1*Department of Zoology, Government Arts and Science College (Autonomous), Karwar, Karnataka, India.
2Government First Grade College, Mundargi, Karnataka, India
3Department of Biotechnology, Government Arts and Science College (Autonomous), Karwar, Karnataka, India.
4Department of Biotechnology, Government Arts and Science College (Autonomous), Karwar, Karnataka, India.
5Department of Biochemistry, Aligarh Muslim University, Aligarh, Uttar Pradesh, India
Corresponding author email: zafarin@gmail.com
Article Publishing History
Received: 24/03/2022
Accepted After Revision: 29/05/2022
Two commercially significant marine Pelecypoda species named Perna viridis (green mussel) and Paphia malabarica (short neck yellow clam) were exposed to different concentrations of Atrataf (commercial brand of atrazine available in India) in an acute toxicity test. The 96 h LC50 values of Atrataf to P. viridis and P. malabarica were 6.10 mg L-1, and 4.90 mg L-1 respectively. This study showed that there is a significant increase in mortality in both species as the dose and duration of Atrataf exposure are increased further, exposure to sublethal concentrations of the Atrataf. Moreover, following 14 days of exposure to sublethal doses of Atrataf, the immunotoxic potential of atrazine was examined by measuring viable haemocytes using the Tryphan Blue Exclusion Assay.
After 14 days of exposure to the highest sublethal doses of Atrataf, the percentage of viable hemocytes decreased to 74.51 (Perna viridis) and 78.39 (Paphia malabarica), relative to the control. Since Haemocytes are the most critical cells in the immune system of Pelecypoda, any decrease in the hemocyte count will have a detrimental impact on the immune system activities. This is the first study of its kind study to investigate and report atrazine as a potential compound, which can induce immunotoxicity in Pelecypoda. The fact that the two studied species of Perna viridis and Paphia malabarica, are both commercially and ecologically important, their selection adds to the study’s significance.
Atrazine, Pelecypoda, Immunotoxicity, In vivo, Tryphan Blue Exclusion Assay.
AN M. Z, Navalgund MA, Manawadi SM, Sanjotha G, Khan M. S. In vivo Immunotoxicity Assessment of Atrazine in two Economically- Important Marine Pelecypoda Species. Biosc.Biotech.Res.Comm. 2022;15(2).
AN M. Z, Navalgund MA, Manawadi SM, Sanjotha G, Khan M. S. In vivo Immunotoxicity Assessment of Atrazine in two Economically-
Important Marine Pelecypoda Species. Biosc.Biotech.Res.Comm. 2022;15(2). Available from: <a href=”https://bit.ly/3z2mfxz“>https://bit.ly/3z2mfxz</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Agricultural chemicals such as pesticides and weedicides contaminate surface water locally but have a tremendous potential to impact at global scale by either entering into the food chain or by bioaccumulation in the soft tissues (Shomar, Muller and Yahya 2006). Pesticide and herbicide toxicity to non-target organisms, particularly aquatic species, has been widely documented in scientific studies (LeBlanc, Bain and Wilson 1997; María et al. 2007; Palma et al. 2008; Flynn and Spellman 2009; Nwani et al. 2010; Tongo and Ezemonye 2015; Ullah, Hasan and Dhama 2015; Westlund et al. 2018; Klementova et al. 2019). Pesticides have long been the focus of toxicological research, while weedicides have gotten less attention. Weeds drastically reduce agriculture produce by tapping available resources, thus deprive crops of indispensable nutrients and vital minerals. Farmers use variety of weedicides to manage this menace but Atrazine (ATZ) is a favourite choice due to its efficacy and ease of application (Opute et al. 2021).
Atrazine is a generic weedicide, which controls weeds by interfering with the protein biomolecules involved in the photosynthetic machinery of plants (Trebst 2008). Atrazine is widely used in paddy fields, other cereal crops, Sugarcane and most of the horticulture-based plants since from almost sixty years (Muller 2008). Regrettably, this weedicide is always used in excess quantity leads to the advent of weedicide tolerant species. Efficacy of the atrazine increases if used in right quantities (Muller 2008; Heap 2014). International survey of herbicide-resistance weed data base has reported development of ATZ related weed-resistance in more than sixty species (Short and Colborn 1999).
ATZ is predominant contaminant of surface runoff due to its extensive use, and its ability to linger in the milieu for a considerably longer duration (Koskinen and Clay 1997; Opute et al. 2021). Toxicological studies carried out in vertebrate species establish it as toxic to the reproductive, endocrine, and immunomodulation systems (Cummings, Rhodes and Cooper 2000; Stoker et al. 2002; Laws et al. 2003; Whale et al. 2003; Filipov et al. 2005; Opute et al. 2021). Established facts from erstwhile studies, paves way for further studies in other important animal models to understand the hazardous effects of the ATZ. Given the widespread usage of ATZ, more research is particularly essential to corroborate its risks to susceptible nontarget organisms (Opute et al. 2021).
Rampant irrational use of this weedicide has the potential to result in the adverse and precarious effects on almost all ecosystems in general, aquatic ecosystems in particular (Flynn and Spellman 2009). Toxic effects of ATZ have already been reported on several species of fishes, amphibians, and reptiles (Solomon et al. 1996; Glen et al. 2014). Bulk of these studies have focused on physiological and biochemical aspects (Ralston et al. 2009; Nwani et al. 2010; Ullah, Hasan and Dhama 2015).
Some studies have reported the influence of ATZ on the aggregation behaviour in freshwater mussels (Flynn and Spellman 2009). Nonetheless marine species are more susceptible to the harmful manifestation of ATZ, which necessitates the need for further research on these species. A special attention is needed on the pelagic Pelecypoda due to their vulnerable habitat that exposes to the high levels of environmental contaminants (Opute et al. 2021).
Given the widespread consumption of mussels, oysters, and clams as food, it is imperative that safety standards be established. Baring seldom reports on the genotoxicity of heavy metals, the risk assessment of environmental contaminants in Pelecypoda is not as exhaustive as it should have been (Prakash and Rao 1995; Sokolova 2004). Other vital aspects such as physiology, particularly those related to immunity, have never been studied. In the present investigation we have evaluated the immunotoxic manifestations of ATZ on two commercially important edible marine pelecypods (Opute et al. 2021). Pelecypoda like any other invertebrate species, solely rely upon innate immunity to defend themselves from pathogens. Haemocytes play a critical role to confer immunity against pathogens to these marine species. (Canesi and Pruzzo 2016; Burgos-Aceves and Faggio 2017; Destoumieux-Garzon et al. 2020; Opute et al. 2021).
Erstwhile studies have provided comprehensive information on the molluscan hemocyte morphology and classification (Cheng 1984). Apart from conferring innate immunity, Haemocytes are responsible for wide range of functions (Cheng 1977; Opute et al. 2021). In this study, we are evaluating the toxic effects of ATZ on the Haemocytes of two commercially important pelecypods viz. Perna viridis (Order: Mytilidae) and Paphia malabarica (Order: Venerida) by Exclusion Assay (Strober 2015). This procedure is simple yet tremendously precise and unswerving to determine the viable hemocyte count. Viable / nonviable Haemocytes can be identified simply by observing clear/blue cytoplasm.
MATERIAL AND METHODS
Atrazine (ATZ) sold under the trade name TATA-Atrataf, was procured from the local dealer of pesticides and weedicides. It was a white amorphous powder, which was soluble in water (7 mg mL-1).
Perna viridis (Order: Mytilidae) were collected from the Kali River estuary and carefully transported them to the laboratory in a container filled with marine water. Green mussels were acclimatized for five days by feeding ad libitum with phytoplankton, Chaetocerous sp. (Iqbal, Khan and Goswami 2008). Paphia malabarica (Order: Veneroida) were excavated through sediments at Kali River estuary and carefully transported to laboratory and acclimatized as described for green mussels.
The ninety-six (96) hrs LC50 calculations were done along with the preparation of sub-lethal doses of Perna viridis and Paphia malabarica. LC50 of TATA-Atrataf (Commercial brand of ATZ) for green mussel was determined according to established procedures (Islam et al. 2019). Perna viridis were treated with 0 mg/L (Control), 2 mg/L, 4 mg/L, 6 mg/L, 8 mg/L, 10 mg/L, and 12 mg/L of ATZ each in 3 replicates. The treated water was replaced with fresh ones after every 24 hours.
Inspection of the experimental setup was done after every six hours to remove the dead animals from experimental containers. Mortality was carefully recorded at 24 hrs., 48 hrs., 72 hrs. and 96 hrs. The data was statistically analyzed by using ANOVA (one way) to determine the differences in mortality between control and experimental groups. Furthermore, the data was also analyzed by the Dunnett T test to compare the control and experimental groups. Based on the mortality, LC50 with 95 % confidence Interval was determined by PROBIT regression analysis. All statistical tests were performed by using IBM SPSS version 24.0
Total of 50 (Fifty) Perna viridis were treated with sublethal doses of 0.00 mg/L (Control), 0.25 mg/L (1/25th of LC50), 0.50 mg/L (1/12th), 0.75 mg/L (1/7th), 1.00 mg/L (1/6th) and 1.25 mg/L (1/5th) atrazine in 25 liters of esturian water, each with three replicates. The experiment was carried out for fourteen (14) days with regular change of water containing sublethal doses after every 24 hours. One hundred short neck yellow clams were exposed to sub lethal doses of 0.00 mg/L (Control), 0.20 mg/L, 0.40 mg/L, 0.60 mg/L, 0.80 mg/L and 1.00 mg/L atrazine in 30 liters of estuarine water, each experimental setup was carried out in three replicates. The experiment was carried out for fourteen (14) days with regular change of water containing sublethal doses after every 24 hours.
Extraction of the hemolymph of the green mussels and clams was done with the help of 5 cc syringe (25-gauge needle) from posterior adductor muscles on 2, 4, 6, 8, 10, 12 and 14 day from control (0.00 mg/L) and five experimental groups (0.25 mg/L, 0.50 mg/L, 0.75 mg/L, 1.00 mg/L and 1.25 mg/L). Exclusion Assay (Tryphan Blue) was done as per the instruction given by the manufacturers instruction (Sigma). Viable Haemocytes with clear cytoplasm and nonviable Haemocytes with blue colored cytoplasm were recorded and tabulated separately. More than two thousand each of viable and nonviable Haemocytes were screened on the 2nd day of the experiment from each of the control and five experimental groups of Perna viridis and Paphia malabarica. Methodology was repeated on 4th, 6th, 8th, 10th, 12th and 14th day of the experiment.
Following calculations were done by using the formulas provided by the manufacturer (Sigma)
Haemocytes mL-1 = Avg count per square × 5 × 10000
Total count = Haemocytes/mL × 10 mL
% VHC=Unstained Haemocytes with clear cytoplasm / Total count×100
% NVHC= Haemocytes with blue colored cytoplasm / Total cells ×100
Percentage of viable and nonviable haemocytes were analyzed statistically to determine the LC50 with 95 % CI by using regression test by using IBM SPSS version 24.0.
RESULTS AND DISCUSSION
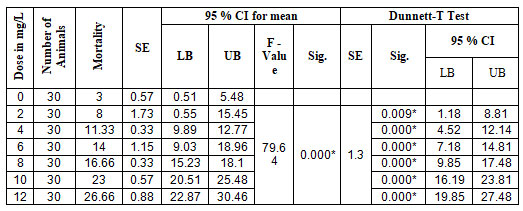
Perna viridis: Acute toxicity test: The ability of ATZ to induce cytotoxicity or immunotoxicity was never been investigated on any pelecypoda. This reports for the first time the acute toxicity of ATZ in two commercially as well as ecologically important edible species of pelecypoda. In 5 experimental groups and a control group, mortality of the mussels was recorded at 24 hrs., 48 hrs., 72 hrs., and 96 hrs. The results of the one-way ANOVA revealed a significant difference in mortality rate between the experimental and control groups, with F=79.64 (P<0.0001), and further analysis of multiple comparisons by using the Dunnett-T test revealed a significant difference (P<0.0001) between the experimental and control groups. The Lowest Observed Effective Concentration (LOEC) was recorded as 2.00 mg/L (P<0.009) with 95 % CI (1.18, 8.81). The results of the one-way ANOVA and the Dunnett-T test are presented in Table 1.
Table 1. ANOVA (One Way) and Dunnet-T test for Acute toxicity in Perna viriids.
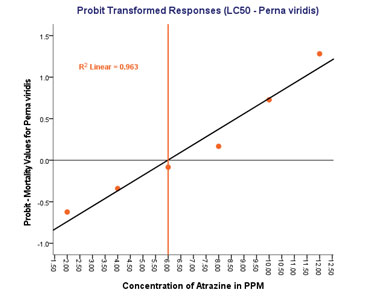
The results of the regression determined LC50 as 6.10 mg/L. Parameter estimates of analysis presented in Table 2.
Table 2. Parameter estimates of analysis in Perna viridis
| Parameter Estimates | |||||||
|
|
Parameter | Estimate | SE | Z | Sig. | 95% CI | |
| LB | UB | ||||||
| a | Concentration | .214 | .028 | 7.733 | .000 | .160 | .269 |
| Intercept | -1.372 | .198 | -6.928 | .000 | -1.570 | -1.174 | |
| a. model: (p) = Intercept + BX | |||||||
Dose- response curve (R2 linear = 0.963) shows that, as the dose and duration of the experimental groups increases, there is a concomitant increase in the rate of mortality (Figure 1).
Figure 1: LC50 determination in Perna viridis
Viable Haemocyte Count Perna viridis: Acute toxicity test was done over 14 days to evaluate the % of viable haemocytes and nonviable haemocytes. Viability and non-viability were determined by simply observing the staining of the cytoplasm, viable haemocytes have clear cytoplasm without any staining whereas nonviable haemocytes have cytoplasm, which is stained as blue. The average count of viable haemocytes (VHC), nonviable haemocytes (NVHC), total haemocytes (TC) and %viability is presented in Table 3.
Table 3. Haemocytes count and Percentage of viability in Perna viridis (× 107 Haemocytes/mL)
| Duration of the Treatment | |||||||||
| 2 days | 4 days | 6 days | 8 days | 10 days | 12 days | 14 days | |||
| Atrazine treatment | 1.25 mg/L | VHC | 1.68 | 1.63 | 1.59 | 1.57 | 1.54 | 1.51 | 1.42 |
| NVHC | 0.40 | 0.40 | 0.40 | 0.40 | 0.42 | 0.44 | 0.49 | ||
| THC | 2.08 | 2.04 | 1.99 | 1.97 | 1.96 | 1.95 | 1.91 | ||
| % | 80.86 | 80.16 | 79.82 | 79.8 | 78.75 | 77.3 | 74.51 | ||
| 1.00 mg/L | VHC | 1.81 | 1.78 | 1.73 | 1.67 | 1.65 | 1.60 | 1.58 | |
| NVHC | 0.31 | 0.33 | 0.34 | 0.35 | 0.38 | 0.39 | 0.40 | ||
| THC | 2.12 | 2.10 | 2.07 | 2.02 | 2.03 | 2.00 | 2.00 | ||
| % | 85.55 | 84.41 | 83.4 | 82.51 | 81.1 | 80.27 | 79.76 | ||
| 0.75 mg/L | VHC | 1.94 | 1.93 | 1.85 | 1.76 | 1.72 | 1.68 | 1.72 | |
| NVHC | 0.28 | 0.29 | 0.32 | 0.35 | 0.36 | 0.37 | 0.40 | ||
| THC | 2.22 | 2.22 | 2.18 | 2.10 | 2.08 | 2.06 | 2.12 | ||
| % | 86.76 | 87.48 | 85.2 | 83.41 | 82.66 | 81.82 | 81.1 | ||
| 0.50 mg/L | VHC | 1.92 | 1.94 | 1.89 | 1.84 | 1.79 | 1.77 | 1.77 | |
| NVHC | 0.27 | 0.23 | 0.28 | 0.30 | 0.32 | 0.33 | 0.33 | ||
| THC | 2.19 | 2.17 | 2.16 | 2.14 | 2.11 | 2.10 | 2.10 | ||
| % | 87.6 | 89.39 | 87.22 | 86.17 | 85.01 | 84.35 | 84.47 | ||
| 0.25 mg/L | VHC | 1.96 | 2.05 | 2.02 | 1.99 | 1.94 | 1.91 | 1.85 | |
| NVHC | 0.20 | 0.19 | 0.20 | 0.22 | 0.24 | 0.28 | 0.32 | ||
| THC | 2.17 | 2.24 | 2.22 | 2.22 | 2.19 | 2.20 | 2.17 | ||
| % | 90.5 | 91.53 | 90.89 | 89.9 | 89.13 | 87.07 | 85.31 | ||
| 0,00 mg/L (Control) | VHC | 2.03 | 1.98 | 1.98 | 1.96 | 1.94 | 1.96 | 1.95 | |
| NVHC | 0.12 | 0.13 | 0.14 | 0.13 | 0.14 | 0.16 | 0.16 | ||
| THC | 2.16 | 2.11 | 2.12 | 2.09 | 2.08 | 2.12 | 2.11 | ||
| % | 94.34 | 93.66 | 93.21 | 93.69 | 93.07 | 92.47 | 92.34 | ||
The counting of VHC and NVHC were done on 2nd, 4th, 6th, 8th, 10th, 12th and 14th day for the experimental and control groups, %of viability was calculated and presented in Figure 3A and 3B.
Figure 2: LC50 determination in Paphia malabarica
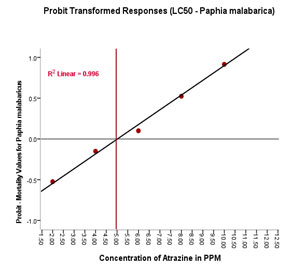
Figure 3 A: % Viable Haemocytes in Perna viridis
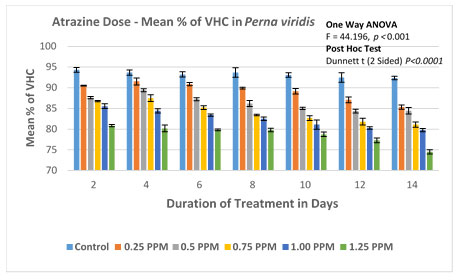
Figure 3B: Atrazine dose – VHC response curve in Perna viridis
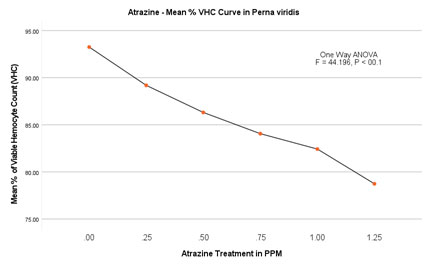
The % of viable haemocytes in the control group was found to be between 94.34 (2.034/mL×107) on the 2nd day to 92.34 (1.952/mL×107) on the 14th day. No significant difference in the rate of mortality was evident within the control group with no ATZ treatment. In experimental groups of lowest observed effective concentration (LOEC) is found to be 0.25 mg/L. The number of viable haemocytes decreased steadily from 90.5 (1.96/mL×107) on the 2nd day to 85.31 (1.85/mL×107) on the 14th day. In the second experimental group with 0.50 mg/L of atrazine, the percentage of viable cells decreased from 87.6 (1.92/mL×107) to 84.47 (1.72/mL×107) on day 2 and day14 respectively.The trend of decrease in % viable haemocytes has continued with the concomitant increase in the doses of 0.75 and 1.00 mg/L. The experimental group with the highest treatment of 1.25 mg/L, % viable haemocytes was recorded as 80.86 (1.68/mL×107) on the 2nd day and further decreased to reach a minimum of 74.51 (1.42×107 ml-1) on 14th day.
ANOVA (One Way) was applied to determine the significance level between the viable haemocyte Count in the Control group and in the experimental groups. This test confirms the significant difference in the viability of haemocytes with P<0.001 (F=28.566). Furthermore, multiple comparisons were performed by the Dunnett-T test, which was found to be significant with P<0.0003 between the groups with 95 % CI.
Nonviable Haemocyte count (NVHC) in Perna viridis: In order to overcome the errors associated with this assay technique, we have also examined the % nonviable haemocytes. The result of this counting of the dark blue-coloured cells clearly shows a drastic increase in the % nonviable haemocytes in Perna viridis. In the control group percentage of nonviable haemocytes on the 2nd day of the experiment was found to be 5.781 (0.122/mL×107) and on the last day of the experiment i.e., on the 14th day 7.66 (0.160/mL×107). The result of this experiment clearly shows no significant change in the % nonviable hemocytes. However, in the case of experimental groups steady increase in the % nonviable haemocytes was clearly evident (Figure 4A and 4B).
Figure 4A: % Nonviable Haemocytes in Perna viridis
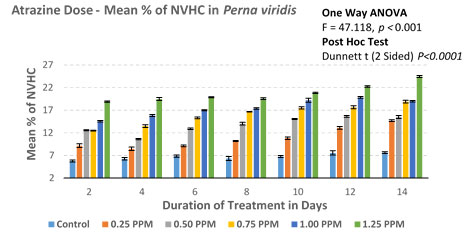
Figure 4B: Atrazine dose – NVHC response curve in Perna viridis
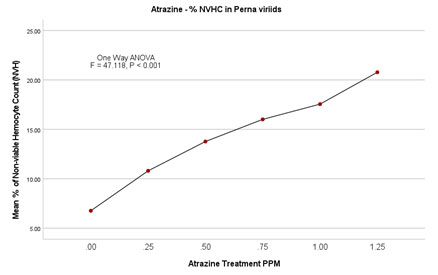
In the case of the lowest observed effective concentration (LOEC) of 0.25 mg/L on the 2nd day, the % nonviable haemocytes were 9.18 (0.206/mL×107) and on the 14th day % went up to 14.68 (0.318 × 107). Similarly, there was an incremental increase in % nonviability in experimental groups of 0.50mg/L, 0.75 mg/L, and 1.00 mg/L. Finally experimental group with highest treatment of 1.25mg/L % nonviability recorded as 18.91 (0.398/ml×107) on 2nd day itself and 24.47 (0.486/ml×107). ANOVA (One Way) test was repeated by using IBM SPSS version 24.0, which showed a significant difference between the groups with P<0.001 (F = 47.118). Further, in the multiple comparisons by using the Dunnett-T test, the mean difference is significant for all the experimental groups when individually compared with the control group (P < 0.001) with 95 % CI.
Paphia malabarica: Acute toxicity studies: In this paper, we reported the LC50 of atrazine and the lowest observed concentration (LOEC) by conducting 96 hrs. of exposure. All experiments were carried out in triplicates. The mortality rate was recorded after 24 hrs., 48 hrs., 72 hrs., and 96 hrs. Results of ANOVA (one way) showed a significant difference in the rate of mortality between control and experimental groups with P < 0.0001 (F = 138.6), and Dunnett-T test results of multiple comparisons also show a significant difference between the experimental groups and control group with P<0.0001. The LOEC in this species was also found to be 2.00mg/L (P< 0.004) with 95 % CI (2.24, 11.76). The results are written in Table. 4.
Table 4. ANOVA (One Way) and Dunnet-T test for Acute toxicity in Paphia malabarica
| Dose in MG/L | Number of Animals | Mean Mortality | SE | 95 % CI for mean | Dunnett T Test | ||||||
| LB | UB | F – Value | Sig. | SE | Sig. | 95 % CI | |||||
| LB | UB | ||||||||||
| 0 | 30 | 8.66 | 0.33 | 7.23 | 10.1 | ||||||
| 2 | 30 | 15.66 | 0.66 | 12.8 | 18.53 | 138.6 | 0.000* | 1.6 | 0.004* | 2.24 | 11.76 |
| 4 | 30 | 21.66 | 0.33 | 20.23 | 23.1 | 0.000* | 8.24 | 17.76 | |||
| 6 | 30 | 27 | 0.57 | 25.51 | 30.48 | 0.000* | 14.58 | 24.08 | |||
| 8 | 30 | 34.66 | 2.6 | 23.46 | 45.87 | 0.000* | 21.24 | 30.76 | |||
| 10 | 30 | 41.66 | 0.33 | 40.23 | 43.1 | 0.000* | 28.24 | 37.76 | |||
| 12 | 30 | 45.66 | 1.2 | 40.49 | 50.83 | 0.000* | 32.24 | 41.76 | |||
The regression test LC50 as 4.90mg/L. Parameter estimates for analysis and Dose- response curve
(R2 linear=0.996) is depicted in Table 5 and Figure 2.
Table 5. Parameter estimates of analysis in Paphia malabarica
| Parameter Estimates | |||||||
| Parameter | Estimate | Std. Error | Z | Sig. | 95% Confidence Interval | ||
| Lower Bound | Upper Bound | ||||||
| a | Concentration | .180 | .024 | 7.337 | .000 | .132 | .228 |
| Intercept | -.904 | .177 | -5.095 | .000 | -1.081 | -.726 | |
| a. model: (p) = Intercept + BX | |||||||
Viable Haemocyte Count (VHC) in Paphia malabarica: The average number of viable haemocytes, nonviable haemocytes, total haemocytes and % viability is shown in Table 6.
Table 6. Haemocytes count and Percentage of viability in Paphia malabarica (×107 Haemocytes/mL)
| Duration of the Treatment | |||||||||||||
| 2 Days | 4 Days | 6 Days | 8 Days | 10 Days | 12 Days | 14 Days | |||||||
| Atrazine treatment | 1.0 mg/L | VHC | 1.35 | 1.34 | 1.31 | 1.31 | 1.29 | 1.26 | 1.23 | ||||
| NVHC | 0.23 | 0.25 | 0.27 | 0.29 | 0.31 | 0.31 | 0.34 | ||||||
| THC | 1.58 | 1.58 | 1.59 | 1.6 | 1.59 | 1.58 | 1.56 | ||||||
| % | 85.68 | 84.46 | 82.79 | 82.11 | 80.82 | 80.08 | 78.39 | ||||||
| 0.8 mg/L | VHC | 1.31 | 1.32 | 1.31 | 1.30 | 1.30 | 1.31 | 1.29 | |||||
| NVHC | 0.22 | 0.24 | 0.26 | 0.27 | 0.28 | 0.28 | 0.31 | ||||||
| THC | 1.54 | 1.56 | 1.56 | 1.57 | 1.59 | 1.59 | 1.59 | ||||||
| % | 85.43 | 84.86 | 83.50 | 82.89 | 82.23 | 82.26 | 80.67 | ||||||
| 0.6 mg/L | VHC | 1.40 | 1.37 | 1.34 | 1.35 | 1.31 | 1.30 | 1.26 | |||||
| NVHC | 0.19 | 0.20 | 0.23 | 0.26 | 0.27 | 0.29 | 0.30 | ||||||
| THC | 1.59 | 1.58 | 1.57 | 1.61 | 1.57 | 1.59 | 1.56 | ||||||
| % | 88.03 | 87.05 | 85.09 | 84.07 | 83.11 | 81.85 | 80.94 | ||||||
| 0.4 mg/L | VHC | 1.43 | 1.41 | 1.36 | 1.35 | 1.34 | 1.33 | 1.33 | |||||
| NVHC | 0.17 | 0.19 | 0.22 | 0.24 | 0.25 | 0.25 | 0.26 | ||||||
| THC | 1.60 | 1.60 | 1.58 | 1.59 | 1.54 | 1.58 | 1.58 | ||||||
| % | 89.49 | 87.97 | 85.94 | 84.78 | 87.27 | 84.07 | 83.71 | ||||||
| 0.2 mg/L | VHC | 1.52 | 1.46 | 1.45 | 1.42 | 1.38 | 1.37 | 1.36 | |||||
| NVHC | 0.16 | 0.17 | 0.19 | 0.19 | 0.21 | 0.23 | 0.23 | ||||||
| THC | 1.67 | 1.62 | 1.64 | 1.61 | 1.59 | 1.60 | 1.60 | ||||||
| % | 90.57 | 89.77 | 88.50 | 88.19 | 87.01 | 85.76 | 85.45 | ||||||
| 0,00 mg/L (Control) | VHC | 1.54 | 1.55 | 1.53 | 1.5 | 1.56 | 1.59 | 1.59 | |||||
| NVHC | 0.08 | 0.08 | 0.09 | 0.10 | 0.11 | 0.10 | 0.10 | ||||||
| THC | 1.61 | 1.63 | 1.63 | 1.65 | 1.67 | 1.69 | 1.68 | ||||||
| % | 95.29 | 94.85 | 94.22 | 93.83 | 93.31 | 94.07 | 94.20 | ||||||
Based on the number of viable haemocytes, the % viability was calculated and depicted in Figure 5B. The mean % viability in the control group without ATZ was found to be 95.29 (1.54/mL×107) on the 2nd day whereas, on the last day of the experiment that is on the 14th day, the % viable haemocytes were calculated to be 94.19 (1.59/mL×107). No significant difference was found in this group over 14 days. Among the experimental groups, LOEC was found to be 0.20mg/L. From this experiment, it is evident that the total number of viable haemocytes has steadily decreased from 90.56 (1.52/mL×107) on the 2nd day to 84.69 (1.36/mL×107) on the last day (14th day) of the experiment. The trend of decrease continued with the concomitant increase in the doses (0.40mg/L, 0.60 mg/L and 0.80mg/L). Finally, at peak treatment of 1.00mg/L, % viable haemocytes were calculated as 85.69 (1.35/mL×107) on the 2nd day and reached a minimum of 78.39 (1.23/mL×107) on the 14th day (Figure 5A and 5B).
Figure 5A: % Viable Haemocytes in Paphia malabarica
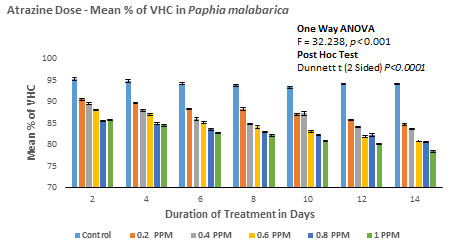
Figure 5B: Atrazine dose – VHC response curve in Paphia malabarica
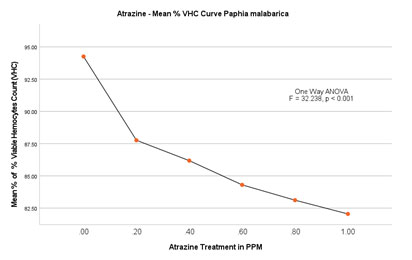
ANOVA (One-Way) test showed significant difference in % viability between the groups with P<0.001 (F = 32.238) but no significant difference within the groups.
Nonviable haemocyte count (NVHC) in Paphia malabarica: In the control group without ATZ, % nonviable haemocytes on the 2nd day of the experiment were calculated as 5.781 (0.122/mL×107), and on the last day of the experiment (14th day) there is a marginal increase in the percentage of haemocytes to about 7.66 (0.160/mL×107). In experimental groups, on the 2nd day of the experiment at 0.20mg/L, % of the nonviable haemocytes was found to be 9.43 (0.16/mL×107) and on the 14th day of the experiment, % of nonviable haemocytes increased to 14.55 (0.23/mL×107). As the dose and the duration of treatment increased, there was a progressive increase in % nonviability in experimental groups of 0.40 mg/L, 0.60 mg/L, and 0.80 mg/L. In the experimental group with the highest exposure of 1.00 mg/L, % nonviability was recorded as high as 14.30 (0.23/mL×107) on the 2nd day itself and a remarkable increase of 21.6 % (0.34mL×107).
ANOVA (One Way) test was applied by using IBM SPSS version 24.0, the results of this test clearly show a significant difference between the groups with P<0.001 (F=34.08). The results are represented in Figure 6A and 6B. Further, results of the Dunnett-T test also show significant increase in the % nonviable haemocytes with P<0.001 with 95 % CI.
Figure 6A: % Nonviable Haemocytes in Paphia malabarica
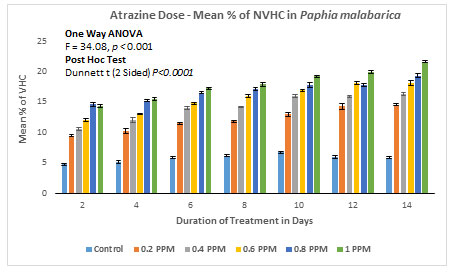
Figure 6B: Atrazine dose – NVHC response curve in Paphia malabarica
Humanity is facing several challenges and one of the most pressing issues of the present time is environmental contaminants. This is not only adversely affecting human health but also deteriorating the quality of each and every ecosystem. Thousands of chemicals are being synthesized on daily basis, which at one point in time gain access to the environment. Most of these chemicals enter the environment without being evaluated for their risk. some of them are subjected to toxicity related studies, others are ignored or insufficiently studied. Perhaps there is no match between new pesticides and weedicides being introduced into the environment and the assessment of their risk.
Scientific reports published focused on the toxicological studies done on laboratory animals such as mice, rats, rabbits and fishes. However, from the perspective of the method of application of some of these chemicals specifically, pesticides and weedicides were taken into consideration aquatic animals are much more vulnerable. Slower degradation of these chemicals leads to long term persistence of these chemicals in surface water, which poses greater challenges to the aquatic animals. Amongst aquatic animals, Pelecypoda species are more likely to be affected by pollutants and environmental contaminants (Opute et al. 2021).
ATZ induced toxicity is reported in several studies already published on fishes, amphibians and mammals. The majority of the studies have reported deleterious effects of ATZ on the aspects like reproduction, endocrinology, developmental biology and the immune system. Some of the toxic manifestations reported on mice models are reproductive organ anomalies, failure of implantations, termination of gestations and delayed onset of puberty (Cummings, Rhodes and Cooper 2000; Stoker et al. 2002; Laws et al. 2003). Some reports implicate ATZ with Immunotoxicity in mice and other mammals. The ATZ is also known to significantly decrease the hematopoietic progenitor cells, reticulocytes and CD4+ lymphocytes in mice and rats (Pistl et al. 2002; Laws et al. 2003; Filipov et al. 2005). Few reports suggest the immunomodulatory functions by significantly increasing the natural killer cells, T cell proliferation, IgM secreting plasma cells, macrophage-mediated phagocytic and cytolytic functions (Filipov et al. 2005; Rowe et al. 2006; Opute et al. 2021).
In fishes, ATZ is reported to have toxic effects on the secondary lymphoid organs in salmonid fishes like Coho salmon (Oncorhynchus kisutch) and lake trout (Salvelinus namaycush) (Zeeman and Brindley 1981). Some reports even suggest ATZ is a potential chemical, which can trigger leucopenia and disintegration of macrophages in fishes (Zeeman and Brindley 1981). Besides immunotoxicity, the ATZ is also known to have endocrine toxicity in catfishes (Opute et al. 2021). In studies done on the amphibians, the ATZ is reported to have an ability to decrease the number of spleenocytes and leucocytes in R. pipines, R. sylvatica and X. laevis (Kiesecker 2002; Christin et al. 2013; Opute et al. 2021). Apart from the aforementioned literature, no other studies have been done to assess the immunotoxic potential of ATZ in invertebrates. Nevertheless, immunotoxicity of other environmental contaminants such as pesticides, heavy metals, and PCBs have been investigated in a few invertebrate species (Ellis et al. 2011; Renault 2015; Destoumieux et al. 2020; Teresa et al. 2021).
Even molecular mechanisms involved have also been discussed in a few species (Gerdol and Venier 2015; Bachere et al. 2015; Burgos-Aceves and Faggio 2017; Teresa et al. 2021). In the present study, we have evaluated the acute toxicity of ATZ on Perna viridis (Order: Mytilida) and Paphia malabarica (Order: Venerida), and we have also examined the effect of sub-lethal doses of ATZ on the viability of haemocytes. The results of this study indicate that the ATZ significantly decreases the number of haemocytes in Pelecypoda. This study relies on an exclusion assay – a simple, economical and reliable assay to identify viable and nonviable haemocytes (Piccinini et al. 2017). This assay requires minimum time and is very convenient for the assessment of a large number of samples in a short period. Analysis by one way ANOVA showed a significant decrease in the viable cells in experimental groups when compared to the control group (P < 0.0001) in both Pelecypoda species studied (Teresa et al. 2021).
In P. viridis, in the control group, the % viability was found to be 94.34 on the 2nd day and 92.34 on the 14th day. In the case of the experimental group at the highest concentration (1.25mg/L) the % viability was recorded as 80.86 on 2nd day and further decreased to 74.51 by 14th day of ATZ treatment. Correspondingly there was an increase in the number of nonviable haemocytes. In the control group the % of nonviability was recorded to be 5.78 on the 2nd day which marginally increased to 7.66 on the 14th day but in the experimental group with the highest concentration of 1.25mg/L, % nonviability was recorded to be 18.91 on day 2 but drastically increased to 24.47 on 14th day. Similarly, in P. malabarica, similar results have been recorded.
CONCLUSION
The findings of the present investigation show the immunotoxic potential of ATZ by decreasing the number of viable haemocytes. Analysis by one way ANOVA showed a significant decrease in the viable cells in experimental groups when compared to the control group (P < 0.0001) in both Pelecypoda species studied. In P. viridis, in the control group, the % viability was found to be 94.34 on the 2nd day and 92.34 on the 14th day. In the case of the experimental group at the highest concentration (1.25mg/L), the % viability was recorded as 80.86 on the 2nd day and further decreased to 74.51 by the 14th day of ATZ treatment. Correspondingly there is an increase in the number of nonviable haemocytes. In the control group the % of nonviability was recorded to be 5.78 on the 2nd day which marginally increased to 7.66 on the 14th day but in the experimental group with the highest concentration of 1.25mg/L, % nonviability was recorded to be 18.91 on day 2 but drastically increased to 24.47 on 14th day. Similarly, in P. malabarica, similar results have been recorded.
ACKNOWLEDGEMENTS
The financial aid for this study has been provided by the University Grants Commission (UGC), New Delhi under the order no. MRP(S)-757/10-11/KAKA088/UGC-SWRO.
Conflict of Interests: Authors declare no conflict of interests to disclose.
REFERENCES
Bachere E, Rosa RD, Schmitt P, et al. (2015). The new insights into the oyster antimicrobial defense: cellular, molecular and genetic view. Fish Shellfish Immunol. (2015) 46:50–64
Burgos-Aceves MA, and Faggio C (2017). An approach to the study of the immunity functions of bivalve haemocytes: Physiology and molecular aspects. Fish Shellfish Immunol. 67:513–7.
Canesi L and Pruzzo C (2016). Specificity of innate immunity in Pelecypoda: a lesson from bacteria. In: Ballarin L, Cammarata M, editors. Lessons in Immunity: From Single-Cell Organisms to Mammals. London: Academic Press. p. 79–91.
Cheng TC (1984). A Classification of Molluscan Haemocytes Based on Functional Evidences. In: Cheng T.C. (eds) Invertebrate Blood. Comparative Pathobiology, vol 6. Springer, Boston, MA.
Cheng TC (1977). Biochemical and ultrastructural evidence for the double role of phagocytosis in molluscs: defense and nutrition. Comp. Pathobiol., 3, 21–30. MA.
Christin MS, Ménard L, Giroux I, et al. (2013). Effects of agricultural pesticides on the health of Rana pipiens frogs sampled from the field. Environ Sci Pollut Res. 20, 601–611.
Cummings AM, Rhodes BE and Cooper RL (2000). Effect of atrazine on implantation and early pregnancy in 4 strains of rats. Toxicol. Sci.58:135–143.
Destoumieux GD, Canesi L, Oyanedel D, et al. (2020), Vibrio–bivalve interactions in health and disease. Environ Microbiol, 22: 4323-4341.
Ellis RP, Parry H, Spicer JI, et al. (2011). Immunological function in marine invertebrates: Responses to environmental perturbation. Fish Shellfish Immunol. 30:1209–22.
Filipov NM, Pinchuk LM, Boyd BL, et al. (2005). Immunotoxic effects of short-term atrazine exposure in young male C57BL/6 mice. Toxicol. Sci. 86:324–332.
Flynn K and Spellman T (2009). Environmental levels of atrazine decrease spatial aggregation in the fresh water mussel, Elliptio complanata. Ecotoxicol Environ Saf. 72: 1228–1233.
Gerdol M and Venier P (2015). An updated molecular basis for mussel immunity, Fish and Shellfish Immunology, Volume 46, Issue 1, Pages 17-38.
Kraak GJVD, Hosmer AJ, Hanson ML et al. (2014). Effects of Atrazine in Fish, Amphibians, and Reptiles: An Analysis Based on Quantitative Weight of Evidence, Critical Reviews in Toxicology, 44:sup5, 1-66.
Heap I (2014). Global perspective of herbicide-resistant weeds. Pest Manag Sci. 70(9):1306-15.
Islam SMM, Rahman MA, Nahar S, et al. (2019). Acute toxicity of an organophosphate insecticide sumithion to striped catfish Pangasianodon hypophthalmus. Toxicol Rep., 6:957-962.
Kiesecker JM (2002). Synergism between trematode infection and pesticide exposure: A link to amphibian limb deformities in nature? Proc. Natl. Acad. Sci. USA 99:9900–9904.
Klementova S, Hornychová L, Šorf M, et al. (2019). Toxicity of atrazine and the products of its homogeneous photocatalytic degradation on the aquatic organisms Lemna minor and Daphnia magna. Environmental Science and Pollution Research, 26, 27259.
Koskinen WC and Clay SA. (1997). Factors affecting atrazine fate in north central U.S. soils. Rev Environ Contam Toxicol., 151:117-65.
Laws SC, Ferrell JM, Stoker TE, et al. (2003). Pubertal development in female Wistar rats following exposure to propazine and atrazine biotransformation by‐products, diamino‐S‐chlorotriazine and hydroxyatrazine. Toxicol. Sci.76:190–200.
LeBlanc GA, Bain LJ and Wilson VS (1997). Pesticides: multiple mechanisms of demasculinization. Moll Cell Endocrinol. 126: 1–5.
Calderón-Segura ME, Gómez-Arroyo S, Molina-Alvarez B, et al. (2007). Metabolic activation of herbicide products by Vicia faba detected in human peripheral lymphocytes using alkaline single cell gel electrophoresis, Toxicology in vitro, Volume 21, Issue 6, Pages 1143-1154.
Iqbal ANMI, Khan MS and Goswami U (2008). Cytogenetic studies in green mussel, Perna viridis (Mytiloida: Pteriomorphia), from West Coast of India. Mar. Biol. (Berl.), 153(5): 987–993.
Muller G (2008). Chapter 2 – History of the Discovery and Development of Triazine Herbicides, Editor(s): Homer M. LeBaron, Janis E. McFarland, Orvin C. Burnside, The Triazine Herbicides, Elsevier, Pages 13-29.
Nwani CD, Lakra WS, Nagpure NS et al. (2010). Toxicity of the Herbicide Atrazine: Effects on Lipid Peroxidation and Activities of Antioxidant Enzymes in the Freshwater Fish Channa Punctatus (Bloch). International Journal of Environmental Research and Public Health 7, 3298.
Opute PA, Oboh IP, Asouzu JE, et al. (2021). Effects of atrazine on the endocrinology and histoarchitecture of the testes in African Catfish, Clarias gariepinus (Burchell, 1822), African Journal of Aquatic Science, 46:3, 361-369.
Palma P, Palma VL, Fernandes RM et al. (2008). Acute Toxicity of Atrazine, Endosulfan Sulphate and Chlorpyrifos to Vibrio fischeri, Thamnocephalus platyurus and Daphnia magna, Relative to Their Concentrations in Surface Waters from the Alentejo Region of Portugal. Bulletin of Environmental Contamination and Toxicology, 81, 485.
Piccinini F, Tesei A, Arienti C et al. (2017). Cell Counting and Viability Assessment of 2D and 3D Cell Cultures: Expected Reliability of the Trypan Blue Assay. Biol Proced Online 19, 8.
Pistl J, Kovalkovicova N, Legath J et al. (2002). Metabolic activity of sheep peripheral blood phagocytes after exposure to selected pesticides in vitro. Bull. Vet. Inst. Pulawy, 46:247–253.
Prakash NT and Rao KSJ (1995). Modulations in antioxidant enzymes in different tissues of marine bivalve Perna viridis during heavy metal exposure. Mol. Cell. Biochem. 146, 107-113.
Ralston-Hooper K, Hardy J, Hahn L et al. (2009). Acute and chronic toxicity of atrazine and its metabolites deethylatrazine and deisopropylatrazine on aquatic organisms. Ecotoxicology, 18, 899.
Renault T (2015). Immunotoxicological effects of environmental contaminants on marine Pelecypoda. Fish and Shellfish Immunology, Volume 46, Issue 1, Pages 88-93, ISSN 1050-4648.
Rowe AM, Brundage KM, Schafer R et al. (2006). Immunomodulatory effects of maternal atrazine exposure on male Balb/c mice. Toxicol Appl Pharmacol,214(1):69-77.
Shomar BH, Muller G, and Yahya A (2006). Occurrence of Pesticides in Groundwater and Topsoil of the Gaza Strip. Water, Air, and Soil Pollution, 171, 237.
Short P and Colborn T (1999). Pesticide use in the U.S. and policy implications: a focus on herbicides. Toxicol Ind Health. Jan-Mar;15(1-2):240-75.
Sokolova IM (2004). Cadmium effects on mitochondrial function are enhanced by elevated temperatures in a marine poikilotherm, Crassostrea virginica Gmelin (Bivalvia: Ostreidae) Jour. Exp. Biol.207, 2639-2648.
Solomon KR, Baker DB, Richards RP et al. (1996). Ecological risk assessment of atrazine in North American surface waters. Environ. Toxicol. Chem.15,31–76.
Stoker TE, Guidici DL, Laws SC, et al. (2002). The effects of atrazine metabolites on puberty and thyroid function in the male Wistar rat. Toxicol Sci., Jun;67(2):198-206.
Strober W (2015). Trypan Blue Exclusion Test of Cell Viability. Curr Protoc Immunol. 2015, 111:A3.B.1-A3.B.3.
Teresa B, Manon A, Caterina C et al. (2021). Immunological Responses of Marine Pelecypoda to Contaminant Exposure: Contribution of the -Omics Approach. Frontiers in Immunology,12, 2021, 286.
Tongo I and Ezemonye L (2015). Human health risks associated with residual pesticide levels in edible tissues of slaughtered cattle in Benin City, Southern Nigeria. Toxicology Reports, 2, 1117.
Trebst A (2008). Chapter 8 – The Mode of Action of Triazine Herbicides in Plants, Editor(s): Homer M. LeBaron, Janis E. McFarland, Orvin C. Burnside, The Triazine Herbicides, Elsevier, Pages 101-110.
Ullah S, Hasan Z and Dhama K (2015). Toxic Effects of Endosulfan on Behaviour, Protein Contents and Antioxidant Enzyme System in Gills, Brain, Liver and Muscle Tissues of Rohu, Labeo rohita. International Journal of Pharmacology, 12, 1.
Westlund P, Nasuhoglu D, Isazadeh S et al. (2018). Investigation of Acute and Chronic Toxicity Trends of Pesticides Using High-Throughput Bioluminescence Assay Based on the Test Organism Vibrio fischeri. Arch Environ Contam Toxicol 74, 557–567.
Whale MM, Loganathan G, Yamashita N, et al. (2003). Immunomodulation of human natural killer cell cytotoxic function by triazine and carbamate pesticides. Chem. Biol. Interact. 145:311– 319.
Zeeman MG and Brindley WA (1981). Effects of toxic agents upon fish immune systems: A review. In: Sharma R.P., ed. Immunologic Consideration in Toxicology, pp. 1–47. CRC Press, Boca Raton, FL.


