1Department of Microbiology, Sant Tukaram Arts and Science College, Parbhani – 431401, Maharashtra, India
2Department of Microbiology, Yogeshwari Mahavidyalaya, Ambajogai – 431517, Maharashtra, India
Article Publishing History
Received: 10/06/2016
Accepted After Revision: 25/06/2016
Fusarium wilt is one of the drastic diseases of Bt-cotton caused by Fusarium oxysporum f.sp. vasinfectum. In order to find effective measure to control this phytopathogen, 114 rhizospheric isolates were screened for in vitro antifungal potential against Fusarium oxysporum f.sp. vasinfectum using dual culture method. Out of 114 isolates tested, 13 rhizospheric isolates inhibited growth of Fusarium oxysporum f.sp. vasinfectum in vitro. Highest antifungal activity was shown by isolate RLS19 (68.89 %) and followed by RLS52 (62.22 %), RLS53 (60.00%), RLS72 (57.78 %), and RLS101 (57.78 %) in dual culture technique against phytopathogen. Characterization of microbial control mechanism, rhizospheric isolates was evaluated by testing the production of volatile metabolites, diffusible metabolites and siderophore production. Rhizospheric isolates was isolate RLS101 inhibit 50 % of growth followed by RLS 52 (20 %) and RLS79 (10 %) by producing volatile metabolites against phytopathogen others were unable produce volatile metabolites. Diffusible metabolites was produced by rhizospheric isolates RLS19, RLS52 and RLS18 which inhibits maximum growth phytopathogen 57.50 %, 55.00 % and 52.50 % respectively. Qualitative siderophore production was detected by modified CAS agar assay method, 8 rhizospheric isolates were able to produce siderophore and highest activity was shown by RLS18, RLS53 and RLS58.
Bt-Cotton, Fusarium Oxysporum F.Sp. Vasinfectum, Volatile Metabolites, Diffusible Metabolites, Siderophore
Raut L. S, Hamde V. S. in Vitro Antifungal Potential of Rhizospheric Isolates Against Fusarium Oxysporum Causing Fusarium Wilt Of Bt-Cotton. Biosc.Biotech.Res.Comm. 2016;9(2).
Introduction
Bt- cotton is one of the most important cash crops cultivated in India. In north zone of India, Maharashtra is state first rank in cultivation area but trails on third rank for production (current cotton scenario, CCI-2013-14) due to different environmental factors and attack of pests. Fusarium wilt is one of major destructive and yield reducing diseases of Bt-cotton caused by the fungal phytopathogen Fusarium oxysporum f.sp. vasinfectum (Atkinson, 1892). All growth stages of Bt-cotton are susceptible to Fusarium wilt disease and widespread in most cotton-growing countries of the world. This disease was first reported in India (Kulkarni, 1934) and spread through infected soil and seed (Bennett et al., 2008).
Present control strategies to control the Fusarium wilt disease is by using chemical pesticides. Chemical control measures create imbalances in the microbial community, which may be unfavorable to the activity of beneficial organisms and could lead to the development of resistant strains of pathogen and pollution of environment.Since, Fusarium wilt is increasingly destructive in cotton production, and to avoid the hazardous effect of chemicals new ways of alternative controlling the diseases need to be searched. The production of siderophores in rhizosphere fungi was higher than those isolated from the contaminated soil. Siderophore production by Fungal M10 strain was studied using CAS blue agar and the iron(III)-chelating compounds, excreted by the microorganism and diffused through the medium producing a colour change from blue to orange. Microbial siderophores production increases virulence power of microorganisms and serve as good biocontrol agents, (Talebi
et al., 2011, Hussein and Joo, 2012, Vinale et al., 2013 and Balado et al. 2015).
![]()
Microbiological control of plant pathogen by rhizospheric microorganisms offers an attractive alternative over chemical control. There are evidences that microorganism can be served as good microbial control candidate to suppress the diseases. U.S. Environmental Protection Agency registered eight species of microorganisms for commercial use against soil borne plant pathogens in the United States (Cook et al., 1996). Researchers from different region reported microorganisms as biocontrol agents such as Pseudomonas sp. (Chernin et al., 2011 Sandheep et al., 2012; ), Bacillus sp. (Saha et al., 2012; Lamsal et al., 2012), Trichoderma sp. ( Ahith and Lakshmidevi, 2010; Otadoh et al., 2011; Sandheep et al., 2012) Serratia sp (Chernin et al., 2011) etc. Several researchers working on biological control of Fusarium wilt of different crops but there is less literature available on microbiological control of Fusarium wilt of Bt- cotton. Biological Control of Fusarium Wilt of Cotton was studied by using endophytic bacteria (Chen et al. 1995), Trichoderma sp (Sivan and Chet, 2008 and Mali and Ramaiah., 2015).
Soil serves as excellent culture medium for all types of microorganisms due to the abundant availability of nutrient and favorable environmental condition. Rhizosphere of plants has been frequently exploited as brilliant source for searching microbial control agents. It has been suggested that microorganisms isolated from the rhizosphere of a particular crop may be better adapted to that crops rhizospheric environment and may provide better control of diseases than the other plant rhizosphere species (Cook, 1993). The present study was aimed to i) Isolation of phytopathogen responsible for causing Fusarium wilt of Bt-cotton, ii) isolation of rhizospheric isolates from healthy Bt-cotton plant iii) in vitro screening of rhizospheric isolates for antifungal activity against Fusarium wilt pathogen and (iii) characterization of in vitro microbial control mechanism of efficient rhizospheric isolates by testing for production of volatile metabolites, diffusible metabolites and siderophore.
Material And Methods
Isolation Of Phytopathogen
Fusarium wilt infected Bt- cotton plants were collected from different fields of Beed, Hingoli and Vasantrao Naik Agricultural University, Parbhani district in sterile polyethylene bags and brought to research laboratory, Department microbiology Yogeshwari Mahavidyalaya, Ambajogai. Infected stems and branches of Bt-cotton were washed with sterile water thoroughly. The infected stem tissues were cut into small pieces (2–5 mm size) and by using flame-sterilized forceps; they were transferred to sterile Petri plates containing 0.1 % mercuric chloride solution for 30-60 s. These surface sterilized pieces of stem tissues are again transferred to another sterile Petri plates containing sterile distilled water to remove the disinfectant solution with 2-3 changes of sterile water. The outer layer of tissues were removed rapidly with sterile surgical blade and small pieces from the central core of tissues in the advancing margin of infection are cut and aseptically transferred to Petri plates containing Potato Dextrose Agar (PDA). Petri plates are incubated at room temperature for 6 days (Narayanasamy, 2011). Isolated pure culture of phytopathogen was maintained on PDA and Kings- B agar plate and also on slant for further use.
Isolation Of Rhizospheric Bacteria
Rhizospheric soil samples from healthy Bt- cotton growing fields of different districts of Marathwada region (Beed, Latur, Osmanabad, Aurangabad, Jalna, Nanded, Hingoli and Parbhani) were collected in sterile polyethylene bags and brought to research laboratory. 1g of each rhizospheric soil sample was mixed with 100 ml sterile distilled water and shaken well for 2 min, and then the content of flask was allowed to settle. Different dilutions 10-1, to10-6 of these samples were prepared by serial dilution technique. Highest dilution 10-4, 10-5 and 10-6 dilutions used for plating propose. 0.5 ml selected dilutions was plated by using pour plate technique. All the plates were incubated at room temperature for 24-48 h.
In Vitro Screening For Microbial Control Agent
114 rhizobacteria isolates were screened for antifungal activity against Fusarium oxysporum f.sp. vasinfectum by using dual culture technique on King B agar plates (Gull and Hafeez, 2012). 5 mm diameter mycelial disc was punched from margin of actively growing mycelium and placed at the center of 9 cm Petri plate and rhizobacterial isolates were inoculated 3 cm apart from the center. Three rhizospheric isolates were placed in a plate along with phytopathogen at the center. Control plate was kept without inoculation of rhizobacteria isolates and the all the plates were incubated at 28 0C for 6 days. The antifungal activity was determined by measuring the inhibition of mycelial growth of Fusarium oxysporum f. sp. vasinfectum.
Mechanism Of Microbial Control Agent
To characterize the mechanism of microbial control agent, the efficient rhizospheric isolates were tested for the production of volatile metabolites, diffusible metabolites and siderophore.
Detection Of Volatile Metabolites
Volatile metabolites detection of efficient rhizospheric isolates was done by using double plate method (Dennis and Webster, 1971). The kings-B agar plate inoculated with a 5 mm mycelial disc of Fusarium oxysporum f.sp. vasinfectum and rhizospheric isolate at the center of 90 mm Petri plate separately. Both the inoculated plates were placed facing each other and sealed with cellophane adhesive tape. Control was kept without inoculation of rhizospheric isolate. Procedure repeated for each efficient rhizospheric isolates in triplicates. All the plates were incubated at 28 0C for 6 days. The production of volatile metabolites was then determined by inhibition of Fusarium oxysporum f.sp. vasinfectum and percentage of radial growth inhibition was calculated by using the formula (Whipps, 1987).
Where, R1 is radial growth of the pathogen alone (a control value) and R2 is radial growth pathogen in the direction of the antagonist pathogen + antagonist (an inhibition value).
Detection Of Diffusible Metabolites
Diffusible antifungal metabolites were detected by well diffusion assay (Schlumbaum et al., 1986). Rhizobacterial isolates showing antifungal activity against Fusarium oxysporum f.sp. vasinfectum during screening were grown in King B broth at room temperature on rotary shaker at 150 rpm for 48 h to obtain cell free culture filtrate. Kings B agar plates were prepared and after solidification with the help of sterile cork borer three wells 3cm apart from the center 90 mm diameter were punched on a plate. These wells were labeled according to the rhizobacteria isolates cell free culture filtrate to be loaded. 5 mm plugs from leading edge of 3 day old culture Fusarium oxysporum f.sp. vasinfectum were punched and kept at the center of the plate. Different rhizobacterial isolate broths were filtered by using Millipore syringe filter 0.22 µ (Hi-media) to prepare filtrate for diffusible metabolites. Each well was loaded with 100 µl cell free culture filtrate aseptically. Control was kept without inoculation of rhizobacterial cell free culture filtrate. All the plates were incubated at 28 0C for 6 days. Plates were observed for zone of inhibition. Percentage of radial growth inhibition of pathogen was calculated by using the formula, (Whipps, 1987).
Qualitative Detection Of Siderophore
Siderophore production was determined by using modified Chrome Azurol S (CAS) assay (Milagres et al., 1999). Initially all the glassware’s are rinsed with distilled water and dried. 60.5 mg of CAS was weighed accurately and dissolved in 50 ml of distilled water and to this add 10 ml of iron solution (1 mM FeCl3.6H2O, in 10 mM HCl). 72.9 mg of Hexa decyl tri methyl-ammonium bromide (HDTMA) dissolved in 40 ml distilled water. CAS and Iron solution mixture was slowly added to 40 ml HDTMA with constant stirring to obtain dark blue colour. Basal Medium containing 30.24 g Pipes, and 12 g of a 50 % (w/w) NaOH to rise the pH to the pKa of Pipes (6.8) and 15 g Agar in 750 ml distilled water. All the contents are separately sterilized by autoclaving at 121 0C for 15 min. After cooling to 50 0C to basal medium 750 ml add 100 ml CAS-Fe-HDTMA mixture along the glass wall and agitated with enough care to avoid foaming. Petri dishes (9.0 cm in diameter) were prepared with 30 ml of Nutrient agar medium for culturing rhizospheric isolates. After solidification, the medium was cut into halves, one of which was replaced by CAS blue agar (15 ml) and allowed to solidify. The halves containing culture medium was inoculated with 24 h old rhizospheric isolate near the borderline of the two medium. The same procedure was repeated for each rhizospheric isolate. Un-inoculated CAS agar plate serves as control. All plates were incubated at room temperature for 5 days and changed CAS agar colour from blue to orange or purple or dark purplish- red indicates the siderophore production.
Results And Discussion
Isolation Of Phytopathogen
Fusarium wilt phytopathogen was isolated from infected Bt-cotton plant. The phytopathogen produced whitish mycelial growth on inoculated PDA plates and growth of fungal mycelium was transferred on fresh PDA and purified (Fig.1). Based on macro and microscopic observations of mycelial growth showed boat-shaped macroconidia, with slightly tapering apical cells and hooked basal cells 4-celled, microconidia ellipsoidal, 1-celled and chlamydospores are globose, usually solitary, phytopathogen was identified as species Fusarium oxysporum (Watanabe, 2010; Gull and Hafeez, 2012). The phytopathogen was transferred on fresh PDA and Kings B agar plates and also on maintained on slants for further use.
 |
Figure 1 |
Isolation Of Rhizospheric Bacteria
114 rhizobacterial isolates from different Bt-cotton fields were isolated from rhizospheric soil samples by serial dilution technique using pour plate method . Isolates were selected on the basis of distinctive morphology, size, shape and colour of the bacterial colony and location of the sample. All the isolates were tentatively labeled as RLS01 to RLS114 and maintained on nutrient agar slants with periodic transfer to fresh medium for future applications.
In Vitro Screening For Microbial Control Agent
All the114 rhizospheric isolates were screened for in vitro antifungal activity against Fusarium oxysporum f.sp. vasinfectum by dual culture technique Gull and Hafeez, 2012). Out of 114, 13 rhizobacterial isolates inhibited mycelial growth in dual culture. These isolates showed significant differences in mycelial growth inhibition. Radial growth of phytopathogen in test and control was measured and recorded (Table 1 and Fig 2). Rhizospheric isolates showed significant inhibition RLS19 (68.89 %), RLS52 (62.22 %), RLS53 (60.00 %), RLS72 (57.78 %) and RLS101 (57.78 %) of Fusarium oxysporum f.sp. vasinfectum in dual culture technique. The highest antifungal activity was shown by rhizospheric isolate RLS19 (68.89 %) and followed by RLS52 (62.22 %), RLS53 (60.00 %).
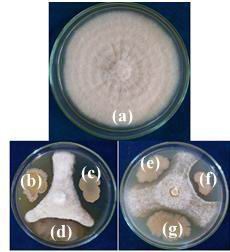 |
Figure 2: In vitro screening of antifungal activity of rhizospheric isolates against Fusarium oxysporum f.sp. vasinfectum (a) Control (b) RLS19 (c) RLS107 (d) RLS58 (e) RLS72 (f) RLS53 and (g) RLS52 |
| Table 1: In vitro screening of antifungal activity of rhizospheric isolates against Fusarium oxysporum f.sp. vasinfectum | ||||
| Sr. No. | Rhizospheric isolate | Growth towards antagonist in mm (R2) | Growth of control in mm (R1) | % of inhibition |
| 1 | RLS18 | 20 | 45 | 55.56 |
| 2 | RLS19 | 14 | 45 | 68.89 |
| 3 | RLS53 | 18 | 45 | 60.00 |
| 4 | RLS52 | 17 | 45 | 62.22 |
| 5 | RLS58 | 20 | 45 | 55.56 |
| 6 | RLS72 | 19 | 45 | 57.78 |
| 7 | RLS76 | 20 | 45 | 55.56 |
| 8 | RLS79 | 22 | 45 | 51.11 |
| 9 | RLS96 | 25 | 45 | 44.44 |
| 10 | RLS101 | 23 | 45 | 48.89 |
| 11 | RLS102 | 22 | 45 | 51.11 |
| 12 | RLS107 | 19 | 45 | 57.78 |
| 13 | RLS106 | 22 | 45 | 51.11 |
| 14 | Control | 45 | 100.00 | |
Similar results were also recorded by other investigators, rhizospheric Pseudomonas isolate, isolated from rhizospheric soil of tomato evaluated against Fusarium oxysporum f.sp. lycopersici by dual culture technique and maximum zone inhibition was 22 mm (Asha et al., 2011). Rhizospheric isolates, Pseudomonas sp. and Bacillus sp. were evaluated for biocontrol potential against Fusarium oxysporum f.sp. ciceris by dual culture technique, where Bacillus subtilis B28 showed 51.16 % inhibition (Karimi et al., 2012). Endophytic bacteria were antagonistic against F. oxysporum, isolates EB1 and EB2 inhibits radial growth 42.60 % and 41.00 % respectively in dual culture test (Edward et al., 2013).Soil isolates SC09-21, SGO9-01, SR04-02 and SR04-16 inhibited the mycelial growth of Fusarium oxysporum f.sp. radicis-lycopersici in vitro by dual culture technique. Isolate SC09-21 showed 12.2 mm and SGO9-01, R04-02 and SR04-16 less than 10 mm zone of inhibition due to antibiosis (Xu and Kim, 2014). Trichoderma harzianum significantly reduce the mycelial growth of Fusarium oxysporum f.sp. ciceri, a wilt pathogen of chickpea ranging from 20.11% to 65.78% (Srivastava et al., 2015).
| Table 2: Production of diffusible antifungal metabolites by rhizospheric isolates against Fusarium oxysporum f.sp. vasinfectum | ||||
| Sr. No. | Rhizospheric isolates | Growth towards antagonist in mm (R2) | Growth of control in mm (R1) | % of inhibition |
| 1 | RLS18 | 19 | 40 | 52.50 |
| 2 | RLS19 | 17 | 40 | 57.50 |
| 3 | RLS53 | 20 | 40 | 50.00 |
| 4 | RLS52 | 18 | 40 | 55.00 |
| 5 | RLS58 | 22 | 40 | 45.00 |
| 6 | RLS72 | 20 | 40 | 50.00 |
| 7 | RLS76 | 21 | 40 | 47.50 |
| 8 | RLS102 | 20 | 40 | 50.00 |
| 9 | Control | nil | 40 | 100.00 |
When results of previous researchers (Xu and Kim, 2014; Asha et al., 2011; Karimi et al., 2012; Edward et al., 2013; Xu and Kim, 2014; Srivastava et al., 2015) compared to our findings, our finding found better than the others, where rhizospheric isolates RLS19 (68.89 %), RLS52 (62.22 %), RLS53 (60.00%) RLS72 (57.78 %), and RLS101 (57.78 %) found better in inhibiting the Fusarium sp. Based on screening results in dual culture technique, eight isolates namely (RLS18, RLS19, RLS52, RLS53, RLS58, RLS72, RLS76 and RLS102) with good inhibition activities of Fusarium oxysporum f.sp. vasinfectum were selected for further study.
| Table 3: Siderophore production of rhizospheric isolates by modified CAS assay | ||
| Sr. No. | Rhizospheric isolate | Siderophore production |
| 1 | RLS18 | +++ |
| 2 | RLS19 | ++ |
| 3 | RLS53 | +++ |
| 4 | RLS52 | ++ |
| 5 | RLS58 | +++ |
| 6 | RLS72 | ++ |
| 7 | RLS79 | ++ |
| 8 | RLS102 | ++ |
| 9 | Control | nil |
| -= No siderophore production, += less siderophore production | ||
| += less siderophore production ++=medium siderophore production +++= high siderophore production | ||
Mechanism Of Microbial Control Agent
While finding the mechanism of the microbial control agent, the rhizospheric isolates were tested for the production of volatile metabolite, diffusible metabolite and siderophore production.
Detection Of Volatile Metabolites
After 6 days incubation it was observed that three rhizospheric isolates were able to produce volatile metabolites and inhibit the radial growth of Fusarium oxysporum f. sp. vasinfectum (Fig. 3 and Fig. 4). Highest Inhibition of phytopathogen by producing volatile metabolites was shown by rhizospheric isolate RLS101 (50 %) followed by RLS52 (20 %) and RLS79 (10 %). Other isolates were unable produce volatile metabolites and there was no inhibition of Fusarium oxysporum f.sp. vasinfectum growth.Previous researcher found that Pseudomonas aueroginosa P12 isolate inhibits 26.30 % radial growth of Fusarium oxysporum f. sp. ciceris by producing volatile metabolites (Karimi et al., 2012). This finding suggests that volatile metabolite production by rhizospheric isolates is one of the mechanism by which phytopathogen can be controlled. Here in present study, rhizospheric isolate RLS101 found better in controlling Fusarium oxysporum f.sp. vasinfectum by producing volatile metabolites than Pseudomonas aueroginosa P12 (Karimi et al., 2012).
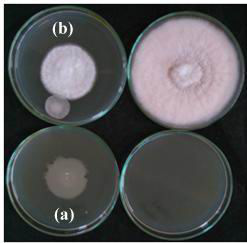 |
Figure 3: Detection of volatile metabolite production of rhizospheric isolate RLS102 against Fusarium oxysporum f.sp. vasinfectum (a) Control (b) Test |
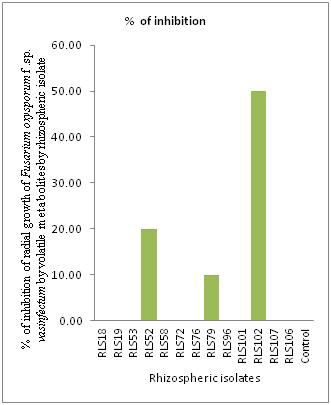 |
Figure 4: Production of volatile antifungal metabolites of rhizospheric isolates against Fusarium oxysporum f.sp. vasinfectum |
Detection Of Diffusible Metabolites
Diffusible antifungal metabolites were studied by well diffusion assay (Schlumbaum et al., 1986). Eight efficient rhizospheric isolates tested showed inhibitory effect on Fusarium oxysporum f.sp. vasinfectum by producing diffusible metabolites (Fig. 5 and Table 2). Rhizospheric isolate RLS19 (57.50 %) showed highest inhibition of phytopathogen by producing diffusible metabolites followed by RLS52 (55.00 %) and RLS 18 (52.05 %). Bacillus subtilis B28 isolate also inhibiting Fusarium oxysporum f.sp. ciceris (78.30 %) by producing diffusible metabolites (Karimi et al., 2012).
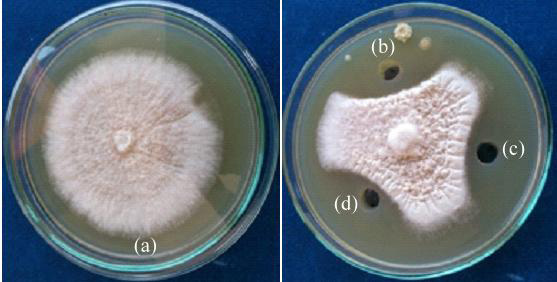 |
Figure 5: Detection of diffusible metabolites production of rhizospheric isolate by agar well diffusion method against Fusarium oxysporum f.sp. vasinfectum (a) Control (b) RLS18 (c) RLS76 (d) RL19 |
Qualitative Detection Of Siderophore
After 5 day incubation, eight isolates produced siderophore on modified CAS plate. Siderophore production was recorded in the form of grades (Fig.6 and Table 3). The highest siderophore production was recorded in the form of change in colour of the medium from blue to purple or orange (Fig. 6). Highest siderophore producing rhizospheric isolates were RLS18, RLS53 and RLS58. Siderophore production was recorded in the form of change in colour from blue to purple or orange (Milagres et al., 1999). CAS agar plate assay indicated that all the rhizobacterial isolates have siderophore production ability, RLS18, RLS53 and RLS58 found superior compared to other rhizospheric isolates. Similar result was reported by other researchers (Chaiharn et al., 2009). The qualitative production of siderophore by B. cereus with orange halos during the exponential growth and Sporulation phases by modified CAS plate assay (Lalloo et al., 2010).
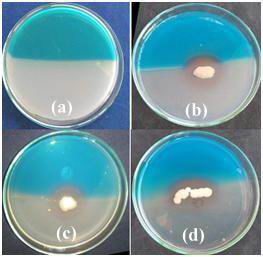 |
Figure 6: Siderophore production of rhizospheric isolates by modified CAS assay (a) Control (b) RLS18 (c) RLS58 (d) RLS53 |
Production of siderophores by different fungal species isolated from heavy metal contaminated and uncontaminated soils was studied by using Chrome azurol sulfonate (CAS) was used for both quantitative and qualitative evaluation of siderophores production. The production of siderophores in rhizosphere fungi was higher than those isolated from the contaminated soil (Hussein and Joo, 2012). Siderophore production by Fungal M10 strain was studied using CAS blue agar and the iron(III)-chelating compounds, excreted by the microorganism and diffused through the medium producing a colour change from blue to orange (Vinale et al., 2013). Microbial Siderophores production increases virulence power of microorganisms and serve as good biocontrol agents (Balado et al. 2015).
Conclusion
Our results suggest that the rhizospheric isolate RLS19, RLS52 RLS53 and RLS72 inhibits the mycelial growth of Fusarium oxysporum f.sp. vasinfectum by producing volatile metabolites diffusible metabolites and siderophore. The cumulative effect of these secondary metabolites resulted in inhibition of mycelial growth of the pathogen causing Fusarium wilt of Bt-cotton and serves as good alternative for chemical control. These four isolate will be serve as excellent microbial control agents against Fusarium wilt disease of Bt-cotton and needs to evaluate in field condition.
Acknowledgements
The authors wish to thank Dr. B. R. Chavan Principal, Yogeshwari Mahavidyalaya, Ambajogai for providing facilities to conduct the research.
References
Ajith P.S. and Lakshmidevi N. (2010) Effect of volatile and non-volatile compounds from Trichoderma spp. against Colletotrichum capsici incitant of anthracnose on bell peppers. Nat Sci. 8(9):265-269.
Asha B.B., Nayaka S.C., Shankar A.C.U., Srinivas C. and Niranjana S.R. (2011) Biological control of F. oxysporum f.sp. lycopersici causing wilt of tomato by Pseudomonas fluorescens. International Journal of Microbiology Research 3(2): 79-84.
Atkinson G.F. (1892) Some diseases of cotton. 3. Frenching. Bulletin of Alabama Agricultural Experimental Station 41: 19-29.
Balado M., Souto A., Vences A., Careaga V.P., Valderrama K., Segade Y., Rodríguez J., Osorio C.R., Jiménez C.and Lemos M.L. (2015) Two catechol siderophores, acinetobactin and amonabactin, are simultaneously produced by Aeromonas salmonicida subsp. salmonicida Sharing part of the biosynthetic pathway. ACS Chem Biol. 10(12): 2850–60.
Bennett R.S., Hutmacher R.B., Davis R.M. and Bennett R.S. (2008) Seed transmission of Fusarium Fusarium oxysporum f.sp. vasinfectum race 4 in California. The Journal of Cotton Science 12: 160-164.
Chaiharn M., Chunhaleuchanon S. and Lumyong S. (2009) Screening siderophore producing bacteria as potential biological control agent for fungal rice pathogens in Thailand. World Journal of Microbiology and Biotechnology 25(11):1919-1928.
Chen C., Bauske E.M., Musson G., Rodriguezkabana R. and Kloepper J.W. (1995) Biological control of Fusarium wilt on cotton by use of endophytic bacteria. Biological control 5(1): 83-91.
Chernin L., Toklikishvili N., Ovadis M., Kim S., Ben‐Ari J., Khmel I. and Vainstein A. (2011) Quorum‐sensing quenching by rhizobacterial volatiles. Environmental microbiology reports 3(6): 698-704.
Cook R.J. (1993) Making greater use of introduced microorganisms for biological control of plant pathogens. Annual review of phytopathology 31(1): 53-80.
Cook R.J., Bruckart W.L., Coulson J.R., Goettel M.S., Humber R.A., Lumsden R. D., and Quimby Jr P.C. (1996) Safety of microorganisms intended for pest and plant disease control: a framework for scientific evaluation. Biological control 7(3):333-351.
Dennis C. and Webster J. (1971) Antagonistic properties of species-groups of Trichoderma: I. Production of non-volatile antibiotics. Transactions of the British Mycological Society 57(1): 25-IN3.
Edward E.J., King W.S., Teck S.L.C., Jiwan, M., Aziz Z.F.A., Kundat F.R., and Majid N.M.A. (2013) Antagonistic activities of endophytic bacteria against Fusarium wilt of black pepper (Piper nigrum). J Agric Biol. 15: 291-296.
Gull M. and Hafeez F.Y. (2012) Characterization of siderophore producing bacterial strain Pseudomonas fluorescens Mst 8.2 as plant growth promoting and biocontrol agent in wheat. African Journal of Microbiology Research 6(33): 6308-6318.
Hussein K.A. and Joo J.H. (2012) Comparison between Siderophores production by fungi isolated from heavy metals polluted and rhizosphere soils. Korean Journal of Soil Science and Fertilizer 45(5):798-804.
Karimi K., Amini J., Harighi B. and Bahramnejad B. (2012) Evaluation of biocontrol potential of Pseudomonas and Bacillus spp. against Fusarium wilt of chickpea. Australian Journal of Crop Science 6(4): 695-703.
Kulkarni G.S. (1934) Studies in the wilt disease of Cotton in the Bombay Presidency. Indian Journal of Agricultural Science 4(6): 976-1048.
Lalloo R., Moonsamy G., Ramchuran S., Görgens J. and Gardiner N. (2010) Competitive exclusion as a mode of action of a novel Bacillus cereus aquaculture biological agent. Letters in applied microbiology 50(6), 563-570.
Lamsal K., Kim S.W., Kim Y.S. and Lee Y.S. (2012) Application of rhizobacteria for plant growth promotion effect and biocontrol of anthracnose caused by Colletotrichum acutatum on pepper. Mycobiology 40(4): 244-251.
Mali R.R. and Ramaiah P.V. (2015) Studies on biocontrol of wilt of cotton caused by Fusarium oxysporum with the help of Trichoderma viride. J Cnem Biol Phys Sci. 5(3): 2885-2890.
Milagres A.M., Machuca A. and Napoleao D. (1999) Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol S (CAS) agar plate assay. Journal of Microbiological Methods 37(1): 1-6.
Narayanasamy P. (2011) Diagnosis of Fungal Diseases of Plants. In Microbial Plant Pathogens-Detection and Disease Diagnosis: (pp. 273-284). Springer Netherlands.
Otadoh J.A., Okoth S.A., Ochanda J., and Kahindi J.P. (2011) Assessment of Trichoderma isolates for virulence efficacy on Fusarium oxysporum f.sp. phaseoli. Tropical and Subtropical Agroecosystems 13: 99-107.
Saha D., Purkayastha G.D., Ghosh A., Isha M., and Saha A. (2012) Isolation and characterization of two new Bacillus strains from the rhizosphere of eggplant as potential biocontrol agents. Journal of Plant Pathology 94(1): 109-118.
Sandheep A.R., Aju K.A. and Jisha M.S. (2012). Biocontrol of Fusarium wilt of vanilla (Vanilla planifolia) using combined inoculation of Trichoderma sp. and Pseudomonas sp. International Journal of Pharma and Bio Sciences 3(3): 706-716.
Schlumbaum A., Mauch F., Vogeli U. and Boller T. (1986) Plant chitinases are potent inhibitors of fungal growth. Nature 324: 365 – 367.
Sivan A. and Chet I. (1986) Biological control of Fusarium spp. in cotton, wheat and muskmelon by Trichoderma harzianum. Journal of Phytopathology 116(1): 39-47.
Srivastava J., Dwivedi S.K. and Prasad C. (2015) Efficacies of some fungal antagonist against chickpea wilt pathogen Fusarium oxysporum f.sp. ciceri. International Journal of Science and Technology 5(3): 8-19.
Talebi K., Ghadamyari M., and Hosseininaveh V. (2011) Ecological impacts of pesticides in agricultural ecosystem. In: Pesticides in the modern world-risks and benefits.143-168 (Ed) by Stoytcheva M. Intech Open Access Publisher, Croatia.
Vinale F., Nigro M., Sivasithamparam K., Flematti G., Ghisalberti E.L., Ruocco M. and Woo S.L. (2013) Harzianic acid: a novel siderophore from Trichoderma harzianum. FEMS microbiology letters 347(2): 123-129.
Watanabe T. (2010) Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species. CRC press 270-276.
Whipps J.M. (1987) Effect of media on growth and interactions between a range of soilborne glasshouse pathogens and antagonistic fungi. New Phytologist 107(1): 127-142.
Xu S.J. and Kim B.S. (2014) Biocontrol of Fusarium crown and Root rot and promotion of growth of tomato by Paenibacillus strains isolated from soil. Mycobiology 42(2):158-166.


