1,3Department of Biotechnology, Nehru Arts and Science College, Coimbatore – 641 105, Tamil Nadu, India.
2Assistant Professor, Department of Biotechnology, SNMV College of Arts and Science, Coimbatore – 641 050, Tamil Nadu, India.
Corresponding author email: anithavarshini22@gmail.com
Article Publishing History
Received: 29/10/2020
Accepted After Revision: 07/12/2020
Plants have endogenous defense mechanisms that can be induced in response to attacking insects and pathogens. Inducing the plant’s own defense mechanisms by prior application of biological inducer is thought to be a novel plant protection strategy. Synthesis and accumulation of PR proteins have been reported to play an important role in plant defense. By considering this, the study was undertaken to assess defense enzymes of PP pathway byS. griseus against challenge inoculation with Fusarium oxysporum f. sp. Lycopersici (FOL). The S. griseus formulations were evaluated for their competence in regulating wilt disease and growth promotion of tomato under greenhouse circumstances. Further, induction of defense proteins against challenge inoculation with FOL in tomato plants were also studied. In the present research, S. griseus has been introduced in to the root system of tomato plants well in advance of Fusarium oxysporum infestation.
A noteworthy reduction in disease severity (19.5%) and improved yield (520.0 g/plant) also significant increase in plant growth over control was observed in tomato plant treated with chitin amended S. griseus (T3; root dipping) against FOL. β – 1, 3 glucanase and chitinase were persuaded to accumulate at elevated level on 15th day of challenge immunization in T3 plants. Correspondingly leaves of S. griseus (T3; root dipping) treated plants expressed higher activity of peroxidase (PO), polyphenol oxidase (PPO) and phenylalanine ammonia-lyase (PAL) after a day and it reached maximum on 15th day of inoculation with FOL. Equally, phenolics, chlorophyll and carbohydrates were found to a mass in bacterized (chitin amended S. griseus) tomato leaf tissues challenged with FOL and reached extreme on 15th day of pathogen S. griseus inoculation. These outcomes put forward that defense enzymes tangled in phenylpropanoid pathway was induced by S. griseus might have contributed the restriction of invasion of F. oxysporum in tomato leads to plant protection from wilt disease facilitated by Fusarium oxysporum f. sp. lycopersici.
Wilt Disease Tomato, S. griseus Treatment, Disease Reduction,
Anitha A, Arunkumar D, Saraswathi R. K. Induction of Defense Proteins in Tomato Treated with Streptomyces griseus Against Fusarium oxysporum f. sp. Lycopersici. Biosc.Biotech.Res.Comm. 2020;13(4).
Anitha A, Arunkumar D, Saraswathi R. K. Induction of Defense Proteins in Tomato Treated with Streptomyces griseus Against Fusarium oxysporum f. sp. Lycopersici. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/38p4s69
Copyright © Anitha et al., This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Tomato (Lycopersicon esculentum) is one of the most significant commercial vegetable crop grown in India. Tomato roots/stem is affected by highly destructive soil borne pathogen Fusarium oxysporum f. sp. lycopersici (FOL), leads to leaf wilting, yellowing, substantial loss in yield and eventually plant death (McGovern, 2015; Bubici, 2018). The features of soil-borne pathogen (i.e., invading through the vascular tissue) make it difficult to control the disease and biological control agents emerge to hold promise in pathogen management (Ben Abdallah et al., 2016). Plant growth promoting rhizobacteria (PGPR) are being exploited commercially for plant protection via colonizes at rhizosphere, plant immunization or induce systemic resistance against pathogens (Dahal et al., 2017). The plant responses involved, expression of increasing level of numerous pathogenesis associated proteins (PR proteins) comprises (a) β-1, 3 glucanases (PR-2 family), chitinase (PR-3 family) which lysis the fungal cell wall; defense gene products including (b) Peroxidases (PO), Polyphenoloxidases (PPO) with the intention of catalyze lignin formation (c) Phenylalanine ammonia-lyase (PAL) concerned in phytoalexins and phenolics synthesis (Zouari et al, 2016; Mhlongo et al, 2018).
Activated induced resistance (via ISR or SAR) is a broad-spectrum and long-term resistance, which usually suppresses a disease up to 20–85% (Abbasi et al., 2019). Actinomycetes, particularly streptomyces sp., encompass a far above the ground potential to control fungal pathogens since it produces antifungal antibiotics, proteins and cell wall degrading enzymes. They may live saprophytically and endophytically in agricultural environments where they colonize the rhizosphere in addition different parts of plant (Saleem et al., 2016). Streptomyces sp., have been reported as PGP and biocontrol agent against Verticillium dahliae (Cao et al., 2016), Phytophthora drechsleri (Sadeghi et al., 2017), Fusarium oxysporum (Abbasi et al., 2019, Hussein and Al-Dulaimi, 2020) Phytophthora capsici (Abbasi et al., 2020).
There is no considerable learning on various defense enzymes of PP pathway and PR proteins owing to Streptomyces griseus treatment. Herewith, the study was undertaken to assess the induction of defense enzymes of PP pathway and accumulation of PR-proteins by S. griseus against challenge inoculation with Fusarium oxysporum f. sp. Lycopersici (FOL).
MATERIAL AND METHODS
Plant material: Tomato variety Co-4 susceptible to wilt disease was analysed in this study.
Isolation of pathogen: The naturally infected tomato plants evidenced for wilt disease, situated at Nachipalayam Village, Coimbatore were collected in sterile polyethylene bags. Diseased stem and root tissues were sliced (1 – 1.5 cm), inoculated in Potato Dextrose Agar plates (PDA) and incubated at 28°C for 5 – 7 days. The hyphal tips raised from the segment was purified by single spore isolation method, identified as Fusarium oxysporum (FOL) and maintained in PDA slants at 4°C until further use (Aileen, 2006).
Pathogenic fungal inoculum: The respective isolates of FOL, was multiplied in sand maize medium (Riker and Riker, 1936). Approximately 2 x 108 cfu/g of inoculum of FOL in sand maize medium was mixed with sterilized soil at 5% (W/W), then infested into greenhouse earthen pots ten days prior to transplanting 45 days old seedling (Larena et al., 2003).
Bacterial isolate: Streptomyces sp., was isolated from prawn cultivated pond soil of Peddapuram Village; East Godavari District using Colloidal Chitin Agar (CCA) plates, identified as Streptomyces griseus (Acc. No. 9723) and deposited in MTCC, Chandigarh. The culture was maintained in actinomyces agar slants at 4°C till further use.
Development of bioformulation of S. griseus: Talc-based bioformulation of S. griseus (Acc. No. 9723) with (i) chitin, (ii) without chitin, (iii) self-fused S. griseus, (iv) chitinase enzyme of S. griseus with Apsa 80 (carrier molecule) were developed as described by Anitha and Rabeeth, (2009). The prepared formulations were filled in polythene bags, sealed and kept back at room temperature for greenhouse studies.
Efficacy of S. griseus against fusarium wilt of tomato under greenhouse conditions: The formulations were evaluated for their competence in regulating wilt disease of tomato under greenhouse circumstances. The treatments were imposed as seed treatment, seedling dip and foliar applications.
Seed bacterization: Seeds of cultivar Co–4 were surface sterilized, soaked in water containing talc-based S. griseus (with or without chitin) – 10 g/kg of seeds (Meena et al., 2002) for 12 hrs. At the end, the seeds were drained off, dried under shade for overnight and sown in (27 x 42 x 7 cm3) trays containing vermiculite and sand [1:1; (V:V)] ratio (Vidhyasekaran et al., 1997).
Seeding dip and foliar spray: For root inoculation, surface sterilized seeds of cultivar Co-4 were sown in nursery trays mentioned above. After 45 days, seedlings were pulled out from the trays and roots were immersed in chitin amended S. grises and self-fused S. griseus (20 g/L) formulations for 30 mins before transplanting the seedlings. Whereas chitinase of S. griseus along Apsa 80 was prearranged as foliar spray to transplanted seedling.
Efficacy of S. griseus on disease severity and experimental design: An experimental design comprises, T1 – plants raised from seeds treated with talc formulation of S. griseus (10 g/kg of seeds), T2 – plants raised from seeds treated with chitin amended talc formulation of S. griseus (10 g/kg of seeds), T3 – plant roots immersed in chitin amended talc formulation (20 g/L) of S. griseus, T4 – plant roots immersed in talc formulation (20 g/L) of self-fused S. griseus, T5 – foliar spray using crude chitinase enzyme (1L) of S. griseus with Apsa 80 (113.3 IU/mL) after planting, T6 – plants raised from carbendazim treated seeds (2 g/kg of seeds), served as chemical check, T7 – plants neither treated with bacterial suspension nor challenged by the pathogen (Healthy Control) and T8 – plants challenged with pathogen (Inoculated Control).
After the treatments, the seedlings were transplanted into greenhouse pots at the rate of four seedlings/pot which is artificially infested with pathogen (Sand: Maize Medium). Ten days after transplanting, foliar spray near the roots of treated plants were given as per (T1 to T6) and irrigation was given consequently. Later 15 days, external symptoms of wilting and yellowing of leaves (Fusarium wilt) were recorded. The Disease Severity (DS) was calculated as Disease Severity (DS) = Number of leaves with symptoms / Total number of leaves X 100 (Pascale et al., 1999).
Three replications were maintained in each treatment; each replicate comprises of six pots and in each pot four plants were maintained. The relative humidity in the glasshouse was maintained around 80%, and the temperature of 26°C (day) and 20°C (night). The experiments were laid out with randomized block design with four replications and the experiments were repeated once under greenhouse bench.
Efficacy of S. griseus on plant growth promotion: After 45 days, two plantlets from each replication were sampled and the efficacy of diverse treatments on root & shoot length, fresh & dry weight of root and shoot, yield of the plant was recorded in all the treatments challenged with FOL in comparison with control.
Efficacy of S. griseus on induction of defense related enzymes: Leaf samples were collected at different time intervals (0, 5, 10, 15 and 20 days) after challenged with FOL pathogen. Three plants were sampled from each replication of the treatment separately (mentioned in experimental design) and maintained for biochemical analysis. Freshly collected leaf samples (0.1g) were washed with sterile distilled water and homogenized in 2 mL of ice-cold phosphate buffer (0.1 M; pH 7.0) at 4°C. The homogenate was centrifuged at 10000 rpm at 4°C for 15 min, the 181 collected supernatant was stored at –20°C until used for biochemical assays. Assay of accumulations of PR proteins and induction of defense enzymes of PP pathway like chitinase (Wen et al., 2002), β-1, 3-glucanase (El–Katatny et al., 2000), peroxidase (PO) (Hammerschmidt et al., 1982)., polyphenol oxidase (PPO) (Mayer et al., 1965), phenylalanine ammonium lyase (PAL) (Dickerson et al., 1984), total phenol (Zieslin and Ben–Zaken, 1993), total chlorophyll and total carbohydrate (Sadasivam and Manickam, 1991) were measured.
Statistical analysis: The data’s were statistically analyzed independently (Gomez and Gomez, 1984). The treatments mean was compared by Duncan’s Multiple Range Test (DMRT) at 5% significant using SPSS software version 7.0.
RESULTS AND DISCUSSION
Plants have endogenous defense mechanisms that can be induced in response to pathogen attack. The defense genes are considered to be inducible and ideal stimuli or signals are preferable. It is considered a novel plant protection strategy to incorporate the plant own defensive mechanisms through biological inducers (Bubici et al., 2019). By considering the above the effectiveness of prior application of S. griseus in controlling the soil-borne diseases has been documented.Talc based formulations of S. griseus with / without chitin supplements were prepared and analyzed against FOL under greenhouse conditions. Talc formulation of P. fluorescens (Pf1) and B. subtilis against rust and leaf spots of groundnut (Meena et al., 2002), sheath blight infection of rice (Radja et al., 2002) and fruit rot of chilli (Bharathi et al., 2004) have been studied. In this line, talk formulation of Streptomyces corchorusii against rice (Tamreihao et al., 2016) Streptomyces rochei against tomato (Zamoum et al., 2017) has been reported as effective against disease management. The host plant provides the habitat and nutrients necessary for the pathogen to become established at the infection site. Fusarium oxysporum is a soil-borne pathogen and can spread through root system and it would be better to protect the infection sites rather than alter the entire soil microbial community. Root application of the biocontrol agents has been approved in the literature (Shishido et al., 2005; Yigit and Dikilitas, 2007, Bibusi et al., 2019).
Henceforth, in the present research, S. griseus has been introduced in to the root system of tomato plants well in advance of Fusarium oxysporum infestation. A noteworthy reduction in disease severity (19.5%) and improved yield (520.0 g/plant) over control was observed in tomato plant treated with chitin amended S. griseus (T3; root dipping) against FOL. Whereas in inoculated control (T8), there was uppermost disease severity (61.1%) with negligible yield of 120.0 g/plant was observed (Table 1). Chemical (carbendazim) treatment had less effect compared to S. griseus treated plants but then the activity was overwhelmed the healthy control (T7). The prior applicant of S. griseus in root system prevents the pathogen entry that resulted in significant reduction in disease severity.
Table 1. Table 1. Efficacy of S. griseus on disease severity and yield of tomato against Fusarium oxysporum f. sp. lycopersici. Values are the mean of triplicates. In a column, means followed by a common letter are not significantly different at 5% level by DMRT.
| Treatments | Disease severity (%) | Yield (g/plant) |
| T1 – S. griseus (Seed treatment – 10 g / kg) | 26.0a | 485.0 |
| T2 – Chitin amended S. griseus (Seed treatment – 10 g/ kg) | 23.1a | 455.0 |
| T3 – Chitin amended S. griseus suspension (Root dipping – 4 x 108 cfu/mL) | 19.1a | 550.0 |
| T4 – Self fusant S. griseus suspension (Root dipping – 4 x 108 cfu/mL) | 19.5a | 520.0 |
| T5 – Foliar spray using crude chitinase enzyme (1L) of S. griseus with Apsa 80 (113.3 IU/mL) after planting | 28.9ab | 416.0 |
| T6 – carbendazim treated seeds (2 g/kg of seeds) | 31.5ab | 425.0 |
| T7 – Healthy Control | 49.3de | 425.0 |
| T8 – Inoculated Control | 61.1 h | 120.0 |
Equally, S. enissocaesilis and S. rochei (Abbasi et al., 2019), Streptomyces sp. and Pseudomonas fluorescence (Hussein and Al-Dulaimi, 2020) lightened the fusarium wilt of tomato (FWT) disease symptoms like chlorosis, stunting and wilting better than carbendazim. Recently, Wu et al., (2019) have reported that a decreased percentage disease index of rice sheath blight caused by Rhizoctonia solani was associated with an increase in streptomyces sp., derived antifungal agent concentration. In contrast, Streptomyces sp., treated banana plantlets (root dipping) showed significant (P<0.05) reduction in wilt severity (47%) compared to untreated plantlets was reported by Getha et al., (2005) and Bubici et al., (2019).
The plant growth was significantly influenced by applying S. griseus at root tissues. Compared with inoculated control (T8), noteworthy development was perceived in T3 plants followed by T4 plants. Though, the sensible growth was observed in carbendazim treated plants (T6), it failed to provide appreciable growth than the S. griseus (T3) treated plants (Table 2). The outcomes indicated that the S. griseus may endorse the growth directly by producing plant growth regulators / stimulating nutrient uptake / synthesizing antibiotics in contradiction of pathogens.
Table 2. Efficacy of S. griseus on growth promotion of tomato against Fusarium oxysporum f. sp. lycopersici. Values are the mean of triplicates. In a column, means followed by a common letter are not significantly different at 5% level by DMRT.
|
Treatments |
Shoot | Root | ||||
| Length (cm) | Fresh wt. (g) | Dry wt. (g) | Length (cm) | Fresh wt. (g) | Dry wt. (g) | |
| Treatment 1 | 43.9ef | 22.8e | 6.3fg | 31.6gh | 14.5f | 2.5d |
| Treatment 2 | 45.7fg | 23.6 f | 6.5gh | 32.5gh | 14.8g | 2.6de |
| Treatment 3 | 47.5g | 25.8h | 6.8gh | 32.7gh | 15.8h | 3.9fg |
| Treatment 4 | 50.6h | 24.7g | 6.7h | 33.0h | 15.6b | 3.8gh |
| Treatment 5 | 38.4d | 21.8 de | 5.8de | 30.6gh | 13.9ef | 2.4ed |
| Treatment 6 | 40.4de | 20.8ef | 5.2b | 29.9g | 13.9e | 2.2ab |
| Treatment 7 | 34.7bc | 19.9 c | 4.5a | 22.7a | 13.9b | 1.9a |
| Treatment 8 | 26.9a | 15.9a | 5.8b | 20.4de | 9.8a | 1.7a |
Correspondingly, inoculation of S. enissocaesilis and S. rochei (Abbasi et al., 2019), actinobacteria (Passari et al., 2019), G. fasiculatum (Anusha and Krishnaraj, 2017) and G. intraradices (Akkopru and Demir, 2005) has encouraged the growth and yield of tomato plant against FOL inoculation was predicted in earlier. Likewise, Streptomyces corchorusii treated rice plants showed significant increase (P ≤ 0.05) in root and shoot lengths, fresh root and shoot, and dry shoot weights over the control was reported by Tamreihao et al., (2016).
Current exploration implies that prior application of S. griseus strengthen the host cell wall resulting in restriction of pathogen invasion in plant tissue (Abdul et al., 2020). In base line, synthesis and accumulation of PR proteins have been reported to play an important role in plant defense (Abbasi et al., 2019; Balint‐Kurti, 2019). However, there is a little information available on defense responses induced by S. griseus in plants against pathogen invasion. The present study clearly figured that the induction of enzymes involved in phenylpropanoid metabolism and accumulation of PR-proteins in tomato leaf tissue have been encouraged by chitin amended S. griseus (T3; root dipping) in response to challenge inoculation with F. oxysporum f. sp. lycopersici over the inoculated control (T8).
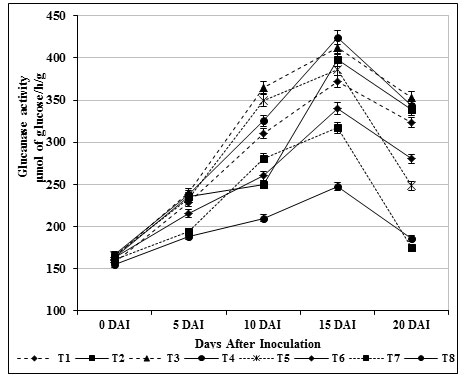
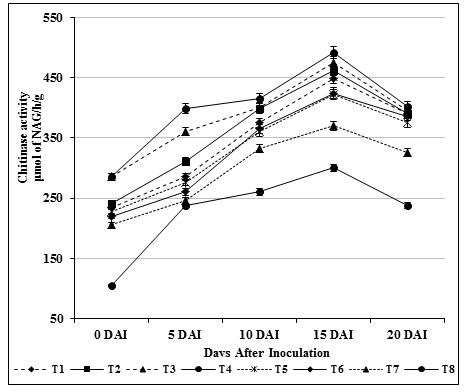
Induction of β-1,3 glucanase and chitinase enzymes play a key role against fungal attack in turn directly connected with the structural relevance of chitin and glucans in the cell wall of fungi (Bubici, 2018). These enzymes act upon the fungal cell wall resulting in loss of inner contents of cells and eventually leads to death. In our study, β-1, 3-glucanase (412.09 μmol/h/g) and chitinase activity (475.25 μmol/h/g) in self fusant S. griseus (T4; root dipping) treated plants was found to be lower than the plants treated chitin amended S. griseus (T3; root dipping) (β-1, 3-glucanase of 424.25 μmol/h/g; chitinase of 492.0 μmol/h/g). Significant activities were observed on 15 days after inoculation (Fig. 1 & 2) for the treated plants, whereas in inoculated control (T8) plants both enzyme activities were renowned at ground level.
Figure 1: Induction b-1, 3 glucanase by S. griseus in tomato plants challenged with the pathogen F. oxysporum f. sp. lycopersici. Vertical bars indicate standard deviations of three replications.
Figure 2: Induction chitinase enzyme by S. griseus in tomato plants challenged with the pathogen F. oxysporum f. sp. lycopersici. Vertical bars indicate standard deviations of three replications
Hence, initiation of enzymes has been occupied in defense against further invasion of the pathogen in root through degrading its physical barrier. In general, accumulation of hydrolytic enzymes is more pronounced in the biocontrol agent’s pretreated plants compared with inoculated control. Similarly, Chandrasekaran and Chun, (2016) and Magesh and Devi (2017) have attributed that induced resistances in tomato, in correlation with an increased activity of chitinase, β-1,3 glucanase and 1-4 glucosidase.
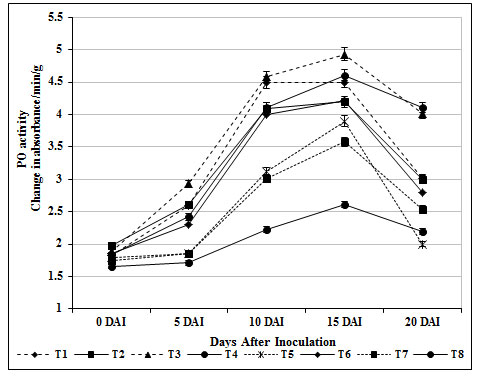
PO is a key enzyme, has been implicated in the regulation of plant cell elongation, phenol oxidation, polysaccharide cross linking, IAA oxidation, cross linking of extension monomers and oxidation of hydrosyl-cinnamyl alcohol into free radical intermediates with wound healing (Magesh and Devi, 2017). The plant resistance phenomenon is associated with biosynthesis of lignin in which PO is playing a key role (Liu et al., 2018). PO activity was significantly increased in T3 plants on 15 days after inoculation with FOL (4.93 absorbance/min/g) whereas the activity was insignificant in inoculated control (T8) (2.23 absorbance/min/g).
PO activity in the leaves of carbendazim treated plants (T6) did not elucidate any remarkable changes throughout the experimental period (Fig. 3). This unique PO induced by S. griseus isolate might have contributed to induce defense in tomato root tissue against FOL invasion. In accordance with the above an increased peroxidase activity in P. fluorescens, Streptomyces sp., and S. rochei treated plants challenged with Fusarium oxysporum – a wilt pathogen was addressed earlier (Nikoo et al., 2014; Salla et al., 2016; Mahesh and Devi, 2016; Abbasi et al., 2019).
Figure 3: Changes in peroxidase activity by S. griseus in tomato plants challenged with the pathogen F. oxysporum f. sp. lycopersici. Vertical bars indicate standard deviations of three replications.
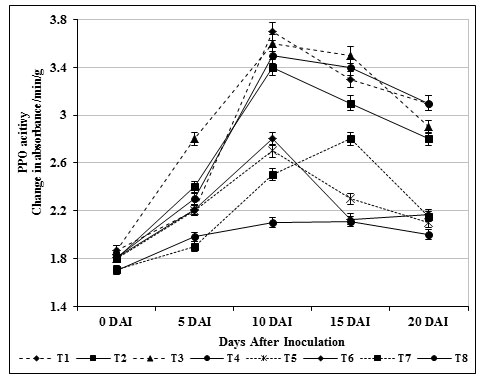
PPO is an important defense related enzyme produced under stress condition and accumulates upon wounding in plants. Correspondingly, the expression of PPO in T3 plants were conferred to be higher when compared with other treatments (T1 to T7) and inoculated control (T8) plants (Fig. 4). The PPO activity in inoculated control plants (T8) was 2.1 absorbance/min/g were as chitin amended S. griseus (T3; root dipping) showed 3.5 absorbance/min/g 15 days after inoculation. In connection with this, carbendazim treated plants (T6) exhibited adequate activity compared to inoculated control (T8) and healthy control (T7) plants but the activity was less compared to T3 plants.
Figure 4: Changes in polyphenol oxidase activity by S. griseus in tomato plants challenged with the pathogen F. oxysporum f. sp. lycopersici. Vertical bars indicate standard deviations of three replications.
The higher expression of PPO might have implicated in induced defense responses against the pathogen invasion. Similarly, higher accumulation PPO in Bacillus subtilis (Chandrasekaran and Chun, 2016) P. fluorescens treated tomato plants (Magesh and Devi, 2017; Ramamoorthy et al., 2002) in response to Fusarium oxysporum induced wilt disease was reported earlier.
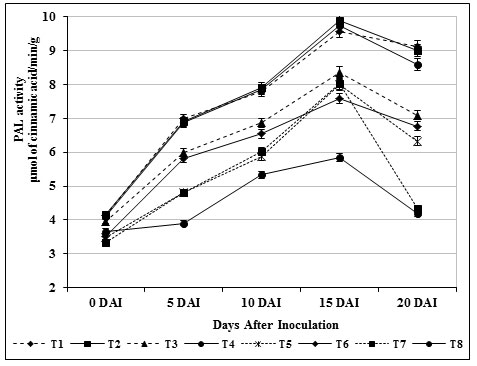
PAL played an important role in the biosynthesis of phenolics and phytolexins (Sharma et al., 2019). It catalyzes the deamination of L-phenylalanine to trans cinnamic acid, which is the first step in biosynthesis of large class of plant natural products based on the phenylpropane skeleton including lignin monomers as well as phytolexins. Experimental results revealed that chitin amended S. griseus (T3) treated plants showed highest activity (9.75 μmol/min/g) of PAL. There was no marked change in carbendazim treatment (T6) during the time course of experimental period and the accumulation of PAL remained higher compared to the untreated control (T8) and healthy control (T7) (Fig. 5).
Figure 5: Accumulation of phenylalanine ammonium lyase activity by S. griseus in tomato plants challenged with the pathogen F. oxysporum f. sp. lycopersici. Vertical bars indicate standard deviations of three replications.
Increased activity of PAL due to S. griseus isolate treatment might have prevented the fungal invasion, and thus, the activity maintained at higher levels. Besides, B. subtilis, Streptomyces sp. and S. rochei treated tomato roots rendered higher PAL activity when compared to untreated control was propounded by Chandrasekaran and Chun, (2016); Magesh and Devi, (2017) and Abbasi et al., (2019).
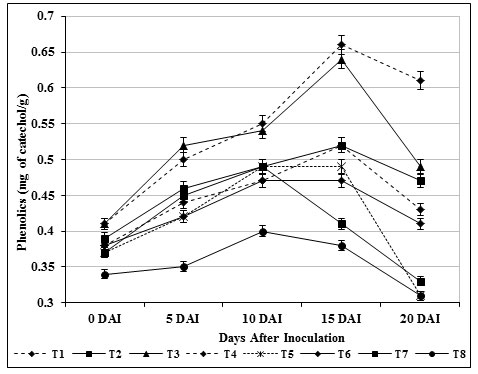
Phenols are fungi toxic in nature and higher accumulation played an important role in plant resistant by enhancing the mechanical strength of host cell wall and by inhibiting the fungal growth (Aoun, 2017). The outcome of the study would demonstrated that quantitative changes in phenolics (0.66 mg catechol/g) were improved in tomato seedlings exposed to chitin supplemented S. griseus (T3) during the experiment against pathogenic F. oxysporum over control indicated that, the response is truly systemic and physiological state of the plant has been altered (Fig. 6). Perhaps the findings of Loganathan et al., (2014) and Panina et al., (2007) were recorded maximum phenol aggregation in various biocontrol agents treated plants. The increased phenolic substances exhibited considerable morphological changes including cytoplasmic disorganization and loss of protoplasmic content of pathogen was reported by Benhamou et al., (2000).
Figure 6: Accumulation of phenols by S. griseus in tomato plants challenged with the pathogen F. oxysporum f. sp. lycopersici. Vertical bars indicate standard deviations of three replications.
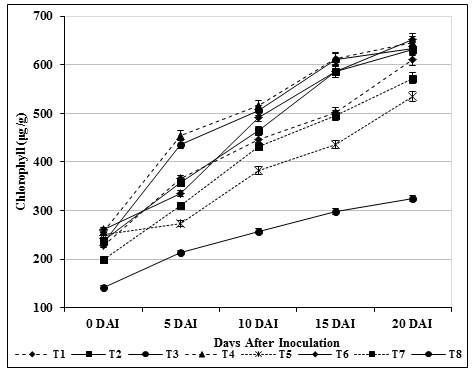
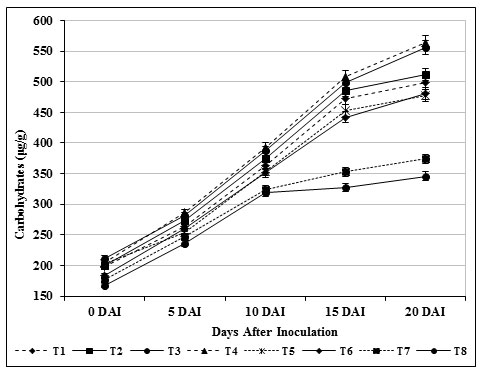
The chlorophyll content in inoculated control plants (T8) was found to be 324 μg/g, which was lower (652 μg/g) than carbendazim treated plants (T6) and other treatments. Total carbohydrate content (564 μg/g) in leaf tissues of treated (T4) tomato plants was found and it was observed as high compared with inoculated control (T8) (Fig. 7). Likewise, 556 μg /g of carbohydrate was observed on 20th day in T3 plants which was found to be higher than the carbendazim treated plants (453 μg/g) and healthy control (T7). The findings specified that the photosynthetic area was confined which consecutively produced higher amount of carbohydrates (Fig. 8).
Figure 7: Accumulation of chlorophyll by S. griseus in tomato plants challenged with the pathogen F. oxysporum f. sp. lycopersici. Vertical bars indicate standard deviations of three replications.
Figure 8: Accumulation of carbohydrates by S. griseus in tomato plants challenged with the pathogen F. oxysporum f. sp. lycopersici. Vertical bars indicate standard deviations of three replications.
This finding is somewhat similar to results reported by Dias et al., (2017) and Passari et al., (2019) indicating that Streptomyces sp. isolate PM5 and Streptomyces thermocarboxydus produced a greater amount of chlorophyll a & b than control plants. Babu et al., (2015) also reported that the rhizobacteria B. subtilis and Azotobacter chroococcum induced higher levels of chlorophyll content in tomato plants, relative to control plants. In contrast, Kandan, (2000) have stated lower sugars leaves in leaves of resistant cultivar.
Under greenhouse conditions, seeds treated with S. griseus (T1 and T2) and the plants treated with crude chitinase enzyme of S. griseus with Apsa 80 (T5; foliar spray – 113.3 IU/mL) did not produced significant amount of defense enzyme and PR protein against wilt disease of tomato plant incited by Fusarium oxysporum than the plants treated with S. griseus (root dipping) (T3 and T4). But then relatively significant effect (defense proteins) was experienced in comparison with carbendazim treated (T6) and inoculated control plants (T8).
CONCLUSION
From this investigation it is clear that S. griseus used along with chitin has been introduced to tomato root systems (root dipping) performed well in the management of FOL collectively appreciable yield and plant growth. The interactions between plant and pathogen have stimulated defensive enzymes initially but later, as the pathogen colonizes the root tissue, defensive enzymes dropped dramatically. At present, higher accumulation of enzymes of phenylpropanoid metabolism and PR-proteins has been instigated in tomato treated with S. griseus (T3 – root dipping) in response to invasion by F. oxysporum f. sp. lycopersici.
In conclusion, biocontrol efficiency could be accomplished by introducing the S. griseus in to the root system well in advance of pathogen infestation evidenced for significant colonization of S. griseus which controls the invasion of fungi and diminishes the pathogen density which simultaneously triggered the plant-mediated defense mechanism might have collectively contributed to induced resistance against FOL. Hence, the contemporary investigation recommended that chitinolytic S. griseus could be used as a promising biocontrol agent in Integrated wilt disease management.
Conflicts of Interest: Authors declare that they have no conflicts of interest.
REFERENCES
Abbasi, S., Safaie, N., Sadeghi, A., & Shamsbakhsh, M. (2019). Streptomyces strains induce resistance to Fusarium oxysporum f. sp. lycopersici Race 3 in tomato through different molecular mechanisms. Front. Microbiol, 10, 1505, 1–16.
Abbasi, S., Safaie, N., Sadeghi, A., & Shamsbakhsh, M. (2020). Tissue-specific synergistic bio-priming of pepper by two Streptomyces sp. against Phytophthora capsici. PloS one, 15(3), e0230531.
Abdul Malik, N. A., Kumar, I. S., & Nadarajah, K. (2020). Elicitor and Receptor Molecules: Orchestrators of Plant Defense and Immunity. Int. J. Mol. Sci, 21(3), 963.
Aileen Reid. (2006). Sampling and testing for plant pathogens. Western Australia. Bulletin, 4683,1833–7244.
Akkopru, A., & Demir, S. (2005). Biological control of Fusarium wilt in tomato caused by Fusarium oxysporum f. sp. lycopersici by AMF Glomus intraradices and some Rhizobacteria. J Phytopathol, 153, 544 – 550.
Anitha, A., & Rabeeth, M. (2009). Control of fusarium wilt of tomato by bioformulation of Streptomyces griseus in green house condition. African J Biol. Appl. Sci, 1(1-2), 9–14.
Anusha Suresh Gadag., & Krishnaraj, P.U. (2017). Effect of Actinobacteria and Glomus fasiculatum against Fusarium oxysporum f. sp. lycopersici in tomato plant. Int. J. Curr. Microbiol. App. Sci, 6(9), 488-498.
Aoun, M. (2017). Host defense mechanisms during fungal pathogenesis and how these are overcome in susceptible plants: A review. Int. J. Bot, 13(2), 82-102.
Babu, A.N., Jogaiah, S., Ito, S.I., Nagaraj, A.K., and Tran, L.S.P. (2015) Improvement of growth, fruit weight and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their beneficial traits and induced biosynthesis of antioxidant peroxidase and polyphenol oxidase. Plant Sci, 623(231), 62–73.
Balint‐Kurti, P. (2019). The plant hypersensitive response: concepts, control and consequences. Mol. Plant Pathol, 20(8), 1163-1178.
Ben Abdallah, R. A., Mokni-Tlili, S., Nefzi, A., Jabnoun-Khiareddine, H., & Daami-Remadi, M. (2016). Biocontrol of Fusarium wilt and growth promotion of tomato plants using endophytic bacteria isolated from Nicotiana glauca organs. Biol. Control, 97, 80–88.
Bharathi, R., Vivekananthan, R., Harish, S., Ramanathan, A., & Samiyappan, R. (2004). Rhizobacteria-based bio-formulations for the management of fruit rot infection in chillies. Crop Prot, 23, 835–843.
Bruce, R. J., & West, C. A. (1989). Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures caster beans. Plant physiol, 91, 889-897.
Bubici, G. (2018). Streptomyces spp. as biocontrol agents against Fusarium species. CAB Reviews, 13, 050, 1-15.
Bubici, G., Kaushal, M., Prigigallo, M. I., Gomez-Lama Cabanás, C., & Mercado-Blanco, J. (2019). Biological control agents against Fusarium wilt of banana. Front. Microb, 10, 616.
Cao, P., Liu, C., Sun, P., Fu, X., Wang, S., Wu, F., & Wang, X. (2016). An endophytic Streptomyces sp. strain DHV3-2 from diseased root as a potential biocontrol agent against Verticillium dahliae and growth elicitor in tomato (Solanum lycopersicum). Anton. Leeuw, 109, 1573–1582.
Chandrasekaran, M., & Chun, S. C. (2016). Expression of PR-protein genes and induction of defense-related enzymes by Bacillus subtilis CBR05 in tomato (Solanum lycopersicum) plants challenged with Erwinia carotovora subsp. carotovora. Biosci Biotech. Biochem, 80(11), 2277-2283.
Dahal, B., NandaKafle, G., Perkins, L., & Brozel, V. S. (2017). Diversity of free-Living nitrogen fixing Streptomyces in soils of the badlands of South Dakota. Microbiol. Res, 195, 31–39.
Dias, M.P., Bastos, M.S., Xavier, V.B., Cassel, E., Astarita, L.V., & Santarém, E.R. (2017). Plant growth and resistance promoted by Streptomyces spp. in tomato. Plant Physiol Biochem, 118, 479–493.
Dickerson, D.P., Pascholati, S.F., Hagerman, A.E., Butler, L.G., & Nicholson, R.L. (1984) Phenylalanine ammonia-lyase and hydroxy cinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol. Plant Pathol, 25, 111–123.
El-Katatny, M. H., Somitsch, W., Robra, K. H., El-Katatny, M. S., & Gubitz, G. M. (2000). Production of chitinase and β-1,3-glucanase by Trichoderma harzianum for control of the phytopathogenic fungus Sclerotium rolfsii. Food Tech. Biotech, 38(3), 173–180.
Gomez, K.A., & Gomez, A.A. (1984). Statistical procedures for agricultural research. John Wiley and Sons, New York. USA.
Hammerschmidt, R., Nuckles, E.M., & Ku´c, J. (1982). Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Pl. Pathol, 20, 73–82.
Kandan, A. (2000). Induction of systemic resistance against tomato spotted wilt virus (TSWV) in tomato by fluorescent Pseudomonas strains. M.Sc. (Ag.) Thesis, Tamil Nadu Agricultural University, Coimbatore, India.
Kavitha, K. (2004). Molecular and biochemical approaches for the selection of biocontrol agent for the ecofriendly management of turmeric rhizome rot. Ph.D. thesis, Tamil Nadu Agricultural University, Coimbatore, India.
Larena, I., Sabuquillo, P., Melgarejo, P., & De Cal, A. (2003). Bio control of fusarium and verticillium wilt of tomato by Penicillium oxalicum under greenhouse and field conditions. J. Phytopathology, 151, 507–512.
Liu, Q., Luo, L., & Zheng, L. (2018). Lignins: biosynthesis and biological functions in plants. Int. J. Mol. Sci, 19(2), 335.
Loganathan, M., Garg, R., Venkataravanappa, V., Saha, S., & Rai, A. B. (2014). Plant growth promoting rhizobacteria (PGPR) induces resistance against Fusarium wilt and improves lycopene content and texture in tomato. Afr. J Microb Res, 8(11), 1105-1111.
Magesh, M., & Devi, P. A. (2017). Management of leaf blight through induction of defense enzymes in tomato. Plant Arch, 17(2), 935-940.
Mayer, A.M., Harel, E., & Shaul, R.B. (1965). Assay of catechol oxidase a critical comparison of methods. Phytochem, 5, 783–789.
McGovern, R.J. (2015). Management of tomato diseases caused by Fusarium oxysporum. Crop Protection, 73, 78-92.
Meena, B., Radhajeyalakshimi, R., Marimuthu, T., Vidyasekaran, P., & Velazhahan, R. (2002). Biological control of groundnut late leaf spot and rust by seed and foliar applications of powder formulation of Pseudomonas fluorescens. Biocontrol Sci. Tech, 12, 195 – 204.
Mhlongo, M.I., Piater, L.A., Madala, N.E., Labuschagne, N., & Dubery, I.A. (2018). The Chemistry of plant–microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Frontiers in Plant Science, 9(112),1-17.
Nikoo, F. S., Sahebani, N., Aminian, H., Mokhtarnejad, L., & Ghaderi, R. (2014). Induction of systemic resistance and defense-related enzymes in tomato plants using Pseudomonas fluorescens CHAO and salicylic acid against root-knot nematode Meloidogyne javanica. J. Plant. Prot. Res, 4, 383–389.
Panina, Y., Fravel, D.R., Baker, C.J., & Shcherbakova, L.A. (2007). Biocontrol and plant pathogenic fusarium oxysporum-induced changes in phenolic compounds in tomato leaves and roots. J. Phytopathol, 155, 475– 481.
Pascale V. Balhadere., Andrew J. Foster., & Nicholas J. Talbot. (1999). Identification of pathogenicity mutants of the rice blast fungus Magnaporthe grisea by insertional mutagenesis. Mol. Plant Microbe Int, 12 (2), 129–142.
Passari, A. K., Upadhyaya, K., Singh, G., Abdel-Azeem, A. M., Thankappan, S., Uthandi, S., & Gupta, V. K. (2019). Enhancement of disease resistance, growth potential, and photosynthesis in tomato (Solanum lycopersicum) by inoculation with an endophytic actinobacterium, Streptomyces thermocarboxydus strain BPSAC147. PloS one, 14(7), e0219014.
Radja Commare, R., Nandakumar, R., Kandan, A., Suresh, S., Bharathi, M., Raguchander, T., & Samiyappan, R. (2002). Pseudomonas fluorescens based bioformulation for the management of sheath blight and leaf folder in rice. Crop Prot, 21, 671–677.
Ramamoorthy, V., Raguchander, T., & Samiyappan, R. (2002). Induction of defense-related proteins in tomato roots treated with Pseudomonas fluorescens Pf1 and Fusarium oxysporum f. sp. lycopersici. Plant and Soil, 239, 55–68.
Riker, A.J., & Riker, R.S. (1936). Introduction to research on plant diseases. John Swift Co., St. Louis, Chicago, P.117.
Sadasivam. S., & Manickam, A. (1991). Biochemical Methoda. Second edition. New Age International (p) Ltd., New Delhi.
Sadeghi, A., Koobaz, P., Azimi, H., Karimi, E., & Akbari, A. R. (2017). Plant growth promotion and suppression of Phytophthora drechsleri damping-off in cucumber by cellulase-producing Streptomyces. Bio Control, 62, 805–819.
Saleem, M., Law, A.D., Moe, L.A. (2016). Nicotiana roots recruit rare rhizosphere taxa as major root-inhabiting microbes. Microb. Ecol, 71, 469–472.
Salla, T. D., Astarita, L. V., & Santarem, E. R. (2016). Defense responses in plants of Eucalyptus elicited by Streptomyces and challenged with Botrytis cinerea. Planta, 243, 1055–1070.
Sharma, A., Shahzad, B., Rehman, A., Bhardwaj, R., Landi, M., & Zheng, B. (2019). Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Mol, 24(13), 2452.
Shishido, M., Miwa, C., Usami, T., Amemiya, Y., & Johnson, K.B. (2005) Biological control efficiency of Fusarium wilt of tomato by nonpathogenic Fusarium oxysporum Fo-B2 in different environments. Phytopathol, 95(9), 1074 – 1080.
Tamreihao, K., Ningthoujam, D. S., Nimaichand, S., Singh, E. S., Reena, P., Singh, S. H., & Nongthomba, U. (2016). Biocontrol and plant growth promoting activities of a Streptomyces corchorusii strain UCR3-16 and preparation of powder formulation for application as biofertilizer agents for rice plant. Microbiol. Res, 192, 260-270.
Wen., C.M., Tseng., C.S., Cheng, C.Y., & Li, Y.K. (2002). Purification, characterization and cloning of a chitinase from Bacillus sp. NCTU 2. Biotechnol. Appl. Biochem, 35, 213 – 219.
Wu, Z., Yang, Y., & Li, K. (2019). Antagonistic activity of a novel antifungalmycin N2 from Streptomyces sp. N2 and its biocontrol efficacy against Rhizoctonia solani. FEMS. Microbiol. Lett, 366, fnz018.
Yigit, F., & Dikilitas, M. (2007). Control of Fusarium wilt of tomato by combination of fluorescent Pseudomonas, non pathogen Fusarium and Trichoderma harzianum T – 22 in green house conditions. Plant Pathol. J, 4(2), 91–95.
Zamoum, M., Goudjal, Y., Sabaou, N., Mathieu, F., & Zitouni, A. (2017). Development of formulations based on Streptomyces rochei strain PTL2 spores for biocontrol of Rhizoctonia solani damping-off of tomato seedlings. Bio Sci. and Tech, 27(6), 723-738.
Zieslin, N., & Ben–Zaken, R. (1993). Peroxidase activity and presence of phenolic substances in peduncles of rose flowers. Plant Physiol. Biochem, 31, 333–339.
Zouari, M., Elloumi, N., Ahmed, C., Delmail, D., Rouina, B., Abdallah, F., & Labrousse, P. (2016) Exogenous proline enhances growth, mineral uptake, antioxidant defense, and reduces cadmium-induced oxidative damage in young date palm (Phoenix dactylifera L.). Ecol. Engg, 86, 202-209.