
| Glucose | Positive | Negative |
| Fructose | Negative | Positive |
| Maltose | Negative | Negative |
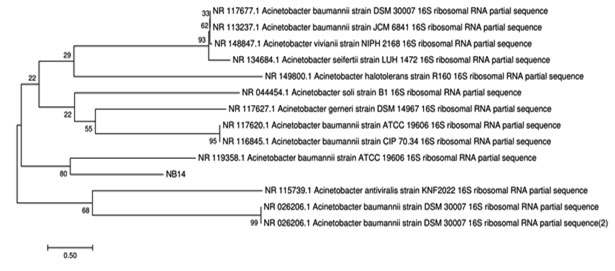
Isolated bacteria were taxonomically identified by 16S rRNA genes. Almost complete 16S rRNA gene [LB6 (1486 bp] and NB14 [1462 bp)] was sequenced and the sequence has been submitted to the GenBank database with accession number Escherichia coli-LB-6 [MK332441] and Acinetobacter baumannii – NB14 [MK332443]. The comparative analysis of the sequences of isolates with the already available database using BLAST (Basic Local Alignment Search Tool) showed that the strains were close to the other members of the genus. Analysis of the phylogenetic tree sequence indicates that strain LB6 closely related to Escherichia coli U5/41 and NB14 to Actenobacter viviani NIPH [Fig 3a and 3b].
Figure 3a: Phylogenetic relationship of LB6 strain with other bacteria
Figure 3.b: Phylogenetic relationship of NB14 with other bacteria
During investigation we have different kind of fatty acids concentration was found in strains Escherichia coli -LB-6 and Acinetobacter baumannii – NB14 [Result illustrated in table 4]. Major fatty acids detected in E.coli-LB-6 strain were 12:0 (Dodecanoic acid) 4.3%, 14:0 (12-Methyltridecanoic acid) 7.5%, 16:0 (Hexadecanoic acid) 25. 3%, 17:0 (10-Methylene-Hexadecanoic acid) 9.37%. Several reviews have been published in the fatty acid biosynthesis in E coli (Janßen and Steinbüche 2014). Where in Acinetobacter baumannii – NB14 strain major fatty acids were 10:0 (3-Hydroydecanoic acid) 8.52%, 13:0 (2-Hydroydodecanoic acid) 8.43%, 16:0 (Hexadecanoic acid) 18.31%. Previously number of fatty acids arachidonic acid (AA) and decosahexaenoic acid (DHA) are highly abundant in Acinetobacter baumannii bacterium (Jihang et al., 2019). A wide range of different fatty acids have been reported from microbial sources.
Table 4. Fatty acid profile of strains LB6 and NB14
|
FATTY ACIDS |
IUPAC/Systemic name | Concentration (%) | |
| L B-6 | NB-14 | ||
| 10:0 | 2-Hydroydecanoic acid | – | 1.31 |
| 10:1 | 3-Hydroydecanoic acid | – | 8.52 |
| 11:0 | 3-Hydroxy-9-Methyldecanoic acid | – | 0.36 |
| 12:0 | 10-Methylundecanoic acid | .28 | – |
| 12:1 | Dodecanoic acid | 4.34 | 5.24 |
| 12:0 | 2-Hydroxydodecanoic acid | – | 8.43 |
| 12:1 | (4Z)-4-Dodecenoic acid | 0.43 | – |
| 12:2 | (8Z)-8-Dodecenoic acid | – | 1.65 |
| 12.3 | 3-Hydroxydodecanoic acid | 5.58 | |
| 14:0 | 12-Methyltridecanoic acid | 7.55 | .89 |
| 14: | 11-Methyltridecanoic acid | – | 0.14 |
| 15:0 | (5Z)-13-Methyl-5-Tetradecenoic acid | 0.62 | 0.87 |
| 15:1 | 12-Methyltetradecanoic acid | 3.18 | 1.86 |
| 15:2 | (10Z)-10-Pentadecenoic acid | 3.76 | 0.56 |
| 15:3 | (6Z)-6-Pentadecenoic acid | – | 0.47 |
| 16:0 | 1-Hexadecanol | 0.39 | 0.29 |
| 16:1 | 14-Methylpentadecanoic acid | 0.49 | – |
| 16:0 | Hexadecanoic acid | 25.36 | 18.31 |
| 16:0 | 10-Methylhexadecanoic acid | 0.57 | – |
| 17:1 ISO W5C | (12Z)-12-Heptadecenoic acid | 3.98 | 0.81 |
| 17:0 ANTEISO | 14-Methylhexadecanoic acid | – | 1.42 |
| 17:1 ANTEISO | (7Z)-13-Methyl-7-Hexadecenoic acid | 0.67 | 0.40 |
| 17:0 | Heptadecanoic acid | 2.71 | 0.42 |
| 17:0 CYCLO | cis-9,10-Methylene-Hexadecanoic acid | 9.37 | 1.57 |
| 18:0 | 16-Methylheptadecanoic acid | 4.81 | 1.70 |
| 18:1W7C11-METHYL | (11Z)-10-Methyl-11-Octadecenoic acid | 1.29 | – |
| 18:3W6C | 12Z)-12-Octadecenoic acid | – | 1.15 |
| 18:1W5C | (13Z)-13-Octadecenoic acid | – | 0.29 |
| 19:0CYCLOW8C | cis-11,12-Methylene-Octadecanoic acid | 4.35 | – |
| 19:0 | Nonadecanoic | 1.20. | – |
| 20:0 ISO | Icosanoic acid | 0.38 | 0.80 |
| 20:2W9C | (11Z)-11-Icosenoic acid | 0.78 | |
| 20:2W6C | (11Z,14Z)-11,14-Icosadienoic acid | – | 0.30 |
Bacterial fatty acid profile represents the physiological responsiveness to the surrounding ecological niche it inhabit and the biochemical keys of taxonomical components (Diamonde et al., 2015). Most of microbial source has been viewed as ideal to explore and isolate commercially essential molecules including poly-unsaturated fatty acids (PUFA) (Tonato et al., 2018). This study alone may have little attributes toward taxonomic identity of the strain, but provides important information about molecular signature of the species and contribute to the understanding of the strain’s physiological and environmental response to variable ecological niche. Several reports suggest that the fatty acid pool of microorganisms undergo changes in response to the surrounding environment and from strain to strain within a species (Ratledge, 2004).
Microbes play a significant role in the arsenic metabolism pathway. They can transform the different forms of inorganic or organic of arsenic forms As (III) and As (V) undergo oxidation and reduction in our ecosystem (Mukhopadhyay et al., 2002). Several researchers have reported on isolating arsenic-resistant bacteria from arsenic-rich environments. Besides that different bacterial species such as Caulobacter, Rhizobium and Sphingomonas (Macur et al., 2001) Yersinia intermedia and Yersinia enterocolitica (Bansal et al., 2000), Listeria, Moraxella and Planococcus (Salam et al., 2009), Acinetobacter, Arthrobacter, Agrobacterium, Comamonas, Pseudomonas, Rhodococcus, and Stenotrophomonas (Cai et al., 2009), Bacillus anthracis and Citrobacter freundii (Shakoori et al., 2010), Brevibacillus brevis (Banerjee et al., 2013), Enterobacter asburiae and Enterobacter cloacae (Selvi et al., 2014) Pseudomonas (Satyapal et al .2018) have been reported from different environments site the world. In the present study, we have a 20th isolates isolated from water samples Brahmaputra river basin.
The 20 isolates were screen in the based on ability to grow on high levels of As (III) and As (V) containing medium. All of the isolates examined in this study were found to be resistant to both As (III) 50mM and As (V) 200mM of concentration in the medium, respectively. Out of them, we have selected two potential isolates LB6 and NB14 based on MIC data. They were found both are high resistant up to As V (100 to 200mM) and As III (10 to 50mM) in the medium. After morphological, biochemical, and 16 rDNA sequence molecular characterization we have confirmed that LB6 strain was Escherichia coli and NB14 strain was Acinetobacter baumannii. Escherichia coli and Acinetobacter broadly represent arsenic resistant bacteria strain isolated from an arsenic-rich environment (Anderson and Cook 2004; Jackson et al., 2005). A bacterium as we know E. coli is a model microorganism.
A huge amount of research has been fields like biotechnology and molecular levels. Similar result reported the Escherichia coli, which are resistant up to 909.79 mg/L As (IV) and 3120.1 mg/L As (III) (Bista and Shakya 2017), Acinetobacter baumannii, which are resistant up to (40 mM to 300 mM As V) and As III (4mM to 25mM As III) reported by (Alaniz et al., 2017). Staphylococcus sp. TA6 was isolated from arsenic contamination sites of groundwater of Jorhat, Assam, and biotransformation of arsenate to arsenite. They suggested the potential isolates will play important role in arsenic geo cycle in Brahmaputra valley (Das & Barooah, 2018b).
A similar study reported the two arsenic resistant bacteria Bacillus sp. and Aneurinibacillus aneurinilyticus, which can arsenic resistant up to As (V) 4500 ppm and As (III) 550 ppm isolated from arsenic affected groundwater of Purbasthali block of Burdwan, West Bengal, India, (Dey et al., 2016). Another study reported the Bacillus and Geobacillus arsenic oxidizing bacterial strain isolated from arsenic-contaminated soil of West Bengal, India. They were found both strains were As III (16-47mM) and As V (167-400mM) concentration is resistant in medium (Majumder et al., 2013). In northeastern India, the presence of arsenic has been identified in 21 districts out of 24 districts of Assam and six in Arunachal Pradesh, one in Manipur, three districts in Tripura, and two in Nagaland (Singh 2004; Mukherjee et al., 2006).
CONCLUSION
The extent, distribution, origin, and mobilization process of arsenic and iron in the aquifers of river Brahmaputra basin, mostly located in the Indian state of Assam, has been largely undocumented, and unexplored. Moreover, the river Brahmaputra basin has a boundary of tea gardens and paddy fields where a huge amount of fertilizers and pesticides are being used. The arsenic forms weak bonds with certain organic material, helps the arsenic to precipitate which may lead to arsenic contamination in groundwater and further decomposed in the same land, concentrating it. This entire study can conclude two potential strains were E.coli-LB6 and Acinetobacter baumannii-NB14 can resistant arsenic As V (100 to 200mM) and As III (10 to 50mM) concentration in the medium. The results suggest that strains, LB6, and NB14 can survive under the arsenic stress and has been identified as a potential candidate for application in bioremediations field.
ACKNOWLEDGMENT
This research was supported by a grant from the Department of Bio Technology (DBT), Govt. of India, New Delhi in the form of Major Research Project vide no. BT/IN/INDOUFOLDSCOPE/39/2015, dated 20th March, 2018. . We thank full Dr. Pankaj Chetia for technical support. We also thankful to Dibrugarh University, for providing the research facility.
Conflict of Interest: On behalf of all authors, the corresponding author states that there is no conflict of interest
REFERENCES
Ahmann, D., Roberts, A.L., Krumholz, L.R. and Morel, F.M., 1994. Microbe grows by reducing arsenic. Nature, 371(6500), pp.750-750.
Alaniz-Andrade, A.L., de León, C.L., Ramírez-Santoyo, R.M., Guzmán-Moreno, J. and Vidales-Rodríguez, L.E., 2017. Arsenic tolerance in bacterial cultures isolated from metal contaminated soil. Acta universitaria, 27(3), pp.9-18.
Anderson, C.R. and Cook, G.M., 2004. Isolation and characterization of arsenate-reducing bacteria from arsenic-contaminated sites in New Zealand. Current microbiology, 48(5), pp.341-347.
Banerjee, S., Majumdar, J., Samal, A.C., Bhattachariya, P. and Santra, S.C., 2013. Biotransformation and bioaccumulation of arsenic by Brevibacillus brevis isolated from arsenic contaminated region of West Bengal. IOSR J Environ Sci Toxicol Food Technol, 3(1), pp.1-10.
Bansal, N., Sinha, I. and Virdi, J.S., 2000. Arsenic and cadmium resistance in environmental isolates of Yersinia enterocolitica and Yersinia intermedia. Canadian journal of microbiology, 46(5), pp.481-484.
Barkay, T. and Poulain, A.J., 2007. Mercury (micro) biogeochemistry in polar environments. FEMS microbiology ecology, 59(2), pp.232-241.
Bhattacharya, P., Frisbie, S.H., Smith, E., Naidu, R., Jacks, G. and Sarkar, B., 2002. Arsenic in the environment: a global perspective. Handbook of heavy metals in the environment. Marcell Dekker Inc., New York, pp.147-215.
Bista, B. and Shakya, S., 2017. Isolation of Arsenic Resistant Escherichia coli from Sewage Water and Its Potential in Arsenic Biotransformation. Journal of Tropical Life Science, 7(1), pp.66-71.
Buyer, J.S., 2002. Identification of bacteria from single colonies by fatty acid analysis. Journal of microbiological methods, 48(2-3), pp.259-265.
Cai, L., Liu, G., Rensing, C. and Wang, G., 2009. Genes involved in arsenic transformation and resistance associated with different levels of arsenic-contaminated soils. BMC microbiology, 9(1), p.4.
Catalona, W.J., Richie, J.P., Ahmann, F.R., Hudson, M.L.A., Scardino, P.T., Flanigan, R.C., DeKernion, J.B., Ratliff, T.L., Kavoussi, L.R., Dalkin, B.L. and Waters, W.B., 1994. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. The Journal of urology, 151(5), pp.1283-1290.
Chakraborti, D., Rahman, M.M., Das, B., Chatterjee, A., Das, D., Nayak, B., Pal, A., Chowdhury, U.K., Ahmed, S., Biswas, B.K. and Sengupta, M.K., 2017. Groundwater arsenic contamination and its health effects in India. Hydrogeology Journal, 25(4), pp.1165-1181.
Chaudhari, M.B., Sutar, Y., Malpathak, S., Hazra, A. and Gnanaprakasam, B., 2017. Transition-metal-free C–H hydroxylation of carbonyl compounds. Organic letters, 19(13), pp.3628-3631.
Chen, H., Tang, Z., Wang, P. and Zhao, F.J., 2018. Geographical variations of cadmium and arsenic concentrations and arsenic speciation in Chinese rice. Environmental Pollution, 238, pp.482-490.
Chowdhury, R., Sen, A.K., Karak, P., Chatterjee, R., Giri, A.K. and Chaudhuri, K., 2009. Isolation and characterization of an arsenic-resistant bacterium from a bore-well in West Bengal, India. Annals of microbiology, 59(2), pp.253-258.
Das, S. and Barooah, M., 2018. Characterization of siderophore producing arsenic-resistant Staphylococcus strain TA6 isolated from contaminated groundwater of Jorhat, Assam and its possible role in arsenic geocycle. BMC microbiology, 18(1), p.104.
Das, S., Bora, S.S., Yadav, R.N.S. and Barooah, M., 2017. A metagenomic approach to decipher the indigenous microbial communities of arsenic contaminated groundwater of Assam. Genomics data, 12, pp.89-96.
Devassy, A., Kumar, R., Shajitha, P.P., John, R., Padmakumar, K.G., Basheer, V.S., Gopalakrishnan, A. and Mathew, L., 2016. Genetic identification and phylogenetic relationships of Indian clariids based on mitochondrial COI sequences. Mitochondrial DNA Part A, 27(5), pp.3777-3780.
Dey, U., Chatterjee, S. and Mondal, N.K., 2016. Isolation and characterization of arsenic-resistant bacteria and possible application in bioremediation. Biotechnology reports, 10, pp.1-7.
Diomandé S. E., Nguyen-The C., Guinebretière M. H., Broussolle V. and Brillard J., 2015. Role of Fatty Acids in Bacillus environmental adaptations .Frontiers in Microbiology, 6:
Ehrlich, H.L., 1978. Inorganic energy sources for chemolithotrophic and mixotrophic bacteria. Geomicrobiology Journal, 1(1), pp.65-83.
Garrity, G.M., 2005. Systematic bacteriology. The Proteobacteria, Part C: The Alpha-, Beta-, Delta-, and Epsilonproteobacteria, Bergey’s Manual Trust, Department of Microbiology and Molecular Genetics,, 2.
Gnanaprakasam, E.T., Lloyd, J.R., Boothman, C., Ahmed, K.M., Choudhury, I., Bostick, B.C., van Geen, A. and Mailloux, B.J., 2017. Microbial community structure and arsenic biogeochemistry in two arsenic-impacted aquifers in Bangladesh. MBio, 8(6).
Jackson, C.R., Harrison, K.G. and Dugas, S.L., 2005. Enumeration and characterization of culturable arsenate resistant bacteria in a large estuary. Systematic and applied microbiology, 28(8), pp.727-734.
Janßen, H.J. and Steinbüchel, A., 2014. Fatty acid synthesis in Escherichia coli and its applications towards the production of fatty acid based biofuels. Biotechnology for biofuels, 7(1), pp.1-26.
Jarosławiecka, A. and Piotrowska-Seget, Z., 2014. Lead resistance in micro-organisms. Microbiology, 160(1), pp.12-25.
Jiang, J.H., Hassan, K.A., Begg, S.L., Rupasinghe, T.W., Naidu, V., Pederick, V.G., Khorvash, M., Whittall, J.J., Paton, J.C., Paulsen, I.T. and McDevitt, C.A., 2019. Identification of novel Acinetobacter baumannii host fatty acid stress adaptation strategies. Mbio, 10(1).
Macur, R.E., Wheeler, J.T., McDermott, T.R. and Inskeep, W.P., 2001. Microbial populations associated with the reduction and enhanced mobilization of arsenic in mine tailings. Environmental science & technology, 35(18), pp.3676-3682.
Majumder, A., Bhattacharyya, K., Bhattacharyya, S. and Kole, S.C., 2013. Arsenic-tolerant, arsenite-oxidising bacterial strains in the contaminated soils of West Bengal, India. Science of the total environment, 463, pp.1006-1014.
Mukherjee, A., Bhattacharya, P., Savage, K., Foster, A. and Bundschuh, J., 2008. Distribution of geogenic arsenic in hydrologic systems: controls and challenges.
Mukhopadhyay, R., Rosen, B.P., Phung, L.T. and Silver, S., 2002. Microbial arsenic: from geocycles to genes and enzymes. FEMS microbiology reviews, 26(3), pp.311-325.
Nevin, K.P., Finneran, K.T. and Lovley, D.R., 2003. Microorganisms associated with uranium bioremediation in a high-salinity subsurface sediment. Applied and environmental microbiology, 69(6), pp.3672-3675.
Nevin, W., Wortman, S. and Wysocki, J., Nevin Informatics LLC, 2003. Health care provider information system. U.S. Patent Application 09/988,234.
Peterson, S.A., Herlihy, A.T., Hughes, R.M., Motter, K.L. and Robbins, J.M., 2002. Level and extent of mercury contamination in Oregon, USA, lotic fish. Environmental Toxicology and Chemistry: An International Journal, 21(10), pp.2157-2164.
Piña, R.G. and Cervantes, C., 1996. Microbial interactions with aluminium. Biometals, 9(3), pp.311-316.
Poulain, A.J., Chadhain, S.M.N., Ariya, P.A., Amyot, M., Garcia, E., Campbell, P.G., Zylstra, G.J. and Barkay, T., 2007. Potential for mercury reduction by microbes in the high arctic. Applied and Environmental Microbiology, 73(7), pp.2230-2238.
Ram, M.S., Singh, L., Suryanarayana, M.V.S. and Alam, S.I., 2000. Effect of iron, nickel and cobalt on bacterial activity and dynamics during anaerobic oxidation of organic matter. Water, Air, and Soil Pollution, 117(1-4), pp.305-312.
Ratledge, C., 2004. Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie, 86(11), pp.807-815.
Ron, E.Z., Minz, D., Finkelstein, N.P. and Rosenberg, E., 1992. Interactions of bacteria with cadmium. In Microorganisms to Combat Pollution(pp. 37-46). Springer, Dordrecht.
Sairam, R.K., Srivastava, G.C. and Saxena, D.C., 2000. Increased antioxidant activity under elevated temperatures: a mechanism of heat stress tolerance in wheat genotypes. Biologia Plantarum, 43(2), pp.245-251.
Salam, M.A., Hossain, M.S., Ali, M.E., Asad, M.A. and Ali, M.H., 2009. Isolation and characterization of arsenic resistant bacteria from different environment in south-west region of Bangladesh. Research Journal of Environmental Sciences, 3(1), pp.110-115.
Satyapal, G.K., Mishra, S.K., Srivastava, A., Ranjan, R.K., Prakash, K., Haque, R. and Kumar, N., 2018. Possible bioremediation of arsenic toxicity by isolating indigenous bacteria from the middle Gangetic plain of Bihar, India. Biotechnology reports, 17, pp.117-125.
Selvi, M.S., Sasikumar, S., Gomathi, S., Rajkumar, P., Sasikumar, P. and Govindan, S., 2014. Isolation and characterization of arsenic resistant bacteria from agricultural soil, and their potential for arsenic bioremediation. International Journal of Agricultural Policy and Research, 2(11), pp.393-405.
Shakoori, F.R., Aziz, I., Rehman, A. and Shakoori, A.R., 2010. Isolation and characterization of arsenic reducing bacteria from industrial effluents and their potential use in bioremediation of wastewater. Pakistan Journal of zoology, 42(3).
Shariatpanahi, M., Anderson, A.C., Abdelghani, A.A., Englande, A.J., Oxley, T.A. and Barry, S., 1983. Biodeterioration.
Shoham, Y., Schwartz, Z., Khasin, A., Gat, O., Zosim, Z. and Rosenberg, E., 1992. Delignification of wood pulp by a thermostable xylanase from Bacillus stearothermophilus strain T-6. In Microorganisms to Combat Pollution (pp. 83-94). Springer, Dordrecht.
Singh, A.K., 2004, November. Arsenic contamination in groundwater of North Eastern India. In Proceedings of 11th national symposium on hydrology with focal theme on water quality, National Institute of Hydrology, Roorkee(pp. 255-262).
Smith, E., Naidu, R. and Alston, A.M., 2002. Chemistry of inorganic arsenic in soils: II. Effect of phosphorus, sodium, and calcium on arsenic sorption. Journal of Environmental Quality, 31(2), pp.557-563.
Tamura, K., Stecher, G., Peterson, D., Filipski, A. and Kumar, S., 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular biology and evolution, 30(12), pp.2725-2729.
Tonato, D., Marcuz, C., Vendruscolo, R.G., Bevilacqua, C., Jacques, R.J., Wagner, R., Kuhn, R.C. and Mazutti, M.A., 2018. Production of polyunsaturated fatty acids by microorganisms isolated in the Brazilian Pampa biome. Brazilian Journal of Chemical Engineering, 35(3), pp.835-846.
Weisburg, W.G., Barns, S.M., Pelletier, D.A. and Lane, D.J., 1991. 16S ribosomal DNA amplification for phylogenetic study. Journal of bacteriology, 173(2), pp.697-703.
Zhang, W., Singh, P., Paling, E. and Delides, S., 2004. Arsenic removal from contaminated water by natural iron ores. Minerals engineering, 17(4), pp.517-524.
Zheng, Y., Shi, Y., Tian, C., Jiang, C., Jin, H., Chen, J., Almasan, A., Tang, H. and Chen, Q., 2004. Essential role of the voltage-dependent anion channel (VDAC) in mitochondrial permeability transition pore opening and cytochrome c release induced by arsenic trioxide. Oncogene, 23(6), pp.1239-1247.