Department of Biotechnology, University Institute of Engineering & Technology, Kurukshetra University, Kurukshetra- 136119, India
Corresponding author Email: amitakuk@gmail.com
Article Publishing History
Received: 27/03/2019
Accepted After Revision: 01/06/2019
Dye industry effluent containing toxic compounds, heavy metals and pigments are polluting agricultural soil and water by incorporating toxic, mutagenic and carcinogenic heavy metals from the dye. This study aims to investigate the heavy metal resistant pattern of bacterial diversity present in effluents of dye industry containing various heavy metal in Faridabad, Haryana (India). After carrying out the primary screening, five bacterial isolates KL1-KL5 were selected on the basis of resistant showed against initial concentration (80 µg) of different heavy metal salts. Strain KL1 was highly resistant to all the test heavy metals Nickel (380 µg/ml), Lithium (420 µg/ml), Copper (360 µg/ml), Ferric (400 µg/ml) and Zinc (300 µg/ml) when cultured on nutrient agar medium. Optimized growth conditions for KL1 were at 35°C, pH 7 and 4% NaCl concentration. Strain KL1 was identified as E.coli on the basis of morphological, physiological and biochemical characteristics from IMTECH, Chandigarh. This study revealed that the isolated bacterial strain KL can be efficiently used in the removal of heavy metals in contaminated industrial effluents.
Antibiotic, carcinogenic, Heavy metals, Mutagenic, Effluent
Kumar T, Gupta R, Mittal A. Isolation and Biochemical Characterization of Heavy Metal Resistant Bacteria from Dye Industry Effluent in Faridabad, Haryana, India. Biosc.Biotech.Res.Comm. 2019;12(2).
Kumar T, Gupta R, Mittal A. Isolation and Biochemical Characterization of Heavy Metal Resistant Bacteria from Dye Industry Effluent in Faridabad, Haryana, India. Biosc.Biotech.Res.Comm. 2019;12(2). Available from: https://bit.ly/31fZK4Q
Copyright © Kumar et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Constantly increasing population and industrialization activities are polluting air, water and soil by depositing heavy metals (Marzan et al., 2017; Shifaw, 2018). Various dyes are being used in the textile industry having toxic properties. Azo dye is one of the main pollutants which contribute up to 70% of the textile and paper dyes. Even after using a number of traditional effluent treatment methods these carcinogenic dyes are non-degradable and discharged into the water resources (Carliell et al., 1995; Karatas and Durusun, 2007; Islam et al., 2017). The textile dye effluent consists of heavy metals such as Cadmium, Lead and Zinc either in free ionic metals or complex metals, said to mostly originate from the dyeing process which are very toxic and carcinogenic (Basha and Rajaganesh, 2014). Natural water resources have been polluted by toxic heavy metal containing effluent. These toxic compounds are accumulated in living organisms including microorganisms, plants, animals and human, resulting into serious threat to the health of living organisms, in the present scenario the dye industry effluent is a major source of heavy metal toxicity, (Sarker et al., 2015, Ayangbenro and Babalola, 2017 and Shifaw, 2018).
Textile dye effluents cause serious environmental problems by absorbing light in receiving water bodies like streams, rivers and lakes etc and ultimately interfering with aquatic biological processes.The water containing textile effluent used for irrigation contains heavy metals like Cd, Pb and Zn, which accumulate in various parts of plans that result in various clinical problems in animals as well as human beings including hepatic and renal system damages, mental retardation and degradation of basal ganglia of brain (Emongor et al., 2005). Water pollution due to toxic heavy metals through textile dye effluent remains a serious environmental and public problem in developing countries, (Forgacs et al.,2004; Hao et al., 2000; Saini, 2017 and Shifaw, 2018 ). .
There are various conventional methods available for removal of toxic compounds, pigments, dyes and heavy metals from the industrial effluents. These traditional methods are less efficient, costly and time consuming. Presently bioremediation using microorganisms proved to be a revolutionary technique for the removal and degradation of these toxic compounds from soil and water (Su et al., 2014). Bacteria, fungi and many other microorganisms have metabolic pathways which can uptake and use toxic compounds as an energy source for their survival. Microorganisms have enzymes that can degrade the toxic contaminant into nontoxic form. Due to their characteristic derivative enzymes they have developed resistance against heavy metals in order to adapt toxic levels of heavy metals evolved diverse mechanisms for maintaining homeostasis and resistance to heavy metals, in order to adapt to toxic metals in the ecosystem (Brar et al., 2006; Wei et al., 2014). The present study was carried out to explore the heavy metal resistance and degradation capabilities of microbial diversity present into dye industry effluent.
Material and Methods
Study Area and Sample Collection
Effluent Samples were collected from various industrial sites in and around Faridabad, Haryana (India). Labeled polyethylene bottles previously washed with 10M HNO3 and distilled water were used for sample collection and a cold chain was maintained while transferring to the laboratory in Kurukshetra University Kurukshetra. Effluent sample was filled in these bottles by making sure that there is no air space left. Collected samples were preserved at 40C for further experiments and analysis. Various parameters like pH, temperature, and color of effluent were documented at the sampling site using methods recommended by APHA (1992).
Isolation and Primary Screening for Heavy Metal Resistant Bacteria: For isolation of heavy metal resistant bacteria, effluent samples were diluted to obtain ten-fold serial dilutions (Azad et al., 2013). 100 µl of undiluted dye effluent sample and dilutions from 10-1 to 10-4 were spread on sterile nutrient agar plates incorporated with initial concentrations (80 µg/ml) of Ferric Chloride (FeCl3), Copper Sulphate (CuSO4), Zinc sulphate (ZnSO4) and Nickel Sulphate (NiSO4) and Lithium Sulphate (Li2SO4). Heavy meatal salts used were of analytical grade and Millipore membranes with a 0.22 μm pore size used to sterilize solutions prepared. Plates were incubated at 30oC for 48 hours and observed for bacterial colonies. To enhance the accuracy and authenticity, this screening experiment was carried out and in triplicate. Minimum inhibitory concentration (MIC) of the selected isolates against increasing concentrations of heavy metals on nutrient agar plates was evaluated until the strain unable to grow colonies even after seven days of incubation. Cultures were stored in glycerol stock solution at -200C.
Multiple Metal Resistance Capacity: For determining the heavy metal resistance spectrum, the bacterial strains isolated after primary screening were separately grown on nutrient agar plates supplemented with selected heavy metals (80 μg/mL) at pH 7.0 and 37 °C for 24 h. After incubation the resistance capacity of multiple heavy metals was assessed.
Determination of Minimum Inhibitory Concentration (MIC): Minimum inhibitory concentration of each heavy metal against isolated bacterial strains was determined by growing them on heavy metal incorporated nutrient agar medium. Concentration of respective heavy metals has been increased gradually until the bacterial strain failed to grow colony. The starting concentration of the heavy metals was 80 μg/ml. The culture growing on initial concentration was then streaked on to the higher concentration of heavy metal. The concentration at which bacterial strain failed to grow colony was considered as MIC.
Determination of Optimal Growth Conditions: Growth conditions for bacterial isolate KL were optimized by growing in nutrient broth medium at different pH (5 to 10), temperature (150C to 650C) and salt concentrations (1%-10%). The optical density of the log phase growing cultures conditions was noted at 600nm to determine the growth.
Effects of Heavy Metals on Microbial Growth in Liquid Medium: Bacterial isolate KL was separately grown in nutrient broth supplemented with Nickel (Ni2+), Lithium (Li+), copper (Cu2+), ferric (Fe3-), and zinc (Zn2+) (80 μg/mL) at pH 7.0 and 37 °C for 24 h. Bacterial cells in exponential phase cells were used to inoculate nutrient broth medium. The concentrations of each heavy metals were increased gradually. The cultures were incubated for 4 days at 370C and agitated at 150 rpm on an orbital shaking incubator (REMI, India). Growth rate of the strain was determined by increasing absorbance at wavelength of 600 nm [OD600] with a Spectronic 200 Spectrophotometer (Thermo Scientific, India). Experiment was done in triplicate.
Morphological and Biochemical Identification: Morphological characteristics of strain KL were examined under the microscope by using Gram Staining technique. For the analysis of biochemical characteristics bacterial culture was sent to IMTECH, Chandigarh.
Antibiotic susceptibility Test: Bacterial strain KL was tested for susceptibility to antimicrobial agent by Disc Diffusion method on Muller-Hinton Agar plates (Gupta et al, 2016). Zone of inhibition was noted after 24h incubation and susceptibility was recorded as positive. Antimicrobial agents used for the study were: Vancomycin (30 µg), Erythromycin (15 µg), Penicillin (10 µg), Minocyclin (30 µg), Gentamycin (10 µg), Rifampicin (5 µg), Ofloxacin (5 µg), Amoxycillin (25 µg), Methicillin (5 µg), Chloramphenicol (30 µg), Spectinomycin (100 µg), Metronidazole (5 µg), Nitronidazole (10 µg), Clindamycin (10 µg), Ampicillin (5 µg) and Deoxycholin (10 µg).
Results and Discussion
On the basis of primary screening, carried out with an initial heavy metal concentration of 80 µg/ml on nutrient agar medium, total 15 bacterial strains were isolated. Visual observation of bacterial colonies on heavy metal containing nutrient agar medium after 3-4 days of incubation at 370C showed that the collected dye effluent sample have metal resistant diversity of bacteria. The bacterial colonies were able to grow well on solid medium supplemented with Nickel (Ni2+), Lithium (Li+), copper (Cu2+), ferric (Fe3), and zinc (Zn2+) at an initial concentrations of 80 µg/ml. Out of these bacterial strains, five (KL1, KL2, KL3, KL4 and KL5) were selected for further studies. Primary screening results for KL1 are shown in figure 1.
 |
Figure 1: Culture plates A, B, C, D and E showing growth of Bacterial strain KL1 on Nutrient Agar medium incorporated with 80 µg/ml of different heavy metals ( (Screening results). |
MIC study of each heavy metal showed that all the five bacterial strains were tolerating heavy metal concentration between 130-420 µg/ml (Table 1). MIC of heavy metals showed that bacterial strain KL1 have highest tolerance to all the heavy metals. Multiple heavy metal resistance and MIC study revealed that all the five selected bacteria have high tendency to tolerate and grow under heavy metal stressed environment. On the basis of this study, potential bacterial strain KL was selected for further studies.
Table 1: Multiple heavy metal resistance capacity and MIC study results of five bacterial isolates.
| Metals | MIC (µg/ml) | ||||
| KL1 | KL2 | KL3 | KL4 | KL5 | |
| Nickel (Ni2+) | 380 | 220 | 130 | 160 | 250 |
| Lithium (Li+) | 420 | 130 | 170 | 200 | 170 |
| Copper (Cu2+) | 360 | 320 | 180 | 180 | 200 |
| Ferric (Fe3+) | 400 | 350 | 240 | 220 | 150 |
| Zinc (Zn2+) | 300 | 220 | 190 | 200 | 180 |
Growth conditions for KL were optimized for pH, tempratute and NaCl concentration in the medium.
The optimization study was carried out in nutrient brtoh medium. Bacterial strain was grown in flasks containing 200 ml sterilized nutrient broth medium at diifferent pH (5-10), temparature (150C-650C) and salt concentration (1%-10%). Results of optimization study showed that pH-7, tempratue-350C and 4% salt concentration were the optimized parameter growth of bacterial strain KL (figure 1).
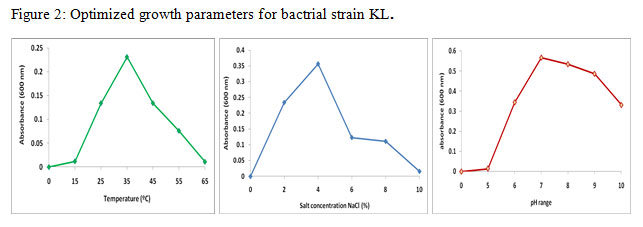 |
Figure 2: Optimized growth parameters for bactrial strain KL. |
After optimizing the growth parameters, bacterial strain KL was investigated for microbial growth in liquid medium (Nutrient Broth) supplemented with Nickel (Ni2+), Lithium (Li+), copper (Cu2+), ferric (Fe3-), and zinc (Zn2+) (80 μg/mL) at pH 7.0 and 35°C for 24 h. Exponential phase cells (24h old) of bacterial strain were used to inoculate nutrient broth medium. The concentrations of each heavy metals were increased gradually. Incubation was given for 12hrs at 350C and agitated at 150 rpm on an orbital shaking incubator. Growth rate of the strain was determined by absorbance at wavelength of 600 nm. With increasing heavy metals of the growth of KL was declined. The most important aspect of this study was that KL can grow in nutrient broth medium containing Cu2+ (280), Ni2+ (320), Fe3- (360) and Li2+ (400) (figure2).
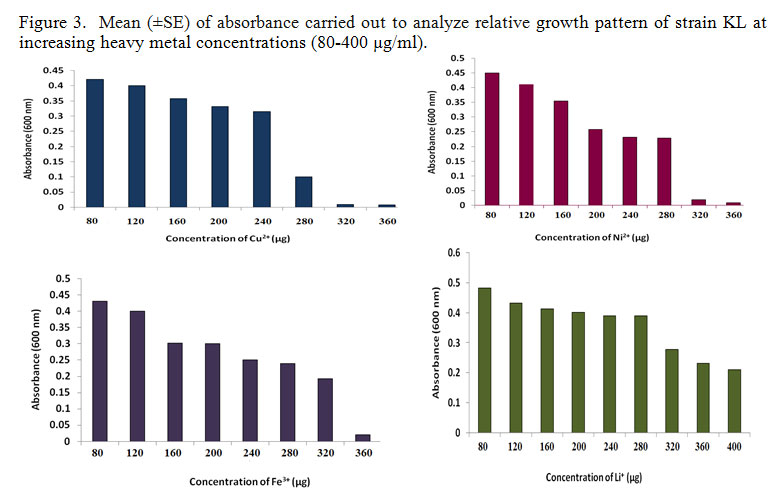 |
Figure 3: Mean (±SE) of absorbance carried out to analyze relative growth pattern of strain KL at increasing heavy metal concentrations (80-400 µg/ml). |
KL was examined under microscope for its morphological characteristics by usnig grams staining technique. Gram staining is a technique used to distinguish two groups of bacteria ( gram +ve and gram –ve) based on their different cell wall constituents. KL was found to be a rod sahpe and gram –ve bacterium. Common physiological and biochemical tests were also performed for classification and identification of bacterial strain KL. Results of morphological, physiological and biochemical cahracteristics are shown in table 2.
Table 2: Morphological, physiological and biochemical analysis performed on strain KL.
| Morphological Test
Results |
Biochemical Tests
Results |
||
| Colony Configuration | Circular | Indole Test | + |
| Cell Shape | Rod | Voges proskauer Test | _ |
| Colony Elevation | Flat | Citrate Utilization | _ |
| Colony Margin | Entire | H2S Production | _ |
| Colony Surface | Smooth | Gas production from Glucose | _ |
| Colony Colour | Creamish | Gelatin Hydrolysis | _ |
| Opacity | Opaque | Casein, Starch and Urea Hydrolysis | + |
| Gram’s staining | -ve | Nitrate Reduction | _ |
| Size | 1.0 | Ornithine and Lysine Decarboxylase | _ |
| Spore | – | Catalase Test | + |
| Motility | Yes | Oxidase Test | _ |
| Shape | Ellipsoidal | Acid Production from | |
| Position | No | Fructose, Arabinose, Galactose, Glucose, Mannitol, Xylose, Sucrose, Rhamnose | + |
| Raffinose, Salicin, Mesoinositol | _ | ||
Bacterial strain KL1 was identified as E.Coli on the basis of morphological, physiological and biochemical analysis report of IMTECH, Chandigarh.
KL1 was investigated for susceptibility towards different antibiotics. This study confirmed that bacterial isolate was susceptible to a wide range of antibiotics (Table 3). We need to incorporate bacterial strain to soil and water for removal of heavy metals that may be pathogenic for other living organisms. Following antibiotics available commercially can be used to treat any infection developed due to bacterial stain KL.
Table 3: Antibiotic susceptible profile of bacterial strain KL.
| Antibiotic | Disc content (µg) | Diameter of inhibition zone (mm) | Susceptibility status |
| Vancomycin | 30 | 10 | Susceptible |
| Erythromycin | 15 | 15 | Susceptible |
| Penicillin | 10 | 17 | Susceptible |
| Minocyclin | 30 | 20 | Susceptible |
| Gentamycin | 10 | 15 | Susceptible |
| Rifampicin | 5 | 12 | Susceptible |
| Oflaxacin | 5 | 13 | Susceptible |
| Amoxycolin | 25 | 18 | Susceptible |
| Methicillin | 5 | No zone | Resistant |
| Chloroamphinicol | 30 | 26 | Susceptible |
| Spectinomycin | 100 | 28 | Susceptible |
| Metronidazole | 5 | No zone | Resistant |
| Nitronidazole | 10 | No zone | Resistant |
| Clindomycin | 10 | 13 | Susceptible |
| Ampicillin | 5 | 15 | Susceptible |
| Deoxycholin | 10 | No zone | Resistant |
Conclusion
There are various techniques available for removal of toxic heavy metals from environment but this study revealed some significant results for metal detoxification by using microorganisms. Results of present study conclude that microorganism isolated from dye industry effluent developed the ability to tolerate heavy metal stress. Bacterial strains isolated in the course of this study can be efficiently used for removal of toxic heavy metals from ecosystem and reinforce the ecological balance. Use of microbes for bioremediation is highly recommended due to its low cost and environmental friendly approach. This nature friendly technique has turned out to be the best available method which is highly efficient under heavy metal stressed environment. Further experimental study is required in the area of gene transfer for developing Genetically Modified organisms using recombinant DNA technology to make this approach more efficient and effective.
Acknowledgements
The authors are grateful of Director of UIET, Kurukshetra University, Kurukshetra, India, for providing instruments and facilities of this research. This research work was potentially supported by TEQIP World Bank project for providing funding.
Conflict of Interest
The authors declare of no conflict of interest in conducting this study.
References
Azad, A.K., Nahar, A., Hasan, M.M., Islam, K., Azim, M.F., Hossain, M.S., Rahman, M.R., Ojha, R.K., Mahmud, G.M.S. and Kayes, R. (2013). Fermentation of municipal solid wastes by bacterial isolates for production of raw protein degrading proteases. Asian J. Microbiol. Biotechnol. Environ. Sci., 15, 365-374.
Ayangbenro, A.S. and Babalola, O.O. (2017). A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Public Health. 14(1):94. Doi: 10.3390/ijerph14010094
Basha, S.A. and Rajaganesh, K. (2014). Microbial Bioremediation of Heavy Metals from Textile Industry Dye Effluents using Isolated Bacterial Strains. Int.J.Curr.Microbiol.App.Sci, 3 (5), 785-794.
Carliell, C.M., Barclay, S.J., Naidoo, N., Buckley, C.A., Mulholland, D.A., Senior, E. (1995). Microbial decolourization of a reactive azo dye under anaerobic conditions. Water SA, 21: 61-69.
Emongor, V.E., Khonga, E.B., Ramolemana,G.M., Marumo, K., Machacha, S. and Motsamai ,T. (2005) . Suitability of Treated Secondary Sewage Effluent for Irrigation of Horticultural Crops in Botswana. Journal of Applied Sciences, 5 (3), 451-454.
Forgacs, E., Crestile, T. and Oros, G. (2004), Removal of synthetic dyes from waste water: A review. Environmental international, 30,953-971.
Gupta, R., Kumar, T. and Mittal, A. (2016). Isolation, identification and characterization of heavy metal resistant bacteria from soil of an iron industry, Haryana (India). Int. J. of Pharma. Sci. and Res., 7(3), 1308-1313.
Hao, O.J., Kim, H. and Chiag, P.C. (2000). Decolourization of waste water. Env. Sci. and Tech., 30, 449-505.
Islam, T., Rahman, M.S. and Hussain, M.S. (2017). Heavy Metal Tolerance Pattern of Textile Dye Degrading Native Bacteria: A Bioremediation Viewpoint. Annals of Medical and Health Sciences Research, 7(1), 67-73.
Karataş, M. and Dursun S. (2007). Bio-decolourization of azo-dye under anaerobic batch conditions J. Int. Env. App. & Sci., 2 (12): 20-25
Marzan, L.W., Hossain, M., Mina, S. A., Akter, Y. and Chowdhury, A.M. (2017). Isolation and biochemical characterization of heavy-metal resistant bacteria from tannery effluent in Chittagong city, Bangladesh: Bioremediation viewpoint. The Egyptian Journal of Aquatic Research, 43(1), 65-74.
Su, C., Jiang, L. and Zhang W. (2014). A review on heavy metal contamination in the soil worldwide: situation, impact and remediation techniques. Environ. Skept. Crit., 3(2), 24-38.
Sarker, B.C., Baten, M.A., Eqram-Ul Haque, M., Das A.K., Hossain, A. and Hasan M.Z. (2015). Heavy Metals Concentration in Textile and Garments Industries’ Wastewater of Bhaluka Industrial Area, Mymensingh, Bangladesh. Curr World Environ, 10(1), 61-66.
Saini, R.D. (2017). Textile Organic Dyes: Polluting effects and Elimination Methods from Textile Waste Water. International Journal of Chemical Engineering Research, 9(1), 121-136.
Shifaw, E. (2018). Review of Heavy Metals Pollution in China in Agricultural and Urban Soils. J Health Pollution, 8 (18), 180607.


