Department of Botany and Microbiology, Andhra Christian College, Guntur, Andhra Pradesh, India
Corresponding author email: phebesarah63@gmail.com
Article Publishing History
Received: 24/10/2020
Accepted After Revision: 12/12/2020
In the present study soil samples were collected from different chilli rhizospheres in the vicinity of Guntur, Andhra Pradesh, India and the filed trails were conducted in the year of 2018-2019. A total of 57 microbial strains were isolated from chilli rhizosphere. All the strains were isolated from potato dextrose agar media by using 10-4 serial dilutions. Fine and clear colonies were picked and transformed into a culture tubes for further studies. For the preliminary screening there are 10 isolates were considered as fungal strains based on spore morphology. The spores are round and irregular in shape with green to light brown in colour. Preliminary identification of these fungal isolates was based on morphological and cultural characters on potato dextrose agar medium. All ten strains showed the maximum indole acetic acid production on Czepakdox agar medium.
Various optimization studies like incubation period, pH, temperature and carbon and nitrogen sources were studied by affecting the indole acetic acid productions i.e., 10 days incubation period, pH 7.0 and 30°C. Carbon and nitrogen sources are also affected the indole acetic acid production in this optimization. A carbon and nitrogen source in the optimal medium plays a major role for the production indole acetic acid. Among them, the strain Aspergillus PB-7 in presence of Glucose and peptone showed the maximum IAA production of 110µg/ml and 75µg/ml. Successful inoculation of agricultural crops with biocontrol plant growth promoters includes the delivery of sufficient inoculum to the target, economical production of large quantities of microorganisms. Rhizospheric microbes especially plant growth promoting microorganism were promising to be developed as multifunctional biofertilizer.
Plant growth promoters (PGP), Indole acetic acid (IAA), Potato Dextrose Agar (PDA).
Ratnam D. P. S. K. Indole 3-Acetic Acid Production by Aspergillus Species Isolated from Chilli Rhizospheres. Biosc.Biotech.Res.Comm. 2020;13(4).
Ratnam D. P. S. K. Indole 3-Acetic Acid Production by Aspergillus Species Isolated from Chilli Rhizospheres. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/2HXkEAM
Copyright © Ratnam This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Chilli can be grown in a wide range of Black, Brown, Red and Clay soils, but black soils which retain moisture for long periods are suitable for rain fed crop whereas well drained soils, deltaic soils and sandy loams are good under irrigated condition. Whereas Sandy and loamy soils poorly supported to chilli production when compare to black soils. Various climatic conditions like humid and hot weather supports to the chilli plants. Sometimes environmental conditions are also adversely affected the chilli growth and fruit production. India is the largest producer and consumer of chilli among other major producers in the world. India contributes about 47 per cent to the total world production, and assumes first position in terms of international trade, exporting 38 per cent of its total production. Chilli production in India is moving northwards on increasing demand from diversified sectors and changing consumption patterns.
Dry chilli production rose by nearly 52 per by nearly 52 per cent from 9.7 lakh tones in 1997-98 to about 18 lakh tonnes. More than 80% of the bacteria isolated from the rhizosphere can produce (IAA) Indole Acetic Acid (Khalid et al., 2004) in the presence of precursor, tryptophan either through root exudates or from the proteins released by the dead bacteria cells (Patten and Glick, 1996). Indole-3-acetic acid (IAA), a plant growth hormone compound, is a natural auxin produced by plants, bacteria, fungi and a diverse group of organisms. It is a metabolite derived from tryptophan by many tryptophan dependant and tryptophan independent pathways in plants as well as bacteria and fungi. These growth improvers act as biocontrol agents. (Pattern and Glick, 2002; Wesam et al., 2017).
Various soil microorganisms including bacteria, fungi (Finnie and Van Staden, 1985) and algae (Stein et al., 1990; Rifat Hayat et al., 2010) are capable of producing physiologically active growth hormones like auxins and gibberellins which may exert prominent effects on plant growth and development. Many PGPR (Plant growth promoting) microorganisms associated in chilli rhizosphere. Chilli is one of the important agriculture produce Andhra Pradesh also. Chillies from Andhra Pradesh are well known for their pungency and good red colour. Several districts like Guntur, Krishna, Prakasam, Nellore, Chittor and Anantapuram are the main chilli growing districts in Andhra Pradesh. The present study was mainly focussed on indole acetic acid production and optimization studies by fungal strains isolated from chilli rhizosphere. These strains were used as bio inoculants in application to the farmer’s fields and it is useful for sustainable agriculture (Wesam et al., 2017).
MATERIAL AND METHODS
Isolation of fungi done using the rhizosphere soil samples from the chilli fields of 10 different areas of Guntur, district of Andhra Pradesh, India were collected for the study. Fungal strains were isolated on Potato Dextrose Agar (PDA) medium by soil dilution plate technique (Rapilly, 1968) using 10-3 to 10-5 dilutions. The plates were incubated at 28 ± 2°C for 5 days. Fungal colonies appeared in the plates were noted and sub cultured. After purified by single spore isolation method and they were maintained on potato dextrose agar (PDA) slants. Identification of Fungal solates was based on culture characters as well as microscopic parameters (conidiophores branching, phialides shape and position, spore size and shape) (Nagamani et al., 2006). The pure cultures were stored in the refrigerator at 4°C for further studies.
Screening of isolates for IAA production was determined based on the method described by Patten and Glick (2002) with slight modifications. One milli liter of supernatant was mixed with 2 ml of Salkowski reagent (1ml of 0.5M FeCl3 in 50mL of 35% HClO4) and incubated for 1hr. Development of pink colour indicated the production of IAA. The quantification of IAA was read at 540 nm in a UV- Vis spectrophotometer. A standard curve was plotted for quantification of IAA solution and uninoculated medium with a reagent as a control. The amount of IAA in the culture was expressed as μg/ml (Gordon and Weber, 1951).
The optimization studies for IAA production to determine the IAA production, different incubation periods (7, 8, 9, 10, 11 and 12 days), pH levels (4, 5, 6,7, 8 and 9) and temperature (4,20, 25, 30, 35 and 40⁰C) were studied. Various carbon (1%) and nitrogen (0.1%) sources were studied by using the above method.
For the statistical analysis, all measurements were carried out in triplicate. Statistical analyses were performed using one-way analysis of variance (ANOVA), and the significance of the difference between means was determined by Duncan’s multiple range tests. Differences at < 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
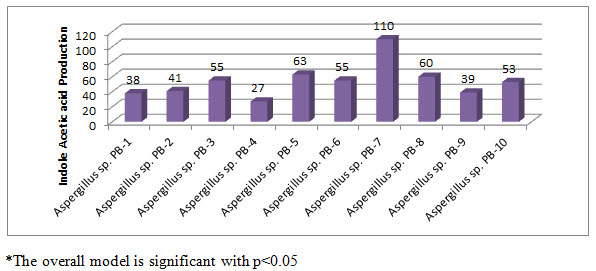
A total of 57 strains were isolated from chilli fields in the vicinity of Guntur district, Andhra Pradesh, India. All the strains were tested for IAA production; among them 10 strains were positive results on Potato dextrose agar medium (Figure-1). The present study mainly reveals the IAA production of the selected 10 fungal strains. Maximum IAA production was observed on Aspergillus sp. PB 7 strain which showed 110 µg/ml of IAA, followed by Aspergillus sp. PB 5 strain (63 µg/ml). Minimum IAA production was recorded in Aspergillus sp. PB 4 with 27 µg/ml. IAA productions by ten fungal strains between the range of 27-110 µg/ml. Aspergillus flavus Promoted the Growth of Soybean and Sunflower Seedlings which was reported by Humayun et al., (2019).
Figure 1: IAA production (µg/ml) by Aspergillus species
Optimization studies for IAA production (µg/ml) by Aspergillus species PB 7: For the optimization studies we selected PB 7 Aspergillus strain which showed maximum IAA production and was tested with the effect of incubation period, pH and temperatures, carbon and nitrogen sources.
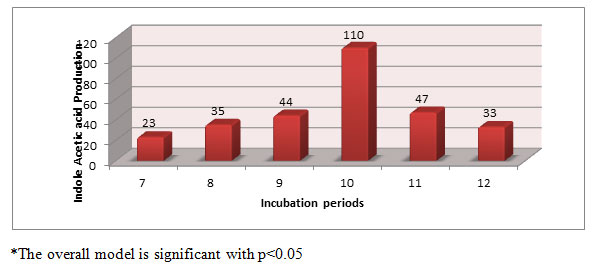
Effect of incubation period on IAA production by Aspergillus species PB 7: The IAA production was started between 7 to 12 days of incubation periods (Figure-2). The maximum IAA production was observed on 10th day of incubation (110 μg/ml). Similarly Unyayar et al., (2000) and Hansan (2002) reported that the maxumum amount of IAA was synthesized during the stationnary phase of growth. May be these reason was that during stationnary phase the bacterium might be able to get maximum tryptophan from dead bacterial mass, which could result in more IAA production. Reduction of IAA production at the later might be due to release of IAA degrading enzymes by the bacteria (Hunter, 1989).
Figure 2: Effect of incubation period on IAA production (µg/ml) by Aspergillus species PB 7
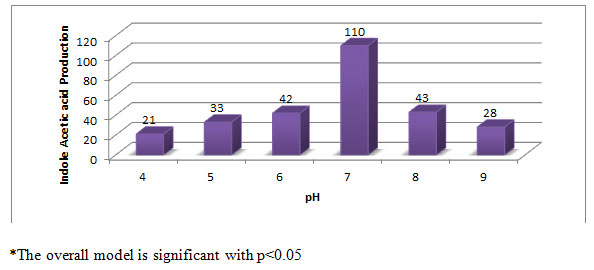
Effect of pH on IAA production (µg/ml) by Aspergillus species PB-7: The pH of the medium showed a significant influence on the production of IAA by fungal strains. The maximum IAA production (110 µg/ml) was observed at pH 7 (Figure-3). For many authors, the optimum pH for IAA production is between 6 to 9. However, the below and above pH 8 the production of IAA was less, because Streptomyces sp population level is more in alkaline soil than the acidic soil (Shirokikh et al., 2007; Mohite, 2013; Bharucha et al., 2013; Dasri et al., 2014).
Figure 3: Effect of pH on IAA production (µg/ml) by Aspergillus species PB-7
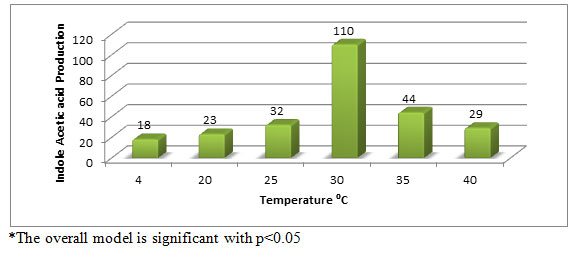
Effect of different temperature on IAA production (µg/ml) by Aspergillus species PB-7: The effect of different temperature on IAA production by fungal strains showed that, the maximum IAA production was obtained at 30°C (65 µg/ml) followed by 35°C (44 µg/ml) and 40°C (29 µg/ml) respectively (Figure-4).
Figure 4: Effect of temperature on IAA production (µg/ml) by Aspergillus species PB-7
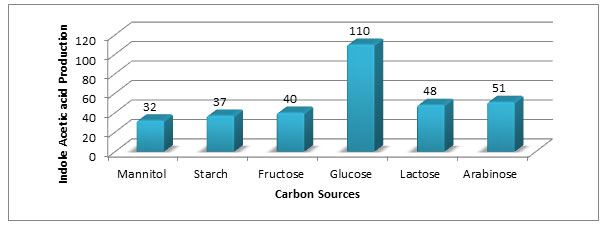
Effect of different carbon source on IAA production (µg/ml) by Aspergillus species PB-7: Different carbon sources (mannitol, Starch, glucose, sucrose, fructose, lactose and Arabinose) were studied for their effect on IAA production by Aspergillus species PB-7 (Figure-5). Glucose in the medium gave maximum IAA production (110 µg/ml) followed by arabinose (51µg/ml) and lactose (48 µg/ml).
Figure 5: Effect of carbon sources on IAA production (µg/ml) by Aspergillus species PB-7
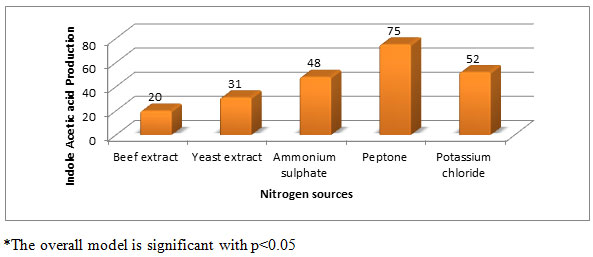
Effect of different nitrogen sources on IAA production (µg/ml) by Aspergillus species PB-7: Various nitrogenous compounds (Ammonium sulphate, potassium chloride, Yeast extract, peptone and beef extract). These nitrogen source have a significant effect on IAA production (Figure-6). Among all the nitrogen sources used, peptone was found to be the best nitrogen source for IAA production. Organic and inorganic nitrogen sources can be utilized by Pseudomonas sp. prefers yeast extract (Balaji, 2012), Pantoea agglomerans PVM prefer beef (Apine and Jadhav, 2011), and Ammonium sulphate was found to be for IAA production of Acetobacter diazotrophicus L1, the most suitable nitrogen source (Nita et al., 2011).
Figure 6: Effect of nitrogen sources on IAA production (µg/ml) by Aspergillus species PB-7
CONCLUSION
From the results, it is clear that Aspergillus species PB 7 isolated form chilli rhizospheres preferred glucose and peptone as carbon and nitrogen sources respectively. The optimum conditions for this culture are incubation 12 days, pH 7.0 and 30°C temperature. There were several plant growth promoting rhizobacterial (PGPR) inoculants that seems to promote plant growth through different mechanism such as plant growth hormone production, nutrient acquisition and plant disease suppression. Thus the optimized microbial inoculants may be useful for the production of multifunctional biofertilizers.
ACKNOWLEDGEMENTS
Author would like to thank the Department of Botany and Andhra Christian College, Guntur for providing necessary facilities to complete this research work.
REFERENCES
Apine, O. A. and Jadhav, J. P. (2011). Optimization of medium for indole-3-acetic acid production using Pantoea agglomerans strain PVM. Journal of Applied Microbiology, 110(5), 1235-44.
Balaji, N., S. S. Lavanya, S. Muthamizhselvi and K. Tamilarasan (2012). Optimization of fermentation condition for indole acetic acid production by Pseudomonas species. International Journal of Advanced Biotechnology and Research, 3, 797-803.
Bharucha U., Patel K., and Trivedi U. B. (2013). Optimization of Indole Acetic Acid Production by Pseudomonas putida UB1 and its Effect as Plant Growth-Promoting Rhizobacteria on Mustard (Brassica nigra). Agric Res.
Dasri, K., Kaewharn, J., Kanso, S. and Sangchanjiradet, S., (2014). Optimization of indole-3-acetic acid (IAA) production by rhizobacteria isolated from epiphytic orchids. Asia-Pacific Journal of Science and Technology, 19: 268-275.
Finnie, J. F. and Van Staden, J. (1985). Effect of seed weed concentrate and applied hormones on in vitro cultured tomato roots. J Plant Physiol. 120: 215- 222.
Gordon, S.A. and Weber, R.P. (1951). Colorimetric estimation of indole acetic acid. Plant physiology, 26(1):192-195.
Hamayun, M., Hussain, A., Afzal Khan, S., Iqbal, A. and Lee, I.J., (2019). Aspergillus flavus promoted the growth of soybean and sunflower seedlings at elevated temperature. BioMed research international, 2019:1-13.
Hansan H.A.H. (2002). Gibberellin and auxin production by plant root fungi and their biosynthesis under salinity-calcium interaction. Rostlinna Vyroba, 48 : 101-106.
Hunter, W.J. (1989). Indole-3-acetic acid production by bacteroids from soybean root nodules. Physiol. Plant. 76 : 31-36.
Khalid A., Tahir, S., Arshad, M. and Zahir, Z.A. (2004). Relative efficiency of rhizobacteria for auxin biosynthesis in rhizosphere and non-rhizosphere soils. Aus. J. Soil Res. 42:921-926.
Mohite B. (2013). Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. Journal of soil science and plant nutrition, 13(3): 638-649.
Nagamani A, Kunwar IK, and Manoharachary C. (2006). Handbook of soil fungi, IK Int Pvt Ltd. New Delhi: pp.425.
Nita B. Patil, Milind Gajbhiye, Sangita S. Ahiwale, Aparna B. Gunjal and Balasaheb P. Kapadnis. (2011). Optimization of Indole 3-acetic acid (IAA) production by Acetobacter diazotrophicus L1 isolated from Sugarcane. International Journal of Environmental Sciences, 2(1), 307-314.
Patten C. L. and Glick B. R. (2002). Regulation of indole acetic acid production in Pseudomonas putida GR12-2 by tryptophan and the stationary-phase sigma factor RpoS. Canadian journal of microbiology, 48(7):635-642.
Patten, C. L., and B.R. Glick. (1996). Bacterial biosynthesis of indole-3-acetic acid. Canadian journal of microbiology 42: 207-220.
Rapilly F. (1968). Les Techniques de mycologie en pathologie végétale. Ann. Epiphyt. 101p. Bruxelles.
Rifat Hayat, Safdar Ali, Ummay Amara, Rabia Khalid, and Iftikhar Ahmed. (2010). Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol. 60: 579- 598.
Shirokikh, I. G., Zenova, G. M., Merzaeva, O.V., Lapygina, E.V., Batalova, G.A. and Lysak, L. V. (2007). Actinomycetes in the prokaryotic complex of the rhizosphere of oats in a soddy podzolic soil. Eurasian Soil Sci. 40: 158-162.
Stein, A., Fortin, J. A. and Vallee, G. (1990). Enhanced rooting of Picea mariana cuttings by ecto mycorrhizal fungi. Can J Bot. 68: 492-498.
Unyayar, S., Unal, E. and Unyayar, A., (2000). Production of auxin and abscisic acid by Phanerochaete chrysosporiumme446 immobilized on polyurethane foam. Turkish Journal of Biology, 24(4):769-774.
Wesam I. A. Saber, Khalid M. Ghoneem, Younes M. Rashad and Abdulaziz A. Al-Askar. (2017). Trichoderma Harzianum WKY1 : an indole acetic acid producer for growth improvement and anthracnose disease control in sorghum. Biocontrol Science and Technology, 27 (5), 654–676.