1School of Sciences, ITM University, Gwalior, Madhya Pradesh, India.
2College of Agriculture, Jawaharlal Nehru Krishi Vishwavidyalaya,
Jabalpur, Madhya Pradesh, India.
Corresponding author email: shivomsingh@itmuniversity.ac.in
Article Publishing History
Received: 15/09/2021
Accepted After Revision: 14/12/2021
Comparative analysis of airborne bacterial load in the rural and urban indoor and outdoor environment is of utmost importance to evaluate the wellbeing hazards linked with co3ntamination of airborne bacteria in the indoor environment. The present study was conducted during December, 2020 to March, 2021 among 50 randomly selected rural and urban (Adupurajagir and Gwalior, respectively) dwellings to determine the indoor and outdoor bacterial load. The mean load of 562.35 CFU/m3 airborne bacteria was recorded in the indoor environment of a modular kitchen in Gwalior city. The mean load of 2593.75 CFU/m3 bacteria was recorded in the indoor environment of the traditional kitchen in Adupurajagir village. In addition, bacterial load of respectively 1215.13 CFU/m3 and 783.03 CFU/m3 was calculated in the open space at both study sites. Based on morphological characteristics five bacterial species (spp.) were identified Staphylococcus aureus spp, Bacillus spp, Coagulase-negative Staphylococcus spp, E-coli spp, and Micrococcus spp.
By gram staining method the most common bacteria were gram-positive (+ve) [n=85, 54.48% (37.17% cocci, 17.94% bacilli)] followed by gram-negative (-ve) [n=71, 45.51% (23.07% cocci, 21.79% bacilli)] identified. Pearson’s correlation coefficient was employed between bacterial load and physical factors of the indoor environment in the rural traditional kitchen. Bacterial load (CFU/m3) showed a significant correlation with temperature (p < 0.001). However, a non-significant correlation was recorded with relative humidity (p > 0.01). High bacterial load was found in the rural traditional kitchen’s indoor environment compared to urban modular kitchen. Outcomes from this study revealed that bioaerosol sampling could deliver fruitful knowledge about the variation of air quality and prevent possible hospital admissions.
Air Quality, Bioaerosol, Gram Staining, Passive Air Sampling, Pearson’s Correlation.
Dixit P, Kumar A, Singh S.Investigations on Bacterial Load in the Rural and Urban Indoor and Outdoor Environment of Gwalior, Madhya Pradesh, India. Biosc.Biotech.Res.Comm. 2021;14(4).
Dixit P, Kumar A, Singh S.Investigations on Bacterial Load in the Rural and Urban Indoor and Outdoor Environment of Gwalior, Madhya Pradesh, India. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/30qmPWT“>https://bit.ly/30qmPWT</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, sources the original author and sources are credited.
INTRODUCTION
People spend 90% of their lives indoors, where the contaminated air in dwellings may be making people sick. In these areas, they are exposed to various airborne microorganisms like bacteria. According to previous studies, approx. 10-35% of indoor air contamination is caused by aerial microbial flora (Mirhoseiniet al. 2016; Fujiyoshi et al. 2017; Andualem et al. 2019; Hui et al. 2019). Therefore, poor indoor air quality (IAQ) can lead to disorders known as sick building syndrome (SBS), building-related illness (BRI), chronic inflammatory response sydrome (CIRS) and numerous negative exposures (Mirhoseini et al. 2016; Hui et al. 2019). The most common bacterial species identified in indoor environment were Bacillus, Staphylococcus, Arthrobacter, Micrococcus, Streptococcus, Diphtheroid, Pseudomonas, Exiguobacterium, Enterobacter, Escherichia coli (E-coli) and Sphingomonas (Mirhoseini et al. 2016; Bolookat et al. 2018; Andualem et al. 2019; Sivagnanasundaram et al. 2019).
It has been investigated that pathogenic bacterium like Staphylococcus aureus (methicillin resistant) and Pseudomonas species were nosocomial infections in nature and developed multi-antibiotic drug resistance which may be accountable ineffective cure (Kunwar et al. 2019). Therefore, indoor air can be more polluted and harmful in terms of health issues including upper respiratory infections (URI) and lower respiratory infections (LRI), etc., over outdoor air (Baldacci et al. 2015; Kim et al. 2018; Smith et al. 2000). Though indoor environments are believed to be safer, but they can pollute with micro-pollutants when their load raised from recommended parameters than associated with outdoor exposure.
A sampling of bioaerosols is a well-known technique to find out the bacterial load, as it permits a significant evaluation, hence the results were evaluated in the form of colony forming unit per cubic meter (CFU/m3). According to the National Institute of Occupational Safety and Health (NIOSH) and the American Conference of Governmental Industrial Hygienists (ACGIH) the recommended parameter of the total count of bioaerosols is 1000 CFU/m3 and the culturable total number of bacteria is 500 CFU/m3 (Cox and Wathes 2020).
Recent researches have exposed the load of bacteria in different indoor environments (Lazaridis et al. 2015; Mirhoseini et al. 2016; Bolookat et al. 2018; Kunwar et al. 2019; Sivagnanasundaram et al. 2019). However, the indoor airborne bacterial concentration is likely enhanced by the physical parameters of the indoor environment (thermal condition, humidity, and ventilation framework) as well as natural activities of human (sneezing, coughing and talking) by spreading micro droplets (Fujiyoshi et al. 2017; Andualem et al. 2019; Hui et al. 2019; Kunwar et al. 2019; Roslund et al. 2019). Therefore, there is need to investigate bacterial load in the indoors, physical parameters, and micro-climatic variations of the indoor environment. Indoor airborne bacteria can affect human health as various skin and respiratory infections (Fujiyoshi et al. 2017; Roslund et al. 2019; Sharma et al. 2020).
Nonetheless, contamination of bacteria in the kitchen may also be contributable to poor indoor air quality. Hence, kitchens are supposed to be another contributing factor in the burden of airborne bacterial infections. It is important to determine the bacterial load in the indoor environment and its comparison with outdoor environment to find out the risk from indoor environment generated bacterial diseases/disorders. The present work focused on the airborne bacterial load in the indoor environment of rural traditional kitchen and urban modular kitchen and their open space environment. Furthermore, it also analyzes the relationship between high bacterial loads with physical factors of indoor environment of rural traditional kitchen.
MATERIAL AND METHODS
The present study was accomplished from December, 2020 to March, 2021 among 50 randomly selected dwellings from rural (Adupurajagir village) and urban (Gwalior city) to compare indoor and outdoor airborne bacterial load to analyze the relationship between high bacterial load with the physical environment and cooking pattern of rural and urban people. Therefore, traditional and modular kitchens were selected from rural and urban dwellings respectively as indoor and their open space as outdoor environments. Gwalior is a major city in Central India in the state of Madhya Pradesh. The city is located at 26.22ο N latitude and 78.18ο E longitudes, 300 km from Delhi. Adupurajagir village is located in the Gwalior districts of Madhya Pradesh. The village is located at 26.113491ο N latitude and 78.226425ο E longitudes (Fig. 1).
Figure 1: Areas in (a) Adupurajagir village and (b) Gwalior city where airborne bacterial
samples were collected. (Source: https://www.google.co.in/maps).
Bacterial samples were collected from the kitchen and open space in morning cooking hours at 9.00-11.30 am. The passive air sampling technique was used, the Petri plate was placed at the center of the sampling area for 1 hour, 1 meter away from the wall, and 1 meter above the height of the human breathing zone (1/1/1 standardized method) (Bolookat et al. 2018; Andualem et al. 2019). Physical parameters such as thermal condition, humidity, and ventilation framework were recorded. To minimize the mixing of outdoor bacterial samples, all ventilations were remained closed and movement of inhabitants prohibited during the indoor sampling.
At the time of sampling, other microbial safety measures were followed (Napoli et al. 2012; Sharpe et al. 2020). After sampling all samples were transported on the same day without any delay to the CTR (Centre for Translational Research) laboratory, Jiwaji University, Gwalior, and incubated at 37 οC for 24-48 hours. Bacterial colonies growing on culture media were expressed and calculated in the form of colony forming unit per cubic meter (CFU/m3) by applying the equation Andualem et al. (2019).
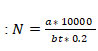
Where: N = Indoor airborne bacterial CFU/m, a = Colony counts per Petri plate, b = Surface area of Petri plate in cm, 2t = Time of air exposure in minutes
Based on microscopic examination, purification by sub-culturing for another 24-48 hours at 37 οC on the same culture media was used to attain pure culture isolates. The identification of the obtained colonies was based on gram staining and their colony formation characteristics such as shape, size, opacity, and color (Becerra et al. 2016; Mirhoseini et al. 2016). A light microscope was used to assess the morphology of bacteria at 100X magnification under oil immersion. The bacterial generic identity was achieved based on the taxonomic classification (Goodfellow et al. 2012).
Descriptive statistics were used to depict the airborne bacterial load. To evaluate comparison, between averages bacterial load in the rural and urban indoor and outdoor environment one-way analysis of variance (ANOVA) was employed. In addition, to analyze the correlation of airborne bacterial load with physical factors of indoor environment Pearson’s correlation coefficients were employed.
RESULTS AND DISCUSSION
Bacterial load: The present study was employed at a preliminary stage to compare the airborne bacterial load in the outdoor and indoor environment of rural traditional and urban modular kitchen among Adupurajagir village and Gwalior city respectively. The minimum and maximum bacterial load were estimated in the urban modular kitchen (511.12 CFU/m3) and a rural traditional kitchen (2621.16 CFU/m3) respectively. The mean bacterial load was 2593.75 CFU/m3 in the rural traditional kitchen, 783.03 CFU/m3 in rural open space, 562.35 CFU/m3 in urban modular kitchen, and 1215.13 CFU/m3 in the urban open space environment. The sum of bacterial load of the indoor environment of rural traditional kitchen among Adupurajagir was found highest (256781.91 CFU/m3), with the mean bacterial load of 2593.75 CFU/m3. Furthermore, the sum of bacterial load of the indoor environment of urban modular kitchen among Gwalior was observed lowest 55672.95 CFU/m3 with the mean bacterial load of 562.35 CFU/m3.
On the other hand, the sum of outdoor bacterial load of Adupurajagir and Gwalior was 77520.32 CFU/m3 and 120297.87 CFU/m3 with the mean bacterial load of 783.03 CFU/m3 and 1215.13 CFU/m3 respectively (Table 1). Although there is no general threshold estimation concerning airborne bacterial load in the indoor environment, the WHO suggested that a total load of microbes in the indoor environment should not surpass 1000 CFU/m3 (Hänninen 2011). The Sanitary Standards of the European Commission for non-industrial premises reported that > 50 CFU/m3, < 100 CFU/m3, < 500 CFU/m3, and < 2000 CFU/m3 is considered very low, low, high, and very high microbial load (Colbeck and Whitby 2019; Kotgire et al. 2020). Taking these standardized data into consideration, the mean bacterial load of the indoor environment in the rural traditional kitchen much higher than that outdoor. Moreover, the mean bacterial load of the indoor environment in the urban modular kitchen shows lower values.
Table 1. Descriptive statistics analysis of airborne bacterial load of indoor air environment in kitchen and open space environment among Adupurajagir village and Gwalior city (n = 50).
| Bacterial CFU/m3 | |||||||
| RK | RO | UK | UO | ||||
| Mean | 2593.75 | Mean | 783.03 | Mean | 562.35 | Mean | 1215.13 |
| Median | 2594.95 | Median | 773.24 | Median | 550.44 | Median | 1192.62 |
| Mode | 2621.16 | Mode | 773.24 | Mode | 537.33 | Mode | 1153.31 |
| Standard Deviation | 23.614 | Standard Deviation | 44.161 | Standard Deviation | 43.992 | Standard Deviation | 68.971 |
| Minimum | 2555.63 | Minimum | 707.71 | Minimum | 511.12 | Minimum | 1048.46 |
| Maximum | 2621.16 | Maximum | 891.19 | Maximum | 655.29 | Maximum | 1310.58 |
RK=Rural Kitchen; RO = Rural Open space; UK = Urban Kitchen; UO = Urban Open space.
One way ANOVA test results was demonstrated to differentiate the mean airborne bacterial load among study sites. Whereas the sum of square between groups was 285805.8 and within groups was 22612981 and total number of mean square was 115951.8 estimated. The ANOVA test reflected that there was a significant mean airborne bacterial load difference among study sites at p < 0.001 with total degree of freedom was 299 (Table 2).
Table 2. One way ANOVA test results on rural and urban airborne bacterial load in
indoor environment of kitchen and open space environment at study sites.
| Analysis of Variance | ||||||
| Source of Variation | SS | df | MS | F | P-value | F crit |
| Between Groups | 285805.8 | 99 | 2886.927 | 0.025533 | < 0.001 | 1.684883 |
| Within Groups | 22612981 | 200 | 113064.9 | |||
| Total | 22898787 | 299 | 115951.8 | |||
SS = Sum of the Square; df = Degree of Freedom; MS = Mean Square.
Morphology analysis: In this study, rural and urban airborne bacterial load in the indoor environment of the kitchen and open space environment contains a diversity of airborne bacteria. Five bacterial species (spp.) were identified as Staphylococcus aureus spp, Bacillus spp, Coagulase-negative Staphylococcus spp, E-coli spp, and Micrococcus spp. Gram +ve bacteria were found maximum than gram -ve (Fig. 2).
Figure 2: Mean of isolated gram +ve and gram -ve bacteria from rural and
urban kitchen and open space environments (n = 156).
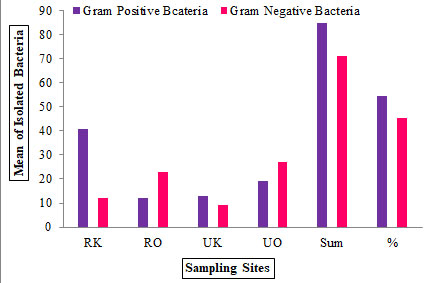
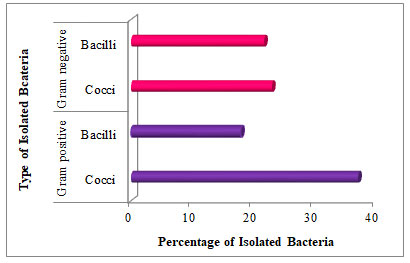
A total of 156 bacterial colonies were isolated from the rural traditional kitchen (53), rural open space (35), urban modular kitchen (22), and urban open space (46). All bacterial isolates were identified on their colony morphology, shape, size, opacity, and color. Among them, 54.48% and 45.51% belong to gram +ve and gram -ve bacteria respectively. Therefore total 84 gram +ve (cocci = 58, bacilli = 26) and total 70 gram -ve (cocci = 36, bacilli = 34) bacteria were isolated from study sites (Fig. 3). In addition, the highest percentage of gram +ve cocci (23.07%) and lowest percentage of gram +ve bacilli were (17.94%) reported in Adupurajagir village.
This was followed by the percentage of gram -ve bacteria in which the highest 23.07% cocci and lowest 21.79% bacilli were reported in Gwalior city. However, the highest percentage of gram +ve cocci were 37.17% and the lowest percentage of gram +ve bacilli was 17.94% estimated in the rural traditional kitchen and outdoor environment among Adupurajagir. Whereas, the mean of 56 gram +ve cocci and 26 bacilli and 36 gram -ve cocci and 34 bacilli were recorded (Table 3). Nonetheless, the high load of gram +ve cocci was observed then gram -ve airborne bacteria. This research also reflects the same outcomes as gram +ve bacteria were isolated more than gram -ve bacteria (Kotgire et al. 2020).
Table 3. Summary of bacterial isolates from rural and urban kitchen and open space environments (n = 156).
| Sampling
site |
No. of
isolates |
Gram +ve | Gram -ve | ||
| Cocci | Bacilli | Cocci | Bacilli | ||
| RK | 53 | 29 | 11 | 4 | 8 |
| RO | 35 | 7 | 5 | 13 | 9 |
| UK | 22 | 9 | 4 | 2 | 7 |
| UO | 46 | 13 | 6 | 17 | 10 |
| Sum | 156 | 58 | 26 | 36 | 34 |
Figure 3: Percentage of isolated gram +ve and gram -ve bacteria on
the basis of their cellular shape (n = 156).
Characterization of identified bacterial species: Five bacterial species were identified Staphylococcus aureus spp, Bacillus spp, Coagulase-negative Staphylococcus spp, E-coli spp, and Micrococcus spp. Staphylococcus aureus spp and Micrococcus spp be owned by the flora of the human dermis and are usually members of the microbiota of the body, it is aptly that this microbiota may be originated from the dermis flora of the inhabitants of their dwellings. Micrococcus spp and Staphylococcus aureus were isolated from all the sampling sites (Fig. 2), it is considered to be an emerging nosocomial pathogen.
Micrococcus spp can cause pneumonia, septic shock, meningitis, and endocarditis. Conversely, Staphylococcus aureus is gram +ve bacteria that may cause disease symptoms through the production of toxins for example food poisoning, human dermis infections, pneumonia, bone infections, urinary tract infections, and diarrhea. Bacillus spp can stay alive in the harsh environmental conditions in the air due to its ability to form spores and show resistance against common disinfectants which are used in daily disinfection practice in dwellings.
This gram +ve bacterium may be found on dirt particles and paper. Its presence on dirt particles in the air may consequence in settling on food or food contact surfaces, hence ensuring its survival in the kitchen and posing a possible threat to women and their children (< 5 years old) because of determined sex disparities across many proportions, women’s household works such as cooking may be more exposed than men’s (Kotgire et al. 2020).
In addition, improper cleaning and poor infrastructure (ceiling, mud walls, roof made by wood or leaves, short height of roof, and gap between wall and roof) were observed during sampling in the traditional kitchen among dwellings of Adupurajagir village, which may lead to serious bioaerosol infectivity of food such as queasiness, vomiting, diarrhea, wound, and central nervous system (CNS) infections and people with the weakened immune system are prone to Bacillus spp. E-coli is the most abundant species in the hospital environment however, in this study it was isolated from the kitchen environment among Adupurajagir village and Gwalior city also.
This gram -ve coliform bacterium of the family Enterobacteriaceae commonly lives in the human intestine. People inside the dwellings among both study sites may be exposed to E-coli from contaminated food and water due to poor hygienic practices were observed. Most strains of this bacterium are not harmful but some strains are contagious by produce toxins that cause illnesses such as septicemia, neonatal meningitis, bloody diarrhea, urinary tract infection, and gastroenteritis. Coagulase-negative Staphylococcus spp is referred from gram +ve (Kotgire et al. 2020).
This species is commonly found as a food-associated saprophyte and also present on the human dermis and mucous membrane. Overcrowded dwellings with poor ventilation framework found in Adupurajagir village. It was observed that villagers believe in living in joint families rather than nuclear families, which is considered to be the main basis of Coagulase-negative Staphylococcus spp occurrence within sampling sites. Coagulase-negative Staphylococcus spp is identified as is one of the main pathogens of nosocomial infection also shows methicillin resistance in nature and developed multi-antibiotic drug resistance which may be responsible for the ineffective treatment.
Findings say that most of the bacterial species are airborne in the residential environment associated with the human dermis. All identified bacterial species have colonized all the sampling sites within the dwellings (Table 3). It should be considered that all the isolated bacterial species are identified as highly infectious and disease-causing or opportunistic pathogenic. Future research would help to find out the possible source of the subsistence of pathogenic bioaerosols within the kitchen and outdoor environment of the dwellings (Magd et al. 2020; Kotgire et al. 2020).
Pearson’s correlation coefficients between bacterial load and physical factors of indoor environment in rural traditional kitchen: During measurement of physical factors of the indoor environment in the rural traditional kitchen, it was observed that all measured rural traditional kitchens did not have a ventilation framework. They use unclean fuel (wood, dung cake, and crop residues) over LPG for cooking. The relative humidity (RH %) and indoor temperature (T οC) ranged from 61% – 90% and 10 οC – 21 οC respectively. Bacterial load (CFU/m3) showed significant correlation with temperature (p < 0.001). However, a non-significant correlation was found with relative humidity (p > 0.01). The thermal condition of the indoor environment exhibited a significant correlation with airborne bacterial load in the rural traditional kitchen (r = 0.9090) while there was a non-significant correlation with the relative humidity (r = 0.0006) (Brągoszewska et al. 2017; Magd et al. 2020).
Hence the airborne bacterial load will increase as the indoor temperature increases and the bacterial load decreased with reduced relative humidity. This is attributed because of decreased metabolism and physiological activities of bioaerosols under dry environmental conditions (Brągoszewska et al. 2017). The difference of bacterial load in the indoor environment of rural and urban kitchen caused by outdoor climate and physical factors of the indoor environment like ventilation framework of kitchens, thermal condition, and relative humidity.
The difference amongst the bacterial load of indoor and outdoor environment at the study sites due to the microclimatic variations, construction material, vehicular pollution, outdoor levels, and daily household activities. The bacterial load of the outdoor environment in these settings reflects the variation of biological sources and the geochemical processes affecting indoor and outdoor relationships of airborne bioaerosols (Nasir et al. 2012; Magd et al. 2020).
Taking the account into consideration, the impact of physical factors of the indoor environment of rural kitchens may significantly affect the spread of diseases, as the lungs of exposed persons are more susceptible to infections due to heavy microbial load. In addition, relative humidity and poorly ventilated indoors also affect their health. There is no prominent evidence but the above-mentioned conditions which are analyzed in rural kitchens might influence the spread of dangerous coronavirus due to the poor health conditions and the increased load of aerosols. Recent research also confirmed that the number of positive cases varied between indoor and outdoor environments among rural and urban areas. Therefore, the indoor environment without a ventilation framework with increased temperature may be more vulnerable to the spread of coronavirus infection among residents (Magd et al. 2020).
CONCLUSION
The study suggests that the microbial air quality analysis of the indoor environment is necessary to provide variation of air quality and prevent possible wellbeing vulnerability allied with it. High bacterial load was found in the indoor environment of the rural traditional kitchen as compared to the urban modular kitchen due to poor ventilation framework and usage of unclean fuel over LPG for cooking. It is important to determine the airborne bacterial load to find out the risk from the indoor environment generated bacterial diseases/disorders. Significance of this study is that bioaerosol sampling could deliver fruitful knowledge about the variation of air quality and in future prevent possible hospital admissions. The study was planned to make a comparison of bacterial load in rural and urban indoor and outdoor environments, to specify the bacterial load in traditional kitchens of rural dwellings.
ACKNOWLEDGEMENTS
The study was financially supported by Madhya Pradesh Council of Science and Technology (MPCST) Bhopal, Madhya Pradesh, India (Grant Ref. No. R&D (Vet.)/17-18/06&28/03/32018). Authors would like to acknowledge Centre for Translational Research laboratory (CTR lab), Jiwaji University, Gwalior, India for their infrastructural support.
Conflict Of Interests: Authors declare that they have no conflict of interests.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
REFERENCES
Andualem Z, Gizaw Z, Bogale L et al. (2019). Indoor bacterial load and its correlation to physical indoor air quality parameters in public primary schools Multidisciplinary Respiratory Medicine Vol 14 No 1.
Baldacci S, Maio S, Cerrai S et al. (2015). Allergy and asthma: Effects of the exposure to particulate matter and biological allergens Respiratory Medicine Vol 109 No 9 Pages 1089–1104.
Becerra S C, Roy D C, Sanchez C J et al. (2016). An optimized staining technique for the detection of Gram positive and Gram negative bacteria within tissue BMC Research Notes Vol 9 No 1.
Bolookat F, Hassanvand M S, Faridi S et al. (2018). Assessment of bioaerosol particle characteristics at different hospital wards and operating theaters: A case study in Tehran MethodsX No 5 Pages1588–1596.
Brągoszewska E, Mainka A, Pastuszka J et al. (2018). Assessment of Bacterial Aerosol in a Preschool, Primary School and High School in Poland Atmosphere Vol 9 No 3 Page 87.
Colbeck I and Whitby C (2019). Biological Particles in the Indoor Environment Issues in Environmental Science and Technology Pages 127–157.
Cox C S and Wathes C M (2020). Bioaerosols Handbook, CRC Press, Pages 15-25.
Fujiyoshi S, Tanaka D and Maruyama F (2017). Transmission of Airborne Bacteria across Built Environments and Its Measurement Standards: A Review Frontiers in Microbiology 8.
Goodfellow M, Kämpfer P, Busse H J et al. (2012). Bergey’s Manual® of Systematic Bacteriology New York NY: Springer New York, Pages 171-206.
Hänninen OO (2011). WHO guidelines for indoor air quality: dampness and mold Fundamentals of mold growth in indoor environments and strategies for healthy living Pages 277–302.
Hui N, Parajuli A, Puhakka R, et al. (2019). Temporal variation in indoor transfer of dirt-associated environmental bacteria in agricultural and urban areas Environment International Vol 132 Page 105069.
Kim K H, Kabir E and Jahan S A (2018). Airborne bioaerosols and their impact on human health Journal of Environmental Sciences Vol 67 Pages 23–35.
Kotgire S, Akhtar R, Damle A et al. (2020). Bioaerosol assessment of indoor air in hospital wards from a tertiary care hospital Indian Journal of Microbiology Research Vol 7 No 1 Pages 28–34.
Kunwar A, Tamrakar S, Poudel S et al. (2019). Bacteriological Assessment of the Indoor Air of Different Hospitals of Kathmandu District International Journal of Microbiology Vol 2019 Pages 1–9.
Lazaridis M, Katsivela E, Kopanakis I et al. (2015). Indoor/outdoor particulate matter concentrations and microbial load in cultural heritage collections Heritage Science Vol 3 No 34.
Magd H, Asmi K and Karyamsetty H (2020). COVID-19 Influencing Factors on Transmission and Incidence Rates-Validation Analysis Journal of Biomedical Research & Environmental Sciences Vol 1 No 7 Pages 277–291.
Mirhoseini S H, Nikaeen M, Satoh K et al. (2016). Assessment of Airborne Particles in Indoor Environments: Applicability of Particle Counting for Prediction of Bioaerosol Concentrations Aerosol and Air Quality Research Vol 16 No 8 Pages 1903–1910.
Napoli C, Marcotrigiano V and Montagna M T (2012). Air sampling procedures to evaluate microbial contamination: a comparison between active and passive methods in operating theatres BMC Public Health Vol 12 No 594.
Nasir Z A, Colbeck I, Sultan S et al. (2012). Bioaerosols in residential micro-environments in low-income countries: A case study from Pakistan Environmental Pollution Vol 168 Pages 15–22.
Roslund M I, Rantala S, Oikarinen S et al. (2019). Endocrine disruption and commensal bacteria alteration associated with gaseous and soil PAH contamination among daycare children Environment International Vol 130 Page 104894.
Sharma G P, Kang S, Sajjad W et al. (2020). Microbial Community Composition Analysis in Spring Aerosols at Urban and Remote Sites over the Tibetan Plateau Atmosphere Vol 11 No 5 Page 527.
Sharpe T, McGill G, Dancer S J et al. (2020). Influence of ventilation use and occupant behaviour on surface microorganisms in contemporary social housing Scientific Reports Vol 10 No 11841.
Sivagnanasundaram P, Amarasekara R W K, Madegedara R M D et al. (2019). Assessment of Airborne Bacterial and Fungal Communities in Selected Areas of Teaching Hospital, Kandy, Sri Lanka BioMed Research International Vol 2019 Pages 1–11.
Smith K R (2000). Indoor air pollution in developing countries and acute lower respiratory infections in children Thorax Vol 55 No 6 Pages 518–532.


