Microbial Biotechnology and Biofertilizer Laboratory, Department of Botany, J.N.V. University, Jodhpur (Rajasthan) India – 342 003
Corresponding author email: mamtasharma019@gmail.com
Article Publishing History
Received: 08/04/2020
Accepted After Revision: 30/05/2020
This present study mainly focused to check the enhance level of various biochemical parameters after giving the treatment of Arbuscular Mycorrhizal (AM) inoculums. Rhizosphere soil of Sorghum bicolour collected from various location contain hyphae with root fragments, spore, vesicles which were used as a propagule for mass multiplication. In the continuation of the research soil were processed for getting AM species and root segment separately. Isolated and screened AM species were mass multiply by trap culture .The increase number of AM propagules use as an inoculums for pot culture of Sorghum. The AM Spore interacts with roots of Sorghum and make hyphal connection. AM colonization enhances the nutrient uptake from soil and provide to plant which directly. AM fungal association with roots of Sorghum bicolor is important for nutrient uptake, growth and biomass production. To study the impact of AM inoculants in Sorghum plant protein, sugar, carotenoid, chlorophyll, phenol, nitrogen and phosphate estimation was done . As a result of successful colonization Sorghum plant shows higher concentration in all biochemical parameter.colonization also help in accumulation of heavy metal and make plant disease resistant. After successful pot culturing plants were analysed for physiochemical characteristic. Without any hazardous effect AM endophyte can change the metabolism of host plant hence it could be considered as a good bio fertilizer tool.
Trap culture, physiological parameter, AM fungal isolates
Sharma M, Vyas A. Influence of Arbuscular Mycorrhizal fungal isolates on Biochemical Parameter in Sorghum bicolor. Biosc.Biotech.Res.Comm. 2020;13(2).
Sharma M, Vyas A. Influence of Arbuscular Mycorrhizal fungal isolates on Biochemical Parameter in Sorghum bicolor. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/2YLO7nm
Copyright © Sharma and Vyas This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Mycorrhizae has a well-established mutual relationship between plant root and fungi found in association with roots of land plants. Mycorrhizae are naturally occurring soil borne fungi which can potentially increase nutrient availability, biomass and make plant stress resistant (Sun et al., 2018).Besides growth promotion the major role of Arbuscular Mycorrhizal (AM) fungi is provide protection against various pathogen (Jung et al., 2012). AM fungi are obligate symbiont that means they required host root for their growth and complete their lifecycle. Once they get successful association, the mycorrhizal fungi starts to form arbuscules, vesicles hyphae and spores. Among all spores and hyphae also found in rhizosphere soil. Arbuscules are branching pattern which provide the place to facilitate the nutrient exchange and also accumulate reserve nutrient. Vesicles are bulbous structure which contains reserve food as glycogen and fat granules. Mycorrhizal hyphae extends deeply in soil in search of water hence it maintain the water level of plant, (Symanchik 2018 Wipf et al., 2019 Montero et al 2019).
Many research have been done to explore the contribution of AM fungi in plant growth and development, plant safety, soil health. In this continuation this research study has been done to evaluate the significant changes in Sorghum when inoculated with AM species. Sorghum is scientifically known as Sorghum bicolor L. Moench,.It is also known as sweet sorghum which belongs to family Poaceae. Sorghum is cultivated all over the world for food, feed, forage, fuel, and ranks fifth among the major cereal crops in terms of production (FAOSTAT, 2015).Sorghum belongs to C4 grass family which has well adaption to grow in arid or semi-arid region and also has a fibrous root system thus it easily interact with AM fungi. Root associations with arbuscular mycorrhizal fungi enhance plant growth by increasing P uptake, N uptake (Gerdemann, 1964; Janos, 1987; Stribely, 1987,Montero et al 2019).
According to Cavagnaro et al. (2015) the nutrient loss from soil can be reduced by potential use of AM fungi due to increasing the absorption zone. It was analysed that Sorghum absorbed more P from soils when colonized with AMF than non mycorrhizal plants (Krishna and Bagyaraj, 1981). For preparing pot culture, sorghum plant treated with AM inoculums which were obtained from trap culture. These AM inoculums belong to genus Glomus Acaulospora Scutellospora Gigaspora. In the meanwhile studied the growth stages of development of mycorrhizal fungi such as infection, apprisoria formation, hyphae development, arbuscule, vesicles and spore formation. From the pot culture study also it has been compared and analysed the effects of AM fungal spore on the plant growth and physiological changes. Besides physiological changes this interaction also enhances root growth, leaf surface area. Besides these AM fungi plays a important role to maintain soil texture, improve quality of soil, nutrient cycling, hence it contribute to balance our ecosystem.AM inoculated Sorghum shows significant increase in all parameter as compare to control. Increased in photosynthetic rate, protein and sugar content, uptake of N P promote overall growth and yield of plant, (Montero et al 2019, Wipf et al 2019).
According to Amiri et al., (2017), increased concentration of N P Fe was found in Pelargonium graveolens. The present research describes the beneficial effect of AM fungi that can improve nutrient status by changing the host plants physiology and improving the biomass.
MATERIAL AND METHODS
A field survey conducted at six sites near Jodhpur area. Rhizosphere soil along with root sample collected in three replicate. As rhizosphere soil considered to be a source of AM spores that’s why AM spores were collected from rhizosphere soil. For the extraction of AM spore wet sieving and decanting technique has been carried out from soil sample. (Gerdemann and Nicolson1963). The isolated AM fungi were identified by key of Trappe and manual of identification of AM fungi of Trappe (1962) Schenck and Perez. (1987). In the present work root samples were collected from the field and were cleared and stained using trypan blue in lactophenol (Phillips and Hayman, (1970).) AM spore percentage and root colonization were evaluated by the gridline intersect method (Giovannetti and Mosse, (1980).).After identification AM spores Glomus fasciculatum, Glomus mossae. Sclerocystis rubiformis, Acaulospora morrowae and Acaulospora leavis, Scutellospora calospora, Gigaspora margarita, were seen mostly. These AM spores were transferred to various pot cultures with host plant Cenchrus ciliaris or Sorghum bicolor for mass multiplication .After one month when AM spores were germinate then infected root segments of host plant with hyphae, vesicles and spore used as inoculum for experimental plant i.e. Sorghum. These pot were kept in isolated condition. The pot which had no AM inoculum served as control. Plants root and rhizospheric soil were collected after 15days, 30 days, 45days, 60days,90 days for biochemical estimation.
BIOCHEMICAL DETERMINATION: The parameters studied include chlorophyll and carotenoid, proteins, total sugar, total phenol, peroxidase, polyphenol oxidase.Chlorophyll and carotenoid content in leaves was estimated using Arnons method (1949). The protein content in leaves of mycorrhizal inoculated and non-mycorrhizal Sorghum plant was estimated using Bradford(1976) method. The total sugar content was estimated in leaves of AM inoculated plant and controlplant using the method Mc. Cready et al,.(1950).The total amount of peroxidase enzyme was estimated from AM inoculated and control plants using modification method proposed by Putter (1974) and Malik and Singh (1980). The total amount of polyphenol oxidase enzyme in root was calculated from mycorrhiza inoculated and control plant using the method proposed by Esterbaner et al(1977) modified by Fujita et al. (1995). Total phenol content was estimated by Mahadevan’s method (1975).
RESULTS AND DISCUSSION
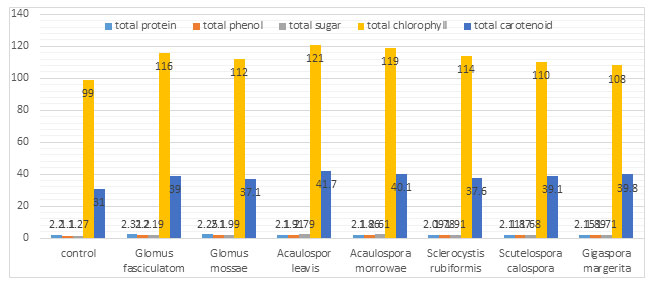
AM fungi association found to be most common relationship in nature which affect cultivated plants as well as the plants growing in natural ecosystems, thus it is most important finding in agriculture research field (Brundrest 2018). AM fungal association with Sorghum plant affects plant growth, nutrient content, and enzymatic activity. In present study result was based on comparison of mycorrhiza treated plant with control plant. Amount measured of chlorophyll and carotenoid from AM inoculated leaf significantly higher as compare to uninoculated control plant.. The highest concentration of chlorophyll were seen in Acaulospora laevis (121 mg/gm fresh wt.) and Acaulospora morrowae (119 mg/gm fresh wt.) while least were observed in Gigaspora margarita that is (108 mg/gm fresh wt.) inoculated Sorghum plant. Carotenoid content were observed higher in (41.7 mg/gm fresh wt.).The increased concentrations of these pigments directly affected the rate of photosynthesis and metabolism in host plants. By the experimental result of (Bhosale and Shinde 2011) the rate of photosynthesis was found to be higher in mycorrhizal treated plants compared to non- mycorrhizal control plants.
Chlorophyll and carotenoids are important plant pigment which plays significant role to accelerate the process of photosynthesis and biomass production. AMF can modified the nutritional value of grains in many agricultural crops and improve the production of carotenoids and certain volatile compounds Bona et al. (2017). When total sugar content of AM treated Sorghum plant compared to control plant then increase concentration in total sugar content was observed in Acaulospora laevis (2.79 mg/gm fresh wt.) inoculated plant followed by Acaulospora morrowae (2.61mg/gm fresh wt.) treated plant.
Baslam et al (2011) used Mycorrhiza as a bioinoculant to test whether the plant shows enhanced accumulation in terms of chlorophyll, carotenoids, total soluble phenol, tocopherols, and various essential nutrients or not. Inoculated plants get better accumulation in contrast to control. Peroxidase activity was analysed for both control and AM inoculated plant which resulted that peroxidase activity is higher in fresh root weight of Glomus fasciculatum inoculated plants. Increasing amount of both enzymes that is Peroxidise and polyphenoloxidase positively correlated with increase in phosphorus concentration because in previous research resulted that AM fungi increases phosphate uptake which ultimately increase peroxidase and polyphenoloxidase activity of host plant. Peroxidase activity observed higher in Glomus fasciculatum that is 21.33 / mg fresh wt and polyphenoloxidase activity also in Glomus fasciculatum that is 23.1 / mg fresh wt. As a result of metabolic activity (ROS) species were formed as a by product thus it is necessary to detoxify reactive oxygen species (ROS) by the enzymes such as superoxide dismutase, catalase, peroxidase, and enzymatic level enhance significantly after mycorrhizal treatment, (Ahanger and Agarwal, 2017 Montero et al., (2019).
The phosphorus availability with in the soil is taken up with phosphate transporter located in the extra-radical hyphae of this fungus (Harrison and Buuren 1995). Glomus fasciculatom (2.2 mg/gm fresh wt.) and Glomus mossae (2.1 mg/gm fresh wt.) came out to be most active in enhancing phenolic accumulation. Total increase in phenol content may be due to an increased phosphorus level and PPO activity. Accumulation of phenol in AM plants which has been reported by (Covacevich and Berbara (2011).As concentration of PRO and PPO enzymes increase they oxidised phenol compound into Quinone or increase in phenyl propane i.e. lignin precursor which are well known for antimicrobial property. It is toxic for attacking pathogen so it is important content of plant defence mechanism.
The findings of Nisha and Kumar (2010) also support these results that higher levels of polyphenoloxidase and peroxidise are observed in mycorrhizal plants. They used seven different types of AM species, inoculated with Wedilla plant and observed increase concentration in enzyme as compare to non inoculated. Protein content in all AM inoculated plant resulted almost similar still The maximum protein content found in Glomus fasciculatum (2.31mg/gm fresh wt. ) and Glomus mossae ( 2.25 mg/gm fresh wt. ) inoculated plant. Hence this AM endophyte can improve the nutritive value as well as enzymatic value of host plant. Through the mycorrhizal symbiosis an increase in protein content in grains of chickpea has also been reported by Pellegrino and Bedini (2014).
The present investigation demonstrate that inoculation with Acaulospora laevis, Acaulospora morrowae and Glomus fasciculatom can enhance the enzymatic activity of Sorghum plant that is good for plant defence system.AM inoculated plant also enhance the accumulation of sugar, chlorophyll, protein which collectively enhance biomass and more highly proteinaceous grain. Occurrence of AM fungi have been reported almost all soil type ( Bernaola, et al.,2018). Any of soil condition semi-arid, arid, humid it can perform vigorously and make a plant nutrient stable for biomass, defence stable for pathogenic attack. The obligatory relationship of plant and fungi are bidirectional because mycorrhizae provides nutritional benefits to the plant in exchange fungus receives carbon compound to complete its life cycle, (Wipf et al 2019). It is very helpful for soil health as well as plant development.
Table 1. Effect of different Arbuscular mycorrhizal fungi on biochemical changes in Sorghum plant after one month
| Treatment | Total protein mg/gm fresh wt. | Total phenol mg/gm fresh wt. | Total sugar mg/gm fresh wt. | Total chlorophyll mg/gm fresh wt. | Total carotenoid mg/gm fresh wt. |
| Control | 2.2 | 1.10 | 1.27 | 99 | 31 |
| Glomus fasciculatum | 2.31 | 2.2 | 2.19 | 116 | 39 |
| Glomus mossae | 2.25 | 2.1 | 1.99 | 112 | 37.1 |
| Acaulospora leavis | 2.1 | 1.91 | 2.79 | 121 | 41.7 |
| Acaulospora morrowae | 2.1 | 1.86 | 2.61 | 119 | 40.1 |
| Sclerocystis rubiformis | 2.09 | 1.78 | 1.91 | 114 | 37.6 |
| Scutellospora calospora | 2.11 | 1.87 | 1.68 | 110 | 39.1 |
| Gigaspora margarita | 2.15 | 1.89 | 1.71 | 108 | 39.8 |
Table 2. Effect of different AM fungi on enzymatic changes in Sorghum plant After one month
| Treatment | PRO activity (Units mg-1 protein) | PPO activity (DA420/100 mg fw) |
| Control | 16 | 17.1 |
| Glomus fasciculatum | 21.33 | 23.1 |
| Glomus mossae | 20.91 | 22.4 |
| Acaulospora leavis | 18.6 | 20.1 |
| Acaulospora morrowae | 19.1 | 21.5 |
| Sclerocystis rubiformis | 18.2 | 20.2 |
| Scutellospora calospora | 19.5 | 20.11 |
| Gigaspora margarita | 19.7 | 22 |
ACKNOWLEDGEMENTS
The authors are thankful to Head of Department of Botany Prof. P.K. Kasera for providing support. Department of Botany JNV University and Microbial Biotechnology and Biofertilizer Lab Jodhpur (Rajasthan) for providing instrumentation facility.
Conflict Of Intrests:There is no conflict of interest.
REFERENCES
Abbasi, H., Ambreen, A. and Rushda, S. (2015). Vesicular Arbuscular Mycorrhizal (VAM) Fungi: A Tool for Sustainable Agriculture. American Journal of Plant Nutrition and Fertilization Technology 5 (2): 40-49.
Ahanger, M. A., and Agarwal, R. M. (2017). Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L.). Protoplasma 254 (4), 1471–1486. doi: 10.1007/ s00709-016-1037-0
Amiri, R., Ali, N., Nematollah, E., and Mohammad, R. S. (2017). Nutritional status, essential oil changes and water-use efficiency of rose geranium in response to arbuscular mycorrhizal fungi and water deficiency stress. Symbiosis 73, 15–25. doi: 10.1007/s13199-016-0466-z
Arnon, D. I. (1949): Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24,1-15.
Baslam, M., Garmendia, I., and Goicoechea, N. (2011). Arbuscular mycorrhizal fungi (AMF) improved growth and nutritional quality of greenhouse grown lettuce. J. Agric. Food Chem. 59, 5504–C5515. doi: 10.1021/jf200501c
Bernaola, L. et al. Natural Colonization of Rice by Arbuscular Mycorrhizal Fungi in Different Production Areas. Rice. Science 25,169–174 (2018).
Bhosale, K. S. and B.P. Shinde 2011. Influence of Arbuscular Mycorrhizal Fungi on Proline and Chlorophyll Content in Zingiber oficinale Rosc Grown Under Water Stress. Indian Journal of Fundamental and Applied Life Sciences, 1(3): 172-176.
Bona, E., Cantamessa, S., Massa, N., Manassero, P., Marsano, F., Copetta, A., et al. (2017). Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: a field study. Mycorrhiza 27, 1–C11. doi: 10.1007/s00572-016-0727-y
Bradford,M.M.1976. A rapid and Sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 72: 248-254
Brundrett, M.C.; Tedersoo, L.(2018) Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 220, 1108–1115.
Chen, M., Arato, M., Borghi, L., Nouri, E., and Reinhardt, D. (2018). Beneficial services of arbuscular mycorrhizal fungi – from ecology to application. Frontiers in Plant Science, 9(September), 1-14
Cobb, A. B., Wilson, G. W. T., and Goad, C. L. (2018 ) Linking sorghum nutrition and production with arbuscular mycorrhizal fungi and alternative soil amendments. Journal of Plant Nutrition and Soil Science, 181(2), 211-219.
Covacevich, F. and Berbara, R.L.L. (2011). Indigenous arbuscular mycorrhizae in areas with different successional stages at a tropical dry forest biome in Brazil. African Journal of Microbiology Research, 5(18): 2697-2705.
Dar, Z.M.; Masood, A.; Asif, M.; Malik, M.A. Review on arbuscular mycorrhizal fungi: An approach to overcome drought adversities in plants. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1040–1049.
Ferlian, O. Hight N Kiver H (2018) et al. Growing research networks on mycorrhizae for mutual benefits. Trends in plant science 20 – 118.
Esterbaner, H., E. Schwarzl and Hayn, M.( 1977). Analytical Biochemistry, 77: 486
Fulekar, J., Pathak, B., and Fulekar, M. H. (2017). Development of Mycorrhizosphere Using Sorghum bicolor for Rhizosphere Biormediation. International Journal of Current Research and Academic Review, 5(6), 42-48.
Gerdemann, J.W. and T.H. Nicolson. (1963). Spores of mycorrhizae Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 46: 235-244.
Giovannetti, M. and B. Mosse, (1980). An evaluation of techniques for measuring vesicular Arbuscular mycorrhizal infection in roots. New Phytol., 84: 489-500.
Harrison M.J, van Buuren M.L, Nature, 1995, 378: 626-629.
Jacott, C., Murray, J., and Ridout, C. (2017). Trade-Offs in Arbuscular Mycorrhizal Symbiosis: Disease Resistance, Growth Responses and Perspectives for Crop Breeding. Agronomy, 7, 75.
Jung, S. C., Martinez-Medina, A., Lopez-Raez, J. A., and Pozo, M. J. (2012). Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 38, 651–664. doi: 10.1007/s10886-012-0134-6
Mahadevan, A. (1975) Methods in Physiological Plant Pathology. Sivakami Publication, Madras, India.
Mathur, N and Vyas, A. (2016) Survival and establishment of exotic plant species in saline areas of Indian Thar Desert by application of mycorrhizal technology. Asian Journal of Plant Science and Research, 2016, 6(3): 1-6
Montero, H.; Choi, J.; Paszkowski, U.(2019) Arbuscular mycorrhizal phenotyping: The dos and don’ts. New Phytol. 221, 1182–1186.
Mustafa, G.; Khong, N.G.; Tisserant, B.; Randoux, B.; Fontaine, J.; Magnin-Robert, M.; Reignault, P.; Sahraoui, A.L. (2017) Defence mechanisms associated with mycorrhiza-induced resistance in wheat against powdery mildew. Funct. Plant Biol. 44, 443–454.
Nisha, M. C. and S. Kumar (2010). Influence of arbuscular mycorrhizal fungi on biochemical changes in Wedilla chinensis (Osbeck) Merril. Ancient science of life, 29(3): 26-29.
Ogar, A., Sobczyk, Ł., and Turnau, K. (2015). Effect of combined microbes on plant tolerance to Zn–Pb contaminations. Environmental Science and Pollution Research, 22(23), 19142-19156.
Pellegrino E and Bedini S (2014) Enhancing ecosystem services in sustainable agriculture: Biofertilization and biofortification of chickpea (Cicer arietinum L.) by arbuscular mycorrhizal fungi. Soil Biol Biochem 68:429-439
Phillips, J.M. and D.S. Hayman, (1970). Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc., 55: 158-161.
Putter, J. (1974). Methods of Enzymatic Analysis, Academic Press, New York 2: 685.
Rajkumar, M., Sandhya, S., Prasad, M. N. V., and Freitas, H.(2012). Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnology Advances, 30(6),1562-1574.
Ryan MH, Graham JH (2018) Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytologist 220,1092–1107.
Sadhana, B. (2014). Arbuscular mycorrhizal fungi (AMF) as a biofertilizers—a review. Int. J. Curr. Microbiol. App. Sci. 3 (4), 384–400.
Schenck, N.C. and Y. Perez. (1987). Manual for the Identification of VA Mycorrhizal Fungi, pp. 11-14. Shapiro, B.M. and E.R. Stadtman. (1970). Glutamine synthetase (Escherichia). Methods Enzymol. 17A: 910-922.
Symanczik, S.; Lehmann, M.F.; Wiemken, A.; Boller, T.; Courty, P.E. Effects of two contrasted arbuscular mycorrhizal fungal isolates on nutrient uptake by Sorghum bicolor under drought. Mycorrhiza 2018, 28, 779–785.
Sun, Z., Song, J., Xin, X., Xie, X., and Zhao, B. (2018). Arbuscular mycorrhizal fungal proteins 14-3-3- are involved in arbuscule formation and responses to abiotic stresses during AM symbiosis. Front. Microbiol. 5, 9–19. doi: 10.3389/ fmicb.2018.00091
Cavagnaro, TR S. Franz Bender, H.R. Asghari, M.G. van der Heijden (2015) The role of arbuscular mycorrhizas in reducing soil nutrient loss Trends Plant Sci., 20, pp. 283-290
Trappe J.M. (1962) Synoptic keys to the genera and species of zygomycetous mycorrhizal fungi. Phytopathology 72: 1102-1108.
Vyas, M and Vyas, A. (2014) Field Response of Capsicum annum dually inoculated with AM fungi and PGPR in western Rajasthan. IJRSB.2(3)21-26 .
Wang, L., Ji, B., Hu, Y., Liu, R., and Sun, W. (2017). A review on in situ phytoremediation of mine tailings. Chemosphere,184, 594–600.
Wipf, D.; Krajinski, F.; van Tuinen, D.; Recorbet, G.; Courty, P.(2019) Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytol. 201 9, 223.