1Department of Botany, Shri Shivaji Arts, Commerce and Science College, Akola (M.S.), India 444003
2Department of Botany, M.S.P. Arts, Science and K.P.T. Commerce College, Manora, Washim (M.S.) India 444404
Corresponding author email: shivdasaher92@gmail.com
Article Publishing History
Received: 02/08/2025
Accepted After Revision: 26/09/2025
The present study investigates the frequency and spectrum of morphological mutations induced by gamma rays, sodium azide (SA), and ethyl methanesulfonate (EMS) in the M2 generation of two chickpea (Cicer arietinum L.) varieties, Vishal and JAKI-9218. Seeds of both varieties were treated with each mutagen, and the resulting M2 populations were systematically screened for macromutations. A diverse array of morphological mutants was identified, including tall, dwarf, bushy, prostrate, one-sided branching, narrow-leaved, broad-leaved, open-flowered, early maturing, and chlorophyll-deficient types. The mutation frequency and spectrum varied notably between the two varieties and among the different mutagen treatments, indicating both genotype-dependent mutagenic response and trait-specific mutagenic efficiency. These findings highlight the effectiveness of gamma rays, SA, and EMS in inducing useful genetic variability in chickpea and demonstrate their potential application in crop improvement programs.
Chickpea, Induced mutations, Morphological mutants, M2 Population.
Aher S. R, Koche D. D. Induced Mutagenesis and Morphological Screening in M2 Generation of Chickpea, Cicer arietinum Varieties Vishal and JAKI-9218. Biosc.Biotech.Res.Comm. 2025;18(3).
Aher S. R, Koche D. D. Induced Mutagenesis and Morphological Screening in M2 Generation of Chickpea, Cicer arietinum Varieties Vishal and JAKI-9218. Biosc.Biotech.Res.Comm. 2024;18(3). Available from: <a href=”https://shorturl.at/LZZvG“>https://shorturl.at/LZZvG</a>
INTRODUCTION
Morphological characterization is a foundational tool in plant breeding and genetic research, involving the systematic assessment of observable traits to identify and classify genotypes. It plays a vital role in detecting genetic variation and mutations within crop populations, thereby supporting both taxonomic classification and the improvement of economically important traits. In chickpea (Cicer arietinum L.), morphological traits serve as key indicators for distinguishing varieties and understanding the genetic basis of agronomic characteristics. As noted by Singh and Dahiya (1974), morphological analysis also offers valuable insights into phylogeny, illuminating the evolutionary development and functional relevance of specific traits across genotypes.
Beyond its taxonomic applications, morphological characterization is integral to practical breeding programs. It enables the identification and selection of superior individuals based on desirable traits, facilitates the assessment of genetic diversity, and complements molecular approaches such as marker-assisted selection. In particular, the evaluation of yield-related traits is crucial for developing high-yielding cultivars. At the local level, morphological traits help guide the selection of varieties suited to specific agro-climatic conditions, while globally, they contribute to germplasm conservation, the assessment of genetic diversity, and the development of climate-resilient cultivars—thereby promoting food security and sustainable agriculture.
Induced mutagenesis, employing physical and chemical mutagens, has emerged as an effective strategy for creating genetic variability and novel allelic combinations. Gamma rays, a commonly used physical mutagen, along with chemical mutagens such as ethyl methanesulfonate (EMS) and sodium azide (SA), have demonstrated high efficiency in inducing mutations and generating phenotypic diversity in a wide range of crops (Borkar and More, 2010 and Koche and Saha 2024).
In this context, the present study was undertaken to induce genetic variability in two chickpea varieties, Vishal and JAKI-9218, through treatment with gamma rays, EMS, and SA. The objective was to generate and characterize morphological mutants in the M2 generation, with the aim of identifying useful variants for potential incorporation into chickpea improvement programs.
MATERIAL AND METHODS
Seeds of two chickpea (Cicer arietinum L.) cultivars, Vishal and JAKI-9218, were obtained from the Pulse Research Station, Dr. Panjabrao Deshmukh Krishi Vidyapeeth (PDKV), Akola, Maharashtra. Uniform, dry, and healthy seeds with 10–12% moisture content were selected for mutagenic treatments.
For physical mutagenesis, seeds were exposed to gamma radiation (Co-60) at doses of 100, 200, 300, and 400 Gy using the gamma irradiation facility at the Central Instrumentation Facility, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur.
For chemical mutagenesis, seeds were pre-soaked in distilled water for 10 hours and then treated with ethyl methanesulfonate (EMS) and sodium azide (SA) at concentrations of 0.1%, 0.2%, and 0.3% for 4 hours under controlled laboratory conditions. Post-treatment, seeds were thoroughly washed under running tap water for one hour to neutralize residual chemicals.
Each treatment consisted of 320 seeds. Treated and control (untreated) seeds were sown in a randomized block design (RBD) with a spacing of 15 cm between plants and 30 cm between rows to raise the M1 generation. Individual M1 plants were harvested separately, and their seeds were used to establish the M2 generation at the experimental field in Jaipur village, Taluka Sengaon, District Hingoli, Maharashtra.
In the M2 generation, plants were systematically screened for morphological mutations. Observations were recorded on five key traits: plant height (cm), growth habit, leaf morphology, branching pattern, and chlorophyll abnormalities. Mutation frequency was calculated, and the collected data were subjected to appropriate statistical analysis to evaluate the significance of induced variation.
RESULTS AND DISCUSSION
The morphological mutants were observed and studied in various traits i.e. Plant height mutants, Growth habit mutants, Leaves variant mutants, Flower mutants, Pod size mutants, Chlorophyll mutants. After recording the total number of mutants from each dose, their values were reported in the form of their frequency per mutagen.
Table 1.1 Effect of mutagens on the frequency of Morphological mutants in M2 generation of Chickpea variety Vishal
| Mutagens
& Dose/ Conc. |
Total Plants observed | PHM | GHM | LVM | FLM | PM | CLM | Freq. (%) of Total
Morpho. Mutants |
| 100 Gy | 437 | 0.1163 | 0.16 | 0.09 | 0.18 | 0.06979 | 0.18 | 8.01 |
| 200 Gy | 428 | 0.1861 | 0.18 | 0.13 | 0.16 | 0.11633 | 0.27 | 10.75 |
| 300 Gy | 425 | 0.2326 | 0.11 | 0.13 | 0.13 | 0.06979 | 0.32 | 10.35 |
| 400 Gy | 418 | 0.2559 | 0.13 | 0.11 | 0.23 | 0.13959 | 0.37 | 12.92 |
| S.A.0.1% | 436 | 0.0465 | 0.06 | 0.06 | 0.67 | 0.06979 | 0.16 | 10.78 |
| S.A.0.2% | 431 | 0.1628 | 0.04 | 0.06 | 1.04 | 0.06979 | 0.18 | 15.78 |
| S.A.0.3% | 427 | 0.2093 | 0.04 | 0.09 | 0.93 | 0.11633 | 0.25 | 16.63 |
| EMS 0.1% | 435 | 0.1395 | 0.11 | 0.09 | 0.37 | 0.09306 | 0.20 | 10.11 |
| EMS 0.2% | 432 | 0.2093 | 0.09 | 0.13 | 0.44 | 0.11633 | 0.27 | 12.73 |
| EMS 0.3% | 429 | 0.2559 | 0.11 | 0.09 | 0.44 | 0.09306 | 0.30 | 13.05 |
| Total | 4298 | 1.8147 | 1.09 | 1.04 | 4.63 | 0.95393 | 2.56 | 12.10 |
Table 1.2: Effect of mutagens on the frequency of Morphological mutants in M2 generation of Chickpea variety Jaki.
| Mutagens
& Dose/ Conc. |
Total Plants observed | PHM | GHM | LVM | FLM | PM | CLM | Freq. (%) of Total
Morpho. Mutants |
| 100 Gy | 427 | 0.0951 | 0.11 | 0.06 | 0.09 | 0.07140 | 0.11 | 5.62 |
| 200 Gy | 418 | 0.1189 | 0.06 | 0.09 | 0.16 | 0.09520 | 0.23 | 7.89 |
| 300 Gy | 415 | 0.1903 | 0.06 | 0.11 | 0.23 | 0.07140 | 0.25 | 9.64 |
| 400 Gy | 416 | 0.2379 | 0.09 | 0.13 | 0.20 | 0.09520 | 0.27 | 10.82 |
| S.A.0.1% | 437 | 0.0475 | 0.06 | 0.06 | 0.39 | 0.04760 | 0.11 | 7.32 |
| S.A.0.2% | 428 | 0.0951 | 0.06 | 0.09 | 0.55 | 0.04760 | 0.21 | 10.75 |
| S.A.0.3% | 427 | 0.0713 | 0.09 | 0.06 | 0.55 | 0.09520 | 0.27 | 11.71 |
| EMS 0.1% | 418 | 0.1189 | 0.06 | 0.11 | 0.34 | 0.07140 | 0.23 | 9.81 |
| EMS 0.2% | 407 | 0.1665 | 0.09 | 0.09 | 0.72 | 0.09520 | 0.32 | 15.48 |
| EMS 0.3% | 409 | 0.2855 | 0.09 | 0.06 | 0.81 | 0.04760 | 0.39 | 17.85 |
| Total | 4202 | 1.4278 | 0.83 | 0.93 | 4.09 | 0.71394 | 2.44 | 10.64 |
Abbreviations used: PHM: Plant height mutants, GHM: Growth habit mutants, LVM: Leaves variant mutant, FLM: Flower mutant, PM: Pod mutants, CLM: Chlorophyll mutants, TMM: Total morphological mutants.
Frequency of Morphological Mutants: The frequency of the morphological mutants in the Gamma rays of variety Vishal is reported from range 8.01%, 10.75%, 10.35% and 12.92% at 100 Gy, 200 Gy, 300 Gy and 400 Gy respectively while in variety Jaki it ranges from 5.62%, 7.89%, 9.64% and 10.82% at the same doses respectively. In SA the frequency in Variety Vishal ranges from 10.78%, 15.78% and 16.63% at 0.1%, 0.2% and 0.3% concentration respectively while in Jaki the values are range from 7.32%, 10.75% and 11.71% at .1%, 0.2% and 0.3% concentration respectively. The values of morphological mutant frequency were recorded in EMS treatment reveals that in variety Vishal it ranges between 10.11% to 13.05% at 0.1% to 0.3% concentration, but in variety Jaki the values ranged from 9.81% to 17.85% at 0.1% to 0.3%. The frequency of morphological mutants in both varieties show increasing order as the concentration or doses of treatments increases. The largest values were reported from variety Vishal (12.10%) as compared to variety Jaki (10.64%) (Table 1.1, 1.2).
Table 1.3: Frequency of different morphological mutants isolated in
M2 generation of Chickpea variety Vishal and Jaki.
| Mutant Type | Var. Vishal | Freq. in Var. Vishal | Var.
Jaki |
Freq. in Var.
Jaki |
| Tall | 36 | 0.84 | 25 | 0.59 |
| Dwarf | 42 | 0.98 | 35 | 0.83 |
| Bushy, Compact | 10 | 0.23 | 05 | 0.12 |
| Prostrate | 04 | 0.09 | 04 | 0.10 |
| Erect | 17 | 0.40 | 08 | 0.19 |
| Spreading | 12 | 0.28 | 15 | 0.36 |
| 1-Sided branching | 04 | 0.09 | 04 | 0.10 |
| Broad-leaved | 11 | 0.26 | 19 | 0.45 |
| Small, narrow leaved | 16 | 0.37 | 10 | 0.24 |
| Altered Leaf Str. | 10 | 0.23 | 07 | 0.17 |
| Elongated Rachis | 07 | 0.16 | 04 | 0.10 |
| Violet/Peach colour flower | 07 | 0.16 | 05 | 0.12 |
| Abnormal flower | 115 | 2.68 | 62 | 1.48 |
| Non-flowering, vegetative | 47 | 1.09 | 85 | 2.02 |
| Early flowering | 16 | 0.37 | 14 | 0.33 |
| Late flowering | 14 | 0.33 | 10 | 0.24 |
| Small pod | 16 | 0.37 | 10 | 0.24 |
| Bold pod | 25 | 0.58 | 20 | 0.48 |
| Albina | 15 | 0.35 | 10 | 0.24 |
| Xantha | 22 | 0.51 | 27 | 0.64 |
| Chlorina | 22 | 0.51 | 17 | 0.40 |
| Viridis | 18 | 0.42 | 21 | 0.50 |
| Tigrina | 33 | 0.77 | 30 | 0.71 |
| Total | 520 | 12.10 | 447 | 10.64 |
Figure 1a: Frequency of Morphological mutants screened from the M2
Population of Mungbean [C. arietinum L., Var- Vijay and Jaki.
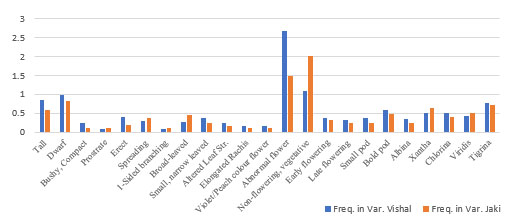
Plant height mutants: The average plant height in control plants of both the selected varieties is ranges from 35 cm to 40 cm. The plant height mutants were reported at maturity in two chickpea varieties (Vishal and Jaki) when pods were fully developed. The average height of tall mutants in Variety Vishal is observed to be 71 cm to 76 cm. while in Jaki the height ranges from 65 cm to 71 cm. The average height in dwarf plants in variety Vishal was found in between 10.00 cm to 20 cm. while in Jaki it ranges from 08 cm to 18 cm.
A total of 78 plant height mutants were reported from variety Vishal, among these 36 (46.15%) were tall and 42 (53.85%) were dwarf, while in variety Jaki total of 60 plant height mutants were reported, among these 25 (41.67%) were tall and 35 (58.33%) were dwarf (Fig. 1-2).
Growth habit mutants: In M2 generation of Chickpea five types of growth habit mutants were reported from both the selected varieties. Growth habit type mutants include; Bushy, Prostrate, Erect, spreading and one- sided branching.
Frequency of growth habit mutants: The frequency of growth habit mutants in both varieties, Vishal and Jaki, was variable in values. In a variety Vishal of Gamma ray treatments, frequency at 100 Gy to 400 Gy is recorded to be 0.16 %, 0.18%, 0.11% and 0.11% subsequently. In variety Jaki the values were ranges from 0.09%, 0.13%, 0.13% and 0.11% at 100 Gy to 400 Gy doses of Gamma ray, 0.06%, 0.06%,0.09% at 0.1% to 0.3% doses of SA subsequently while 0.09%, 0.13%, 0.09% at 0.1% to 0.3% of EMS doses subsequently (Fig. 3-6).
Leaves variant mutants: Leave Variant mutants also contribute in the spectrum of total morphological mutants which were isolated from M2 Population of Chickpea from both the varieties. The overall frequency of leaves Variant mutants in variety Vishal (1.04%) was observed more in value than variety Jaki (0.93%). A total four types of leaf variants mutants were reported from M2 population of Chickpea from both the selected varieties i.e. Broad leaves mutants, Narrow leaves mutants, altered leaf structure mutants and elongated rachis mutants (Fig. 7-10).
Flower mutants: Flowers are the reproductive structure of any plant, which affects the overall yield of crops. The changes in the flower structure were observed in both the selected varieties of Chickpea. From the progeny of M2 generation, five kinds of flower mutants were recorded i.e. Flower Colour, Abnormal flower, Non-flowering, Early flower and late flowering mutants. Cumulatively, the frequency of flower mutants was represented from each selected variety of Chickpea. The highest frequency (4.63%) was reported from variety Vishal as compared to variety Jaki (4.09%) (Fig.11-18).
Pod size mutants: Pod size is a remarkable trait that effects on yield of plant. As compared to pods of Control, significant variations occur in the pod size in some mutant plants. Pod size mutants were reported from different doses of both the selected varieties of chickpea. The size of pods corresponded to the size of leaflets. The larger the leaflet size more bolder the pod size whereas smaller the leaflet size in smaller pod size. The frequency of total pod mutants from variety Vishal (0.95393) was higher as compared to Jaki (0.71394) (Fig. 19-24).
Chlorophyll mutants: Chlorophyll mutants are more common in mutational breeding program. Alternation in the pigments may occurs due to change in gene sequence caused by mutations. In Chickpea leaf colour were changed in mutants of different doses. Different kinds of chlorophyll mutants were reported from both selected varieties of chickpea. Isolated Chlorophyll mutants include; Albina, Xantha, Chlorina, Viridis and Tigrina. The frequency of total chlorophyll mutants from variety Vishal was analysed as 2.56% whereas frequency was 2.44% from variety Jaki. In variety Vishal the highest number (16) of chlorophyll mutants were reported from 400 Gy dose and lowest number (7) was reported from S.A. 0.1% dose. Among all identified chlorophyll mutants from every dose, the highest number (33) was reported from Tigrina types while lowest number (15) from Alibina (Fig. 25-29).
Figures 1- 29

The present study successfully demonstrated the effectiveness of gamma rays, ethyl methanesulfonate (EMS), and sodium azide (SA) in inducing a broad spectrum of morphological mutations in the M2 generation of chickpea (Cicer arietinum L.) cultivars Vishal and JAKI-9218. The induced variability affected several traits, including plant height, growth habit, leaf morphology, floral characteristics, pod size, and chlorophyll pigmentation—traits of agronomic importance in chickpea improvement.
A clear dose-dependent relationship was observed in most traits, with higher concentrations of mutagens generally resulting in increased mutation frequencies. In cultivar Vishal, the highest frequency of morphological mutations was recorded at 0.3% SA, while in JAKI-9218, the highest mutation frequency was induced by 0.3% EMS. Conversely, the lowest mutation rates were recorded at 100 Gy gamma radiation in both varieties, suggesting that the effectiveness of mutagens is both dose- and genotype-dependent. These findings align with earlier reports of genotypic differences in mutagenic sensitivity (Lal & Mishra, 2006; Khan & Goyal, 2009; Lavanya et al., 2011).
Tall mutants were predominantly observed at higher doses of gamma rays and SA in Vishal, and across 100–300 Gy gamma ray treatments in JAKI-9218. These increases in plant height are likely attributed to enhanced internodal elongation, as reported in previous studies on black gram, lentil, and sesame (Jana, 1963; Sudharani, 1990; Begum et al., 1995). In contrast, dwarf mutants were more frequent under SA and EMS treatments in Vishal and showed a dose-dependent increase under gamma radiation in JAKI-9218. Dwarfism may be due to impaired cell division and elongation caused by mutagen-induced disruptions, consistent with findings by Sonavane (2000) and Dahiya et al. (1984).
Leaf morphological mutants, including broad, narrow, elongated rachis, and altered leaf structures, were observed in both varieties. These anomalies may be the result of chromosomal aberrations or reduced mitotic activity induced by mutagenic stress, as noted by Wani and Anis (2008). Variants in growth habit—such as bushy, prostrate, erect, spreading, and one-sided branching—were detected across treatments, with bushy types being most frequent at the lowest gamma dose (100 Gy). The inconsistent distribution of these mutants suggests polygenic control and complex genetic interactions influencing plant architecture, similar to brachytic mutants reported by Gaur et al. (2008).
Floral mutants, including early and late flowering, non-flowering, and aberrant floral forms, were more prevalent under higher doses of EMS and SA in JAKI-9218. No clear dose-response trend was observed in Vishal, indicating genotype-specific floral sensitivity. Similar mutagen-induced floral alterations have been documented in legumes (Sonavane, 2000; Kulthe, 2003). Pod size mutations showed variable trends: small pod types were frequent at lower mutagen doses, while bold pod mutants appeared more commonly at higher doses. These observations corroborate findings in urdbean and chickpea by More (2004), Wani and Anis (2008) and Bogawar et al., (2017).
Chlorophyll-deficient mutants—such as albina, xantha, chlorina, viridis, and tigrina—exhibited a clear dose-dependent increase across all treatments, with JAKI-9218 recording higher frequencies than Vishal. These mutants, which reflect disruptions in the chlorophyll biosynthesis pathway, are widely recognized as sensitive indicators of mutagenic effects (Sarkar & Kundagrami, 2018; Ahir et al., 2023).
Overall, the results indicate that chemical mutagens, particularly EMS and SA, were more effective than gamma rays in inducing morphological variability. The observed genotypic differences in mutation frequencies and spectra emphasize the need to consider varietal responses in mutation breeding. These findings reinforce the utility of induced mutagenesis as a valuable tool for generating genetic variability, which can be harnessed for chickpea improvement through the selection of desirable mutants in subsequent generations.
CONCLUSION
This study demonstrated that induced mutagenesis using gamma rays, EMS, and sodium azide effectively generated a broad spectrum of morphological mutations in chickpea (Cicer arietinum L.). Significant variability was observed in key agronomic traits, including plant height, growth habit, leaf morphology, floral structure, pod size, and chlorophyll pigmentation.
Among the two cultivars, JAKI-9218 exhibited greater sensitivity to mutagenic treatments than Vishal, with the highest mutation frequencies recorded at 0.3% EMS and 0.3% SA. While gamma irradiation was comparatively less effective, it still produced useful mutations at moderate doses. The morphological mutants obtained—especially those influencing plant architecture and yield-related characteristics—hold considerable potential for use in chickpea improvement programs. Further evaluation and selection of promising lines could facilitate the development of superior chickpea varieties with improved agronomic performance, genetic diversity, and adaptability to diverse agro-climatic conditions.
ACKNOWLEDGEMENTS
The authors are grateful to Dr. R. M. Bhise, Principal of Shri Shivaji College of Arts, Commerce and Science Akola (MS) and Dr. N. S. Thakare, Principal, MSP and KPT College Manora District, Washim (MS), for their support in carrying out this collaborative work.
Conflict of Interest: The authors declared no conflicts of interest with this research work.
Data Availability: All data will be available with the corresponding author on reasonable request.
REFERENCES
Aher, S. R. and Koche, D. K. (2022). Induced physical and chemical mutagenic studies in M1 generation of chickpea (Cicer arietinum L.). International Journal of Food Nutrition Science, 11(7), 3370-3376.
Ahir, D. K., Kumar, R., Chetariya, C. P., Upadhyay, V., Pandey, A., & Chaitanya, A. K. (2023). Induced Mutations a Tool to Create New Variations: A Case Study of M3 Generation of Green Gram [Vigna radiata (L.) R. Wilczek]. Emerging Issues in Agricultural Sciences, 6, 104-123.
Begum S., Majid M. A. and Shaikh M. A. Q. (1995). Selection of promising lentil mutants derived through gamma irradiation. Lens Newsletter, 22(1/2), 5-8.
Bogawar, P. R., Aher, S. R., Joshi Saha, A. and Koche, D. K. (2017). Genetic variability of Morphological Mutants induced by Gamma Rays and EMS in Chickpea (Variety- Vijay). International Journal of Current Research, 9(8), 56320-56324.
Borkar, A. T. and More, A. D. (2010). Mutations in Phaseolus vulgaris Linn. through Physical and Chemical Mutagens. Advances in Bioresearch, 1 (1), 22 – 28.
Deshmukh A. G. and Koche D. K. (2023). Induced Morphological Variation in Mutagen-treated Mungbean [Vigna radiata (L.) Wilczek] Cultivars in M2 Generation. Aspect in Plant Biology book,
Brilliant Publications House, Pg. No. 117-124, ISBN No.- 978-0-99-702549-1.
Gaikwad, N. B. (2002). Genetic improvement of lentil (Lens culinaris medica) through mutagenesis. Ph. D. Thesis, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, India.
Gaur, P. M., Gour, V. K. and Singh, K. (2004). Induction and genetics of a variegated leaf and an apical chlorosis mutant in chickpea. Indian J. Genetics. 64, 208–211.
Gaur, P. M., Samineni, S., Tripathi, S. et al. (2016). Allelic relationships of flowering time genes in chickpea. Euphytica, 203,295–308.
Gaur, P. M., Gour, V. K., and Srinivasan, S. (2008). An induced brachytic mutant of chickpea and its possible use in ideotype breeding. Euphytica, 159(1–2), 35–41.
Gautam, V., Swaminathan, M., Akilan, M., Gurusamy, A., Suresh, M., Kaithamalai, B. and John Joel, A. (2021). Early flowering, good grain quality mutants through gamma rays and EMS for enhancing per day productivity in rice (Oryza sativa L.). International Journal of Radiational Biology 97 (12), 1716-1730.
Hakande, T. P. (1992). Cytogenetical studies in Psophocarpus tetragonolobus (L.) DC., Ph. D. Thesis, Marathwada University, Aurangabad, India.
Hasib, K. M. (2022). Induction of Chlorophyll and Morphological Mutations through Gamma Ray in Traditional Aromatic Cultivar Tulai panja. Biosciences Biotechnology Research Asia 19(3).
Jana. M. K. (1963). X-ray induced mutations of Phaseolus mungo L. I. Chlorophyll mutations. Caryologia. 16: 685-692.
Kar, U. C., and Swain, D. (2003). Induced chlorophyll and macro-mutational spectrum and frequency in sesame cv. B67. Madras Agricultural Journal. Kashikar, S. G. and Khalatkar, A. S. (1979). Induced mutations in Petunia hybrida, Hort. Proc. Symp. on “The role of induced mutations in crop improvement”. 78, 433 – 443.
Khan, S. and Goyal, S. (2009). Improvement of mung bean varieties through induced mutations. African Journal of Plant Science 3: 174-180.
Kharkwal, M. C. (1999). Induced mutations in chickpea (Cicer arietinum L.) III. Frequency and spectrum of viable mutations. Indian Journal of Genetics and Plant Breeding. 59, 451–464.
Khazaei, H., Carlson-Nilsson, U., and Schulman, A. H. (2024), The Jan Sjödin faba bean mutant collection: morphological and molecular characterization. Hereditas, 161(1), 37.
Koche, D. K. and Saha, A. J. (2024). Isolation of high yielding, nutritionally improved chickpea mutant lines through induced mutagenesis using gamma rays and EMS. Journal of Applied Biology and Agriculture 1(2), 103-118.
Kulthe M. P. (2003). Induced mutational and biochemical studies in winged bean (Psophocarpus tetragonolobus (L.) DC.). Ph. D. Thesis, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, India.
Lal, N., and Mishra, R. (2006). Induced genetic variability and divergence in M3 generation in mung bean. Indian Journal of Pulses Research, 19(1), 47.
Lavanya, G. R., Yadav, L., Babu, G. S. and Paul, P. J. (2011). Sodium azide mutagenic effect on biological parameters and induced genetic variability in mung bean. Journal of Food Legume, 24 (1), 46 -49.
Mishra, D., Singh, B., and Sahu, R. (2013). Gamma ray induced macro mutations in green gram (Vigna radiata (L.) Wilczek). International Journal of Agriculture and Forestry, 3(3), 105-109.
More S. B. (2004). Genetic improvement of chickpea (Cicer arietinum L.) through mutations. Ph. D. Thesis, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, India.
Sarkar, M., and Kundagrami, S. (2018). Selection of high yielding, extra short duration lines of mung bean derived through gamma radiation. Indian Journal of Genetics and Plant Breeding, 78(02), 233-241.
Savant K. D. (2008). Genetic improvement of sesame (Sesamum indicum L.) through induced mutations. Ph. D. Thesis, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, India.
Singh, Habans and B. S. Dahiya (1974). A note on inheritance of the bushy mutant in chickpea. Current Science. 43: 731-732.
Sinjushin, A., Semenova, E., and Vishnyakova, M. (2022). Usage of Morphological Mutations for Improvement of a Garden Pea (Pisum sativum): The Experience of Breeding in Russia. Agronomy, 12(3), 544.
Sonavane A. S. (2000). Genetic improvement of winged bean through mutation breeding. Ph. D. Thesis, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad, India.
Sudharani, T. (1990). Genetical studies in induced mutants of Phaseolus mungo L. Ph.D. Thesis Osmania uni. Hyderabad.
Umavathi, S. and Mullainathan, L. (2020). Frequency and spectrum of morphological mutants induced by gamma rays and EMS in M2 generation of Chick pea. Universe International Journal of Interdisciplinary Research. 1(2), 273-281.
Wani, A. A. and Anis, M. (2008). Gamma ray and EMS induced bold seeded high yielding mutants in chickpea (Cicer arietinum L.). Turkish Journal of Biology 32 (3), 161-166.


